Abstract
The protein kinase D (PKD) family of serine/threonine kinases, which can be activated by gastrointestinal hormones, consists of three distinct isoforms that modulate a variety of cellular processes including intracellular protein transport as well as constitutive and regulated secretion. Although isoform-specific functions have been identified in a variety of cell lines, the expression and function of PKD isoforms in normal, differentiated secretory tissues is unknown. Here, we demonstrate that PKD isoforms are differentially expressed in the exocrine and endocrine cells of the pancreas. Specifically, PKD3 is the predominant isoform expressed in exocrine cells of the mouse and human pancreas, whereas PKD1 and PKD2 are more abundantly expressed in the pancreatic islets. Within isolated mouse pancreatic acinar cells, PKD3 undergoes rapid membrane translocation, trans-activating phosphorylation, and kinase activation after gastrointestinal hormone or cholinergic stimulation. PKD phosphorylation in pancreatic acinar cells occurs viaaCa2+-independent, diacylglycerol- and protein kinase C-dependent mechanism. PKD phosphorylation can also be induced by physiologic concentrations of secretagogues and by in vivo stimulation of the pancreas. Furthermore, activation of PKD3 potentiates MEK/ERK/RSK (RSK, ribosomal S6 kinase) signaling and significantly enhances cholecystokinin-mediated pancreatic amylase secretion. These findings reveal a novel distinction between the exocrine and endocrine cells of the pancreas and further identify PKD3 as a signaling molecule that promotes hormone-stimulated amylase secretion.
Protein kinase D (PKD),2 a serine/threonine kinase family with a catalytic domain homologous to the Ca2+/calmodulin-dependent kinase domain and two cysteine-rich phorbol ester binding domains similar to those of protein kinase C (PKC), is a physiologically important downstream mediator of diacylglycerol (DAG) signal transduction (1, 2). The mammalian PKDs include three members, PKD1, PKD2, and PKD3, which demonstrate different expression patterns and functions depending on the cell type and external signal stimuli. PKDs are ubiquitously expressed, but levels of individual isoforms vary with developmental stage and cell type (3). PKD proteins are reported to localize in the cytosol, Golgi, nucleus, and vesicle structures (4-9). Activation of PKDs results in a dynamic translocation among subcellular compartments (10, 11). Expression of multiple isoforms in different cell types and in different subcellular localizations suggests that individual PKD isoforms may serve specific functions. The majority of findings demonstrating the diverse expression patterns and functions of PKD have been described using established cell lines (4-9, 12). However, little is known about PKD isoform expression and function in normal differentiated cells and tissues.
Recent functional studies have shown that PKD isoforms differentially regulate exocytic protein trafficking and cargo specificity (9, 12-14). Furthermore, PKD isoforms are differentially activated by oxidative stress signaling via PKCδ-mediated tyrosine phosphorylation (15). In each of these studies, PKD3 was found to have a regulatory mechanism or cellular function distinct from that of PKD1 and PKD2. Unlike the other two isoforms, PKD3 lacks the N terminus hydrophobic domain or the C terminus PDZ binding motif and contains divergent PH (pleckstrin homology) and C1 domains, which are important for regulating its catalytic activity (12, 16, 17). Current knowledge of the physiologic function of PKD3 is limited. It has been demonstrated using kinase-inactive mutants that PKD3 activity is required for basolateral exocytosis in Madin-Darby canine kidney cells (13). PKD3 has also been implicated in the epigenetic control of chromatin by regulating class II histone deacetylases in B lymphocytes (18). Furthermore, PKD3 was found to be a specific regulator of glucose transport in skeletal muscle cells (19).
The exocrine pancreas is highly specialized for the synthesis, storage, and exocrine secretion of digestive enzymes and bicarbonate-rich fluid (20). More than 90% of the newly synthesized proteins in the pancreas is targeted to the secretory pathway (21). In addition, the pancreas contains a variety of endocrine cells localized to the islets which secrete peptide hormones. Numerous steps in the secretory pathway are modulated by DAG signaling, which promotes secretion by maintaining Golgi function and/or activating DAG receptor kinases such as PKCs, which are regulators of exocytic proteins (1, 22-25). PKD is also critical for DAG-mediated secretion, as it is recruited by DAG to the trans-Golgi network, where it phosphorylates the lipid kinase phosphatidylinositol 4-kinase to initiate the process of vesicle fission (9, 26). Gastrointestinal (GI) hormones such as cholecystokinin (CCK), gastrin, neurotensin (NT), and bombesin (BBS)/gastrin-releasing peptide are potent regulatory peptides that modulate pancreatic function (27, 28). They are known to activate PKDs to promote cell proliferation and survival in gut epithelial cells (29-32); however, the role of PKDs in modulating the secretory actions of GI hormones is unknown.
Although the PKD isoforms have been reported to be expressed in secretory tissues such as salivary glands, adrenal glands, intestinal mucosa, and the pituitary (3, 5, 33), the role of PKD in the process of regulated secretion remains poorly understood. Previously, we demonstrated that PKD1 mediates NT peptide secretion from a pancreas-derived neuroendocrine cell line, BON, and that PKD1 activation is regulated by PKC and Rho/Rho kinase pathways (4); PKD1 and PKD2 isoforms are highly expressed in this endocrine cell line with little to no PKD3 expression, thus suggesting that PKD1/2 may be the predominant isoforms for endocrine secretion. The distribution and role of PKD isoforms in the pancreas, an organ with both exocrine and endocrine functions, is not known. Interestingly, we demonstrate that in both human and mouse pancreas, PKD3 is the predominant PKD isoform expressed in the exocrine acini, whereas PKD1 and PKD2 are more highly expressed in endocrine islets. PKD3 is catalytically activated by GI hormone stimulation of the pancreas, and its activation is dependent on CCK1/2 receptor binding and on DAG/PKC activity. PKD3 overexpression in mouse pancreatic acinar cells significantly increased CCK-mediated pancreatic amylase secretion, suggesting that PKD3, in concert with other signaling molecules, contributes to stimulated amylase secretion. Our findings reveal a distinct expression pattern in the exocrine and endocrine cells of the mouse and human pancreas and identify PKD3 as a novel DAG-activated mediator of the exocrine secretory process in response to GI hormone signaling.
EXPERIMENTAL PROCEDURES
Materials—All reagents and antibodies used in this study are described in supplemental Table 1.
Tissues and Immunohistochemistry (IHC)—Mouse pancreas and salivary glands from C57BL/6 and Swiss-Webster male mice (4-6 months old) and aged C57BL/6 mice (22-24 months old) were used for RT-PCR, Western blotting, and IHC analysis. Pancreas from Sprague-Dawley male rats (100-150 g) were used for RT-PCR and Western blotting. All tissues were immediately frozen in liquid nitrogen after resection and stored at -80 °C. All procedures were approved by the Institutional Animal Care and Use Committee and the Internal Review Board for Use of Human Materials at the University of Texas Medical Branch and are in accordance with National Institutes of Health guidelines. IHC staining was performed by the dextran polymer method using Dako EnVision+ system as we have previously reported (34). Pancreatic or salivary tissues were dissected and fixed in 10% neutral-buffered formaldehyde for 2 days and embedded in paraffin. Serial sections of paraffin-embedded specimens were deparaffinized in xylene, hydrated in 100% ethanol, and placed in Tris-buffered saline. Antigen retrieval was performed by incubating the specimens in 10 mm citrate (pH 6.0) in a heated microwave. Endogenous peroxidase was blocked by treatment with 0.03% H2O2 for 5 min. Serial sections of pancreatic tissues were incubated overnight with the anti-PKD1/2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) (1:50-1:200) or the anti-PKD3 antibody (Bethyl Laboratories, Montgomery, TX) (1:50-1:200). After rinsing in Tris-buffered saline, the specimens were then incubated with peroxidase-labeled secondary antibody for 30 min, washed, and then treated with DAB chromogen solution. After washing in distilled water, specimens were counterstained with hematoxylin.
RNA Isolation and RT-PCR—Total RNA was prepared from resected whole mouse pancreas or isolated pancreatic acini using the Qiagen RNeasy RNA isolation kit (Valencia, CA) with DNase digestion. RNA samples are quantified using a Nanodrop Spectrophotometer (Wilmington, DE) and qualified using the Agilent 2100 Bioanalyzer (Santa Clara, CA). Conventional RT-PCR was performed using the Roche Titan One Tube RT-PCR system (Indianapolis, IN) following the manufacturer's recommendations. Reverse transcription was conducted at 50 °C for 30 min from 500 ng of purified RNA followed by 30 cycles of standard PCR (95 °C, 30 s; 54 °C, 30 s; 68 °C, 40 s). RT-quantitative PCR was performed by previously published methods (35). Briefly, cDNA synthesis was performed using the reagents in the Taqman RT kit (25 °C, 10 min; 48 °C, 30 min; 95 °C, 5 min). Quantitative-PCR amplifications (performed in triplicate) were performed using 2 μl of cDNA with 250 nm TaqMan MGB probes and 900 nm primers. PCR assays were conducted in the ABI Prism 7000 System (50 °C, 2 min; 95 °C, 10 min; 40 cycles of 95 °C, 15 s and 60 °C, 1 min). Relative quantifications using either the standard curve method (primer set 1) or the comparative Ct method (primer set 2) were performed following the manufacturer's recommendations. The relative efficiency of RT-PCR reactions from primer pair set 1 was controlled by comparing the Ct values of each reaction to a standard curve constructed using known amounts of synthetic Neil2 transcript. For the comparative Ct method, endogenous 18 S RNA levels were used as an active reference to normalize for different amounts of RNA. The PCR primer sequences are listed in supplemental Table 2.
Isolation of Mouse, Rat, and Human Pancreatic Acini—Pancreatic acini were isolated from C57BL/6 or Swiss-Webster male mice (4-6 months old) or from Sprague-Dawley male rats (100-150 g) as reported previously using collagenase digestion (34). The perfused pancreas was dissected, minced, and transferred to prewarmed oxygenated phosphate buffered saline with Ca2+ and Mg2+ containing 0.1% bovine serum albumin, 0.01% (w/v) soybean trypsin inhibitor, and type IV collagenase (0.25 mg/ml). The minced pancreas was incubated at 37 °C for 8 min with vigorous pipeting and then washed with fresh phosphate-buffered saline and filtered through 100-μm mesh. For secretions studies, acini were cultured in suspension in DMEM with 0.1% fetal bovine serum (FBS), 0.25 mg/ml soybean trypsin inhibitor. For all other studies, isolated acini were cultured on laminin-coated dishes. The isolated human pancreatic acini and pancreatic islets were obtained from the National Diseases Research Interchange (Philadelphia, PA); cell isolations were from deceased donors with no history of pancreatic disease. The cells were received and cultured in DMEM (acini) or RPMI (islets) ∼10 h after isolation.
Cell Culture and Transfections—The AR42J, Panc-1, and HEK293 cell lines were obtained from the American Type Culture Collection (Manassas, VA). Plasmids encoding wild type PKD1 (pME-Py-GST-PKD1-WT), PKD2 (pME-Py-GST-PKD2-WT), and PKD3 (pME-Py-GST-PKD3-WT) were kindly provided by Dr. Vivek Malhotra (University of California San Diego, La Jolla, CA). HEK293 cells were stably transfected with CCK2 receptor construct using Lipofectamine Plus Reagent (Invitrogen) and selected using a fluorescence-activated cell sorter-Vantage cell sorter as described previously (36). HEK293 CCK1R cells were kindly provided by Dr. Vincent Wu (UCLA, Los Angeles, CA). Stably transfected HEK293 cells were subsequently maintained in G418 (400 μg/ml). BON cells were cultured in a 1:1 mixture of DMEM and nutrient mixture, F12K, supplemented with 5% heat-inactivated FBS. MC-26 cells and isolated human islets were cultured in RPMI 1640 supplemented with glutamine (2 mm) and 10% FBS. AR42J, Panc-1, and HEK293 cells were cultured in DMEM supplemented with 10% FBS. All cells were cultured at 37 °C in a humidified 95% air, 5% CO2 atmosphere.
Viral Vectors and Infection—Recombinant adenovirus expressing wild type (WT)-PKD3 was kindly provided by Dr. Q. Jane Wang (University of Pittsburgh, Pittsburgh, PA). Isolated acini were infected with PKD3 adenovirus at titers of between 105 and 108 infectious units/ml in serum-free DMEM. After 90 min of incubation at 37 °C, 0.1% FBS DMEM was added, and the acini cultures were continued for 15-24 h before harvesting for assays. An adenovirus expressing the green fluorescent protein was used as a transfection control, and a null-adenovirus without a transgene and Ad-CMV-LacZ adenovirus expressing the β-galactosidase transgene were used as experimental controls. Recombinant short hairpin RNA lentivirus expressing PKD3-specific RNA interference (RNAi) target sequences were obtained from Sigma. Initial screening for RNAi activity was performed by transducing MC-26 cells and selecting stable cell lines with puromycin according to standard techniques (37). The selected target sequence for acinar cell transduction was CCGGGCATTTATGTACCCACCAAATCTCGAGATTTGGTGGGTACATAAATGCTTTTT. The non-targeting negative control short hairpin RNA lentivirus was obtained from Sigma. Isolated pancreatic acinar cells seeded in 12-well plates were transduced with either control or target sequence after infection with lentivirus (0.5 to 1.5 × 106 transducing units/well). Acinar cell culture medium was changed to fresh medium approximately every 12 h. Transduction efficiency and target protein knockdown were determined by Western blotting after 24-48 h.
Immunoprecipitation and PKD Kinase Assays—Acinar cell proteins (50 μg) were incubated with PKD3 antibody (1:50) on a shaker for 2 h at 4 °C followed by another 2 h of incubation with 30 μl of protein A-Sepharose beads at 4 °C. The immunocomplexes were suspended in 20 μl of kinase buffer. The kinase reaction, with or without 2.5 μg of syntide-2 as peptide substrate, was started by adding 5 μCi of [γ-32P]ATP and incubated for 10 min at 30 °C as we have previously described (4). Phosphorylation of peptide substrates was then evaluated by adsorption onto P81 phosphocellulose paper followed by scintillation counting to measure radioisotope incorporation.
Immunofluorescence Staining and Confocal Microscopy—Cells were cultured on laminin-coated glass cover slips. After agonist treatment, cells were subjected to immunofluorescent staining and analysis by confocal microscopy as described previously (4). Cells were fixed with 4% paraformaldehyde for 20 min at 37 °C, permeabilized with 0.3% Triton, and then incubated with primary antibodies for 1 h at room temperature, washed, and incubated with Alexa 488-conjugated secondary antibodies for 30 min. The fluorescence of immunoreactivity was observed by a LSM 510 META confocal system configured with a fluorescent and an Axiovert 200M inverted microscope (Zeiss, Jena, Germany). The image acquisition was performed using the Zeiss LSM510 work-station (Version 3.0) and the Zeiss Image Browser (Version 3.1) software.
Acinar Cell Secretion Assays—CCK-stimulated secretions were collected and assayed as described previously (38). Briefly, infected and control acini were washed and resuspended in Krebs-Henseleit buffer (KHB) (142 mm NaCl, 23.8 mm NaHCO3, 4.83 mm KCl, 0.96 mm KH2PO4, 1.20 mm MgSO4, 12.5 mm HEPES, 5 mm glucose, and 2.2 mm CaCl2, pH 7.4). After 30 min of incubation in fresh KHB, acini were divided into background, basal (non-stimulated) secretion, and stimulated secretion groups. Background medium was collected at time 0 after resuspension in KHB. Basal and stimulated secretion media were collected after 30 min at 37 °C. Acinar cell pellets were collected and sonicated in KHB and then diluted 1:10 for determination of total amylase activity. Amylase activity was measured using the Phadebas reagent (39). Basal or stimulated amylase activities were determined after subtracting the zero-minute background amylase activity. Results were expressed as percentage of total amylase activity.
Intracellular Calcium Imaging—Single acinar cell recordings of agonist-mediated increases in intracellular calcium ([Ca2+]i) was performed as described previously (40). Briefly, cells were washed in KHB and then loaded with 2.5 μm concentrations of the calcium sensing dye Fura-2 AM ester (Molecular Probes, Eugene, OR) with 0.055 Pluronic F-127 (w/v) for 50 min at 25 °C. Changes in [Ca2+]i were monitored with a Nikon Diaphot inverted microscope (Nikon Instrument Group, Garden City, NY) equipped with a Nikon 40× (1.3NA) Neofluor objective. The fluorescent light source was a PTI DeltaScan RD-1 ratio fluorescence spectrometer system equipped with a light-path chopper and dual-excitation monochromators. The Fura-2 was alternately excited at 340 and 380 nm, and fluorescence emission was monitored through a 510-nm bandpass filter (Omega) with a Roper Coolsnap EZ digital camera (Photometrics, Tucson, AZ).
Protein Preparation and Western Blotting—Cultured cells were washed with phosphate-buffered saline, lysed with Tris lysis buffer, and subjected to Western blotting as described previously (34). Protein lysates were resolved on NuPAGE 4-12% Bis-Tris gels and electrophoretically transferred to polyvinylidene difluoride membranes. Nonspecific binding sites were blocked with 5% dried skimmed milk in Tris-buffered saline-Tween for 1 h at room temperature, then membranes were incubated with primary antibodies overnight at 4 °C followed by secondary antibodies (1:10,000) conjugated with horseradish peroxidase. Membranes were developed using the ECL detection system. For densitometric analysis, immunoblots were captured and analyzed using the Image J software (National Institutes of Health).
Statistical Analysis—Amylase release expressed in percentage of total amylase was analyzed using analysis of variance for a two-factor factorial experiment. The two factors were virus (PKD3, β-galactosidase, mock) and CCK concentration (none, 10-11, 10-10, 10-9, 10-8 m CCK). Main effects and interaction were assessed at the 0.05 level of significance. Multiple comparisons were conducted using Fisher's least significant difference procedure with Bonferroni adjustment for the number of comparisons. Statistical computations were carried out using SAS 9.1® (SAS Institute Inc.).
RESULTS
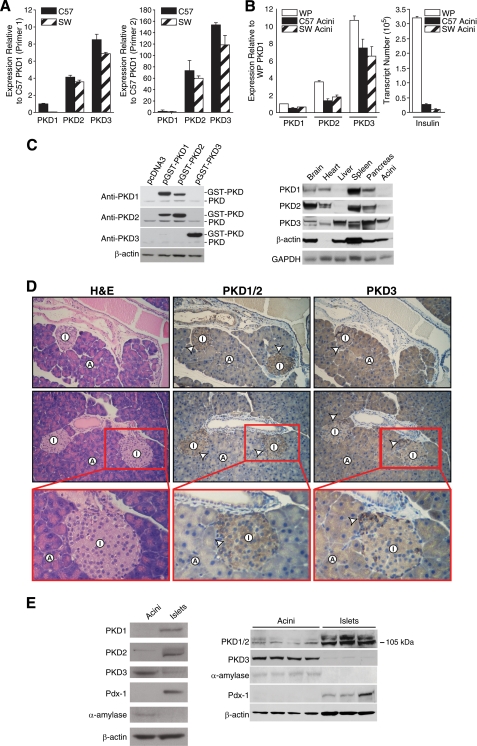
Differential Expression of PKD Isoforms in the Pancreas—The novel PKD proteins have important roles in constitutive and regulated secretion (4, 13, 14). The pancreas is a complex secretory tissue that contains both endocrine cells, which secrete hormones (e.g. insulin, glucagon), and exocrine cells, which secrete digestive enzymes (e.g. amylase). We have previously identified a critical role for PKD1 in stimulated hormone secretion from the BON endocrine cell line (4). To extend these findings and to delineate the potential roles of the PKD isoforms in the pancreas, we first examined PKD isoform expression in the pancreas. Total RNA was purified from C57BL/6 and Swiss-Webster mice pancreas for real time RT-PCR analysis. Quantification using the standard curve method revealed consistent differences in the relative abundance of each PKD isoform. The results were confirmed using two different primer sets that expand non-overlapping regions of each PKD transcript (Fig. 1A). Surprisingly, PKD3 was noted to be the most abundant isoform, whereas PKD1 and PKD2 were found to be expressed at relatively low levels in the pancreas, although all three transcripts were detectable by conventional RT-PCR (data not shown). Because the majority of pancreatic parenchyma consists of exocrine acini, high levels of PKD3 expression may reflect its distribution in the acinar cells. Consistent with this hypothesis, PKD3 expression levels were significantly higher than PKD1 and PKD2 levels in isolated acini (Fig. 1B, left panel); insulin gene expression was analyzed to quantify the purity of the isolated acini (Fig. 1B, right panel). A similar pattern of expression was noted in both young (4-6-month) and aged (22-24-month) C57BL/6 mice, demonstrating that age did not affect PKD expression in the pancreas. Our data show that pancreatic acini mainly express the PKD3 isoform, and this is reflected in the high level of PKD3 gene expression in whole pancreas.
FIGURE 1.
Expression pattern of PKD isoforms in the mouse pancreas. A, quantitative RT-PCR analysis of C57BL/6 (C57) and Swiss-Webster (SW) mouse pancreas using two different primer sets specific for PKD1-3 genes. Results from primer set 1 (left panel) and primer set 2 (right panel) are shown. Results are the means ± S.E. for triplicate assays from each animal. Similar expression patterns were observed in at least five C57 and five SW mice. B, quantitative RT-PCR analysis of whole pancreas (WP) and isolated acini using primer set 1. WP RNA was isolated from C57 pancreas. Insulin-1 gene expression was used to detect islet cell contamination. Results represent means ± S.E. for triplicate assays. Similar pattern of expression was observed in at least five acinar preparations. C, Western blot analysis of PKD protein expression in mouse tissues. The specificity of anti-PKD antibodies was examined by probing the overexpressed proteins from plasmids coding for pcDNA3, pGST-PKD1, pGST-PKD2, or pGST-PKD3 in BON cells (left panel). Homogenized proteins (100 μg) were obtained from perfused C57 mouse brain, heart, liver, spleen, pancreas, and isolated pancreatic acini and analyzed by Western blotting. Total cell lysates were probed with anti-PKD antibodies on parallel blots. β-Actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were probed as loading controls (right panel). D, immunohistochemical staining detects the expression of PKD1/2 in islets and the expression of PKD3 in acini plus islets in C57 mouse pancreas. Results are representative of at least 10 animals. H&E, hematoxylin and eosin. Arrowhead, positive-staining islet cells; A, acinar cell; I, islet (original magnification 100×; enlarged magnification 200×). E, Western blot analysis of PKD proteins in isolated human pancreatic acini and islets from two different donors. Cells were cultured in DMEM (Acini) or RPMI (Islets) for 4 h and then sonicated in lysis buffer. Total cell lysates were probed with anti-PKD antibodies on parallel blots along with acinar (α-amylase) and islet (Pdx-1) loading controls.
Next, we performed Western blotting and IHC utilizing PKD isoform-specific antibodies. The specificity of various PKD antibodies was tested against exogenous expression of different members of the PKD family using Western blot analysis (Fig. 1C, left panel). Anti-PKD1 and PKD2 antibodies detected their respective proteins, but significant cross-reaction was noted. In contrast, neither anti-PKD1 nor anti-PKD2 detected GST-PKD3, whereas the PKD3 antibody only recognized GST-PKD3. We then utilized these antibodies to examine tissue-specific expression of PKD proteins in the mouse. As shown in Fig. 1C (right panel), endogenous PKD1 and PKD2 expression was noted in the brain, heart, spleen, and pancreas, but not in the liver or pancreatic acini. In contrast, PKD3 was the main isoform in the liver and pancreatic acini. This result was confirmed by using two different antibodies for PKD1/2 to demonstrate the same pattern of tissue expression in both young and aged C57BL/6 mice (data not shown). Our data suggest differential distribution of PKD proteins in acini versus non-acinar components of the pancreas. We postulate that PKD3 is expressed in the exocrine acinar cells, whereas PKD1/2 is mainly expressed in non-acinar cells such as endocrine islets.
To examine the distribution pattern of PKDs in intact pancreatic tissues, IHC was performed to compare expression in the acinar, islet, and ductal cell types. In the mouse pancreas, the anti-PKD1/2 antibody stained mainly the endocrine islets with weak staining in the surrounding acinar compartments (Fig. 1D). In contrast, the anti-PKD3 antibody stained strongly in the acinar compartment and the islet clusters. This staining pattern was noticeably different from that of PKD1/2 staining. Duct cell epithelia and surrounding stroma were largely negative for PKD3 staining. The differential pattern of staining was observed in both C57BL/6 and Swiss-Webster mice. The IHC analysis of mouse tissues demonstrates the islet-specific expression of PKD1/2 and the strong expression of PKD3 in pancreatic acini.
To directly confirm the differential distribution of PKD proteins in human pancreas, we utilized freshly isolated human pancreatic acini and isolated pancreatic islets obtained from organ donors provided by the National Diseases Research Interchange. The purity of human pancreatic cell isolates was confirmed by the specific expression of α-amylase in isolated acini and Pdx-1, a transcription factor normally restricted to the islets of Langerhans in the adult pancreas (42), in isolated islets. As shown in Fig. 1E, PKD1 and PKD2 proteins were more strongly expressed in human islets than in acini, whereas PKD3 was predominantly expressed in acini with lower expression in islets. Taken together, our results demonstrate that PKD isoforms are differentially expressed in exocrine and endocrine cells of normal mouse and human pancreas, with PKD3 as the predominant isoform in exocrine acini.
The finding of relatively low levels of PKD1 expression in the mouse and human pancreas was surprising considering the recent report that PKD1 is expressed and activated in rat pancreatic acinar cells (43). To examine PKD expression pattern in the rat pancreas, we utilized RT-PCR and Western blotting. Similar to the findings by Berna et al. (43), PKD1 was the most abundant PKD isoform in the rat pancreas (supplemental Fig. 1A). PKD3 expression was noted in the whole pancreas but not in the isolated acini (supplemental Fig. 1B). Therefore, it appears that the PKD expression pattern is different in the rat compared with the mouse and human pancreas.
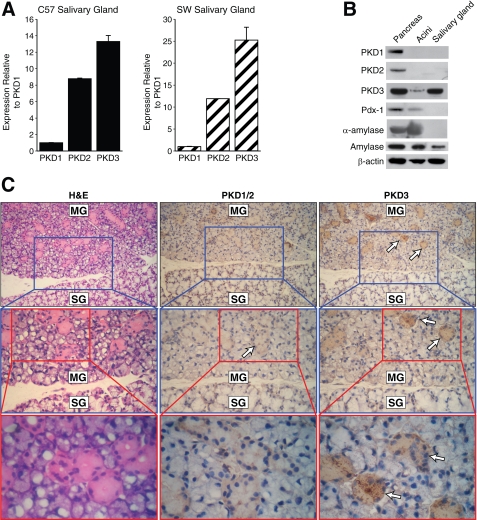
PKD3 Is the Predominant Isoform in Exocrine Salivary Gland Acini—Given our results regarding the marked PKD3 expression in the mouse pancreas, we hypothesized that a similar expression pattern would be noted in the salivary glands which, like the pancreas, contain exocrine acini that secrete amylase. The three salivary glands (parotid, submandibular, and submaxillary glands) from mice were excised and analyzed by RT-PCR, Western blotting, and IHC. Consistent with our findings regarding PKD in the exocrine pancreas, PKD3 is also the predominant PKD gene expressed in the salivary glands of C57BL/6 and Swiss-Webster mice (Fig. 2A). Furthermore, Western blot analysis of salivary glands revealed an expression pattern similar that of pancreatic acini with PKD3 the most abundantly expressed PKD protein and minimal to no PKD1 or PKD2 (Fig. 2B). IHC analysis of submandibular gland tissues demonstrated that PKD3 protein expression was localized in the serous acini, which secrete amylase, but not the mucous acini (Fig. 2C). The finding that PKD3 is the abundant isoform found in salivary glands and that PKD1 or PKD2 is not strongly expressed in these tissues is consistent with our hypothesis that PKD3 is the predominant isoform in mouse exocrine tissues.
FIGURE 2.
Expression pattern of PKD isoform in exocrine salivary glands. A, quantitative RT-PCR analysis of C57BL/6 (C57) and Swiss-Webster (SW) male mouse salivary glands using primer set 1 targeting PKD1-3 genes. Results represent the means ± S.E. for triplicate assays from each animal. B, Western blot analysis of PKD protein expression in salivary glands. Whole pancreas, isolated acini, and salivary tissue lysates from C57 mice were probed with anti-PKD antibodies on parallel blots. Two different anti-amylase antibodies and the β-actin antibody were used as loading controls. C, immunohistochemical staining detects the expression of PKD3 in the serous acini of submandibular salivary glands. Arrows, positive-staining acini; MG, submandibular gland; SG, sublingual gland (original magnification 100×, 1st enlarged magnification 200×; 2nd enlarged magnification 400×). All results are representative of three different animals. H&E, hematoxylin and eosin.
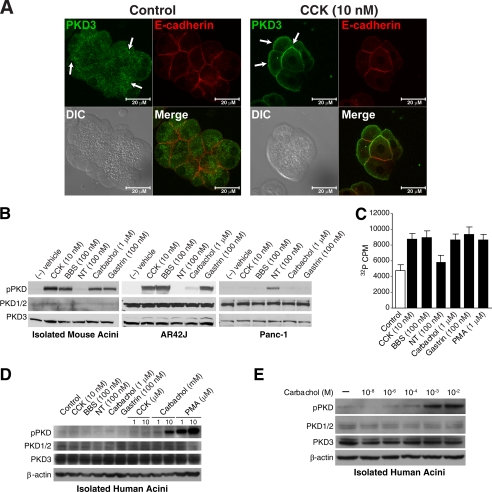
Secretagogue Stimulation Promotes PKD3 Translocation, Phosphorylation, and Kinase Activation in Pancreatic Acinar Cells—PKD3 is a novel member of the DAG-activated PKD family and is the least well characterized isoform. GI hormones such as CCK, gastrin, NT, and BBS/gastrin-releasing peptide can activate the DAG/PKC pathway in pancreatic acinar cells to regulate proliferation and secretion (27, 28). To determine whether PKD3 can be activated by GI hormone stimulation, we examined three processes that occur upon PKD activation; that is, translocation to cellular membranes, activation-loop phosphorylation, and increases in serine/threonine kinase activity (11, 17). First, immunocytochemistry was performed by immunofluoresence staining using the PKD3-specific antibody and visualized by confocal microscopy. PKD3 is reported to localize in the nucleus, trans-Golgi network, and vesicle structures (7, 13, 44). Within non-stimulated pancreatic acinar cells, PKD3 showed minimal staining at the cell membrane or nucleus but showed overlap with VAMP2 staining, indicating partial co-localization of these proteins in the secretory vesicles (supplemental Fig. 2A). VAMP2 contributes to the process of exocytosis (45, 46) and is expressed on zymogen granule membranes in pancreatic acinar cells (47). By comparison, PKD3 immunoreactivity in the cytosol showed little association with Golgi markers (supplemental Fig. 2B). After stimulation with CCK (10 nm) for 5 min, distinct PKD3 staining was noted in the plasma membranes of pancreatic acini (Fig. 3A). This plasma membrane staining was confirmed by partial co-localization of PKD3 with the adherens-junction membrane marker E-cadherin in CCK-stimulated cells. Our results indicate that PKD3 undergoes rapid membrane translocation in response to CCK stimulation.
FIGURE 3.
Secretagogue stimulation promotes PKD3 translocation, phosphorylation, and kinase activation in pancreatic acinar cells. A, isolated mouse pancreatic acini were incubated with control media or media containing CCK (10 nm) for 5 min and then immunostained using antibodies against PKD3 (1:100) and E-cadherin (1:50). Co-localization was qualitatively determined by overlap of green (PKD3) and red (E-cadherin) fluorescence. Arrows, translocation of PKD3 positive-staining; the size bar equals 20 μm. DIC, differential interference contrast. B, isolated mouse pancreatic acini, AR42J cells, and Panc-1 cells were treated with GI hormones at the indicated concentration for 30 min and then analyzed by Western blotting using anti-phospho (pPKD)-Ser-744/748 PKD and anti-total PKD antibodies on parallel blots. C, isolated mouse acini were treated with secretagogues at the indicated concentrations for 30 min and then lysed. PKD3 was immunoprecipitated from whole cell lysates, and kinase activity assay was performed on immunoprecipitates using syntide-2 as substrate. Results represent means ± S.E. for three separate kinase assays. PMA, phorbol 12-myristate 13-acetate. D, isolated human pancreatic acini were treated with secretagogues of the indicated concentration for 30 min and analyzed by Western blotting using anti-phospho-Ser-744/748 PKD and anti-total PKD antibodies on parallel blots. β-Actin was probed as a loading control. E, isolated human pancreatic acini from a different donor were treated with carbachol of the indicated concentration for 30 min and analyzed by Western blotting using anti-phospho-PKD and total PKD antibodies on parallel blots.
In addition to membrane translocation, PKD3 activation involves binding of DAG to its regulatory domain and phosphorylation of two activation-loop serine residues in the catalytic domain (44, 48). To examine the effect of GI hormones on PKD phosphorylation, we utilized an activation-loop antibody which specifically recognizes Ser(P)744/748 in PKD1, Ser(P)707/711 in PKD2, and Ser(P)730/734 in PKD3. As shown in Fig. 3B (left panel), CCK (10 nm), BBS (100 nm), carbachol (1 μm), or gastrin (100 nm) each induced phosphorylation of PKD in mouse pancreatic acini. A similar phosphorylation pattern was observed in the rat acinar tumor cell line AR42J (Fig. 3B, middle panel). However, unlike the primary isolated acini, the AR42J cell line demonstrates strong total PKD1/2 expression; thus, PKD activation in AR42J may be mediated by PKD1/2. The human pancreatic cancer cell line, Panc-1, which is derived from pancreatic duct cell origin, responded to 100 nm NT with PKD phosphorylation but not to the other agonists (Fig. 3B, right panel). These findings suggest that, in terms of PKD signaling, cells of acinar origin respond differently to GI hormones compared with cells of ductal origin. The activation of individual GI hormone receptors on different pancreatic cell types may determine whether PKD is phosphorylated in response to hormone stimulation.
Next, to evaluate PKD3 catalytic activity, we performed immunoprecipitation on acinar cell lysates using the PKD3-specific antibody and analyzed in vitro kinase activity using the synthetic peptide substrate syntide-2. Stimulation of acini with CCK (10 nm), BBS (100 nm), carbachol (1 μm), gastrin (100 nm), or the phorbol ester PMA (1 μm) caused an ∼2-fold increase in PKD3 kinase activity, as demonstrated by increased phosphorylation of the synthetic peptide substrate (Fig. 3C). This result indicates that PKD3 is catalytically activated after GI hormone stimulation in mouse pancreatic acini.
Next we examined PKD phosphorylation in isolated human pancreatic acini, which express high levels of PKD3 and relatively lower levels of PKD1/2. Stimulation with increasing concentrations of carbachol and PMA induced PKD phosphorylation (Fig. 3D). However, the same concentrations of GI hormones used to stimulate mouse pancreatic acini failed to activate PKD in the human acini. CCK failed to stimulate PKD phosphorylation in human acini, which is consistent with previous findings that human acini lack functional responses to CCK due to low level of receptor expression (49). Furthermore, it appears that high concentrations of carbachol are required to induce PKD phosphorylation in the human acini. To further investigate this response to carbachol, we obtained isolated human pancreatic acini from an additional donor and examined the effects of increasing concentrations of carbachol on PKD phosphorylation. As shown in Fig. 3E, carbachol, at a concentration of 1-100 μm, produced only a minimal increase in PKD phosphorylation, but carbachol (1 to 10 mm) significantly induced PKD phosphorylation. Our results demonstrate for the first time that PKD3 undergoes rapid translocation, phosphorylation, and kinase activation after GI hormone stimulation in mouse pancreatic acini and that cholinergic stimulation can lead to PKD phosphorylation in human pancreatic acini.
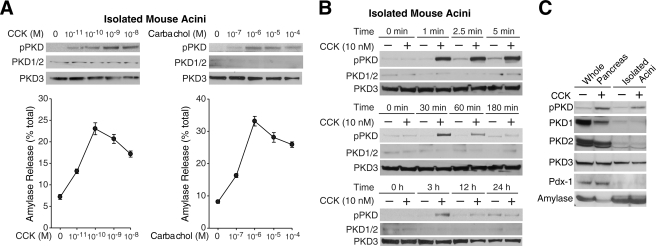
PKD Can Be Activated by Physiologic Concentrations of Secretagogues and by in Vivo Stimulation of the Pancreas—The GI hormone CCK and the neurotransmitter acetylcholine are the principle physiologic stimuli of pancreatic enzyme secretion (50). Physiologic CCK concentrations that maximally stimulate rodent pancreatic acinar cell secretion are usually in the picomolar range (51, 52), whereas acetylcholine concentrations are in the micromolar range (53). To determine whether physiologic concentrations of secretagogues that induce amylase secretion can also induce PKD phosphorylation, we examined the dose-response curve for CCK and carbachol. As shown in Fig. 4A, left panel, an increase in PKD phosphorylation was detectable with CCK at a concentration of 10 pm or greater. PKD phosphorylation reached a maximum at 1-10 nm CCK. Carbachol stimulation increased PKD phosphorylation at greater than 100 nm carbachol, reaching maximum stimulation at 1-100 μm carbachol (Fig. 4A, right panel). Thus, secretagogue-stimulated PKD phosphorylation can occur over a similar range of concentrations as secretagogue-stimulated secretion, suggesting that PKD activation is associated with the physiologic effects of CCK and carbachol. CCK-induced PKD phosphorylation also occurs in a rapid and transient manner as phosphorylation increased within 1 min of stimulation and reached maximal levels between 5 and 30 min of stimulation (Fig. 4B). The rapid onset of PKD signaling is consistent with the kinetics of CCK-induced enzyme secretion in acinar cells (54). CCK classically exerts its effects on the pancreas through the systemic circulation (51). Systemic administration of CCK by intraperitoneal injection also increased PKD phosphorylation in the whole pancreas within 30 min of stimulus, which is similar to the in vitro CCK stimulation of isolated pancreatic acini (Fig. 4C). In summary, PKD phosphorylation can be induced by physiologic concentrations of secretagogues and by in vitro and in vivo stimulation of the mouse pancreas.
FIGURE 4.
PKD phosphorylation is induced by physiologic concentrations of secretagogues and by in vivo stimulation of the pancreas. A, isolated mouse acini were treated with increasing concentrations of CCK or carbachol for 30 min. Top panels, protein was extracted from each sample and analyzed by Western blotting using anti-phospho (pPKD)-Ser744/748 PKD and anti-total PKD antibodies on parallel blots. Bottom panels, amylase release was measured from each sample medium. Results represent the means ± S.E. for four independent experiments. B, isolated acini were treated with CCK (10 nm) for the indicated time periods. Protein was extracted from each sample and analyzed by Western blotting. C, to obtain whole pancreas, mice were treated with either vehicle (saline) or CCK (50 mg/kg) by intraperitoneal injection, and after 30 min, the pancreas was harvested for protein extraction. Isolated acini were treated with either control media or media containing CCK (10 nm). Protein lysates from whole pancreas and isolated acini were probed with anti-PKD antibodies along with anti-Pdx-1 and amylase antibodies for loading control. Blots shown are representative of at least three independent experiments.
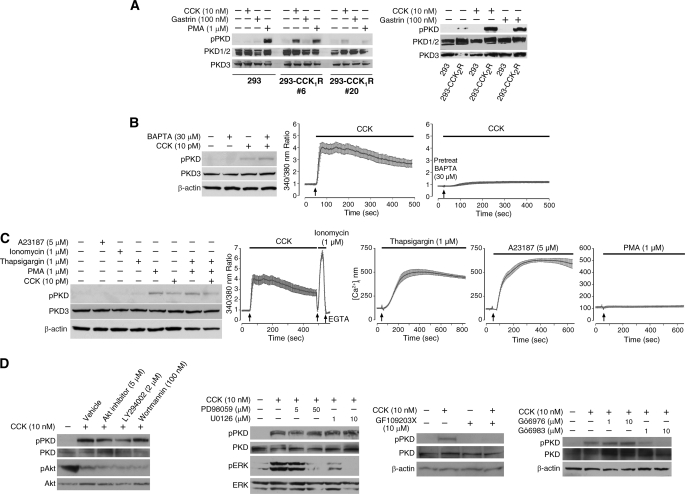
CCK-induced PKD Phosphorylation Is Mediated by CCK1/2 Receptors via the DAG/PKC Pathway but Is Not Dependent on Ca2+ Mobilization—Binding of CCK to its receptor causes activation of PLC-β, leading to an increase of intracellular Ca2+ levels and generation of inositol 1,4,5-trisphosphate and DAG (54). To analyze the role of CCK receptors on PKD phosphorylation, we utilized HEK293 cells stably transfected with either the CCK1 or CCK2 receptors. In human pancreas, CCK2 receptors are reported to be the main CCK receptor, whereas in rodent pancreas, CCK1 receptors appear to be the predominant CCK receptor (51). As shown in Fig. 5A (left panel), parental HEK293 cells did not respond to CCK or gastrin stimulation. Only the phorbol ester PMA induced PKD phosphorylation in these cells. However, CCK alone induced PKD phosphorylation in HEK293 cells that overexpress the CCK1 receptor which preferentially binds CCK compared with gastrin (55). CCK or gastrin each induced PKD phosphorylation in HEK293 cells that overexpress the CCK2 receptor, whereas the same hormones did not stimulate PKD phosphorylation in HEK293 cells lacking the CCK2 receptor (Fig. 5A, right panel). Thus, agonist binding of CCK1 or CCK2 receptors can promote PKD phosphorylation in different cell types.
FIGURE 5.
CCK-induced PKD phosphorylation is mediated by CCK1/2 receptors and by DAG/PKC. A, the parental HEK293 cells and two clones (293-CCK1R #6 and #20) expressing exogenous CCK1 receptors (left panel) and one clone expressing exogenous CCK2 receptors (right panel) were treated with vehicle, CCK, gastrin, or PMA for 30 min, and then cellular proteins were analyzed by Western blotting using anti-phospho-PKD (pPKD) and anti-total PKD antibodies on parallel blots. B, left panel, pancreatic acini were pretreated with BAPTA for 30 min and then treated with vehicle or CCK for 30 min; protein was extracted and analyzed by Western blotting. Right panels, intact acini were incubated with Fura-2 AM for 50 min and then treated with BAPTA or CCK as indicated. Intracellular calcium concentration was measured in a dual wavelength (340 and 380 nm) imaging work station and plotted as the means ± S.D. for 18 acinar cells. C, left panel, pancreatic acini were treated with A23187, ionomycin, thapsigargin alone, or in combination with either PMA or CCK for 10 min; protein was extracted and analyzed by Western blotting. Right panels, intact acini were incubated with Fura-2 AM for 50 min and then treated with the indicated agents. Intracellular calcium concentration was measured and plotted as means ± S.D. for 13-18 acinar cells. D, pancreatic acini were pretreated with vehicle (DMSO), Akt inhibitor, LY294002, wortmannin, PD98059, U0126, GF109203X, Gö6983, or Gö6976 at the indicated concentrations for 30 min. Acini were then incubated with CCK (10 nm) in the presence of inhibitors for an additional 30 min. Proteins from each sample were extracted and subjected to Western blotting. Blots shown are representative of at least three independent experiments.
To analyze the roles of Ca2+ in CCK- or carbachol-induced PKD activation, we utilized the Ca2+ chelator BAPTA. Pretreatment with BAPTA caused a complete inhibition of CCK-mediated Ca2+ mobilization but did not block CCK- or carbachol-induced phospho-PKD (Fig. 5B). This suggests that the upstream mechanisms leading to secretagogue-induced PKD phosphorylation is not dependent on Ca2+ mobilization. Furthermore, to determine whether an increase in cytoplasmic free Ca2+ may contribute to PKD phosphorylation, we treated acini with the Ca2+ ionophores (ionomycin or A23187) or with thapsigargin. Ionomycin, A23187, and thapsigargin each induced rapid increases in intracellular Ca2+, but each agent failed to increase phospho-PKD in acinar cells (Fig. 5C). Thus, Ca2+ mobilization alone is not sufficient to promote PKD phosphorylation in acinar cells. To further identify potential upstream signaling kinases that contribute to PKD phosphorylation, we analyzed the effects of inhibiting three pathways (i.e. PI3K/Akt, MEK/ERK, PKC) that are activated by CCK stimulation of pancreatic acini (54). As shown in Fig. 5D, the PI3K/Akt (wortmannin, LY294002, or an Akt inhibitor) and MEK inhibitors (PD98059 or U0126) failed to block CCK-induced PKD phosphorylation. By comparison, CCK-induced PKD phosphorylation was inhibited by preincubation of pancreatic acini with GF109203X and Gö6983, inhibitors of several PKC isozymes (56, 57). The efficacy of each inhibitor treatment was examined by measuring the levels of phospho-Akt or phospho-ERK. Stimulation of acini with CCK decreased Akt phosphorylation which was further decreased by the PI3K inhibitors LY294002 (10 μm) or wortmannin (100 nm). Stimulation of acini with CCK increased ERK phosphorylation which was effectively blocked by the MEK inhibitors PD98059 (50 μm) and U0126 (10 μm). In summary, our results indicate that CCK-induced PKD phosphorylation is dependent on PKC activity but independent of Ca2+ mobilization.
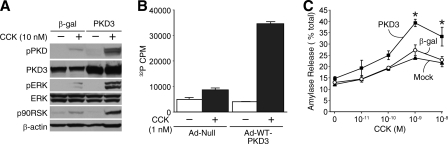
PKD3 Potentiates CCK-induced ERK Signaling and Amylase Release in Pancreatic Acinar Cells—CCK stimulation can increase the tyrosine phosphorylation and kinase activity of ERK1/2 in the pancreas and in isolated acini (54). To study the role of PKD3 in CCK-induced ERK activation, we utilized adenovirus-mediated overexpression of PKD3 in pancreatic acinar cells. First, isolated mouse acini were infected with control adenovirus expressing β-galactosidase or wild type PKD3 adenovirus for 15 h; acini were then stimulated with incubation media containing either vehicle or CCK (10 nm) for 30 min. As shown in Fig. 6A, exogenous PKD3 was detected by increased total PKD3 protein levels. Compared with the control, the PKD3 adenovirus transduction resulted in increased CCK-induced phospho-PKD, phospho-ERK, and phospho-RSK, a direct downstream target of activated ERK. These results show that increased PKD3 activation is associated with increased MEK/ERK/RSK pathway activation. Next, to confirm that the PKD overexpression correlates with increased PKD3 kinase activity, we performed in vitro kinase assays on adenoviral-infected acini. Overexpression of PKD3 significantly increased CCK-stimulated PKD3 kinase activity compared with null controls in acinar cell lysates (Fig. 6B).
FIGURE 6.
Overexpression of PKD3 potentiates MEK/ERK/RSK signaling and promotes CCK-induced amylase release. A, isolated mouse acini were infected with control adenovirus expressing β-galactosidase (β-gal) or adenovirus expressing wild-type PKD3 (PKD3) for 15 h. Acini were then treated with vehicle or CCK (10 nm) for 30 min; protein was extracted and analyzed by Western blotting. B, acini were infected with control null-adenovirus (Ad-Null) or Ad-WT-PKD3 for 24 h. Acini were then treated with vehicle or CCK (10 nm) for 30 min. Cellular proteins were immunoprecipitated using the PKD3-specific antibody and then analyzed by in vitro kinase assay. Results represent the means ± S.E. for three separate kinase assays. C, acini were subjected to mock infection or infection with 108 infectious units/ml adenovirus expressing β-galactosidase (β-gal) or WT-PKD3 for 15 h. Acini were then treated with increasing concentrations of CCK for 30 min. The media supernatant from each sample was collected and analyzed for amylase activity. Results represent the means ± S.E. for four independent experiments. Three pairwise comparisons were carried out for mock versus β-galactosidase versus WT-PKD3 comparisons for each CCK concentration; the asterisk indicates that the mean of WT-PKD3 is significantly greater than β-gal and mock infection (p < 0.05) at each CCK concentration.
The principle biologic effects of CCK on rodent pancreatic acinar cells include the stimulation of enzyme secretion, induction of protein synthesis, and promotion of cell growth (54). To delineate the effects of PKD3 activation on CCK-stimulated enzyme secretion, we performed amylase secretion assays on acini overexpressing PKD3. As demonstrated in Fig. 6C, amylase release from acinar cells infected with control virus expressing β-galactosidase was similar to CCK-induced amylase release in mock-infected cells (no virus), indicating that adenoviral infection itself did not affect basal or stimulated amylase release. In contrast, acinar cells infected with PKD3 adenovirus demonstrated enhanced amylase secretion compared with acinar cells infected with the control (β-galactosidase) adenovirus. CCK-stimulated amylase release was enhanced by PKD3 overexpression, with statistical significance noted at 10-9-10-8 m CCK. Amylase release associated with PKD3 virus was significantly higher than both control virus and no virus at the same CCK concentrations. Furthermore, PKD3 overexpression did not affect the biphasic nature of the CCK dose-response curve. To further delineate the physiologic requirement of endogenous PKD3 in hormone-induced amylase secretion, we tested the feasibility of PKD3 knockdown in acinar cells by lentiviral-mediated RNA interference. However, PKD3 gene silencing failed to inhibit CCK-stimulated amylase release (data not shown), suggesting that whereas PKD3 activity is sufficient to enhance amylase secretion, PKD3 alone is not required for amylase secretion. Nonetheless, the enhancing effect of PKD3 overexpression indicates that PKD3 positively regulates the secretory process and further emphasizes that PKD3, which is abundantly expressed in mouse and human acinar cells, contributes to hormone-stimulated pancreatic exocrine secretion.
DISCUSSION
The novel PKD isoforms are known to regulate various cellular functions such as proliferation and differentiation (32, 58, 59), survival and apoptosis (60, 61), motility and metastasis (32, 62, 63), protein secretion, and Golgi organization (3, 13, 64). Previously, we identified a role for PKD1 and PKD2 isoforms in secretagogue-mediated hormone secretion (4, 14). In our current study we extend previous findings regarding PKD in endocrine secretion to explore the role of PKD in pancreatic exocrine secretion. Here, we show that PKD3 is the predominant PKD isoform expressed in mouse and human pancreatic acinar cells, whereas PKD1 and PKD2 are more strongly expressed in pancreatic islets. We further provide evidence that PKD3 is activated by in vitro and in vivo GI hormone stimulation and that PKD3 activity promotes CCK-induced amylase secretion from mouse pancreatic acinar cells.
Recent data indicate that the three PKD proteins show isoform-specific expression patterns and functions in mediating protein trafficking and oxidative stress response (12, 13, 15). In our study we identify a unique expression pattern in mouse and human pancreas where the PKD3 isoform is mainly found in the acinar cells. PKD3 is also the predominant isoform in mouse salivary acinar cells which have similar functions as pancreatic acinar cells. The strong localization of PKD3 to pancreatic and salivary acinar cells suggests a role for this protein in exocrine enzyme secretion. Indeed, overexpression of wild-type PKD3 in mouse pancreatic acinar cells increases CCK-induced PKD3 kinase activity and potentiates CCK-induced amylase release, demonstrating that PKD3 activity is sufficient for promoting stimulated amylase secretion. Although PKD3 overexpression promotes amylase secretion, knockdown of PKD3 by RNA interference-induced gene silencing did not significantly reduce CCK-mediated amylase release. A possible explanation for this finding is our observation that low levels of PKD1 are also expressed in the mouse pancreatic acini and are activated by CCK and carbachol. Berna et al. (43) have also reported that PKD1 is activated by CCK in rat pancreatic acinar cells. PKD1 and PKD3 may have overlapping functions as well; therefore, activation of PKD1 may account for the enhanced amylase secretion despite PKD3 gene knockdown. Our results suggest that PKD3 alone is not required for amylase secretion but, in combination with other signaling components, contributes to stimulated amylase release.
PKD3 have been previously implicated in regulating protein trafficking in the polarized Madin-Darby canine kidney cells, where it promotes cargo-specific transport via the basolateral but not the apical route (9). In polarized pancreatic acinar cells, physiologic CCK stimulation leads to apical exocytosis, whereas supramaximal CCK stimulation results in basolateral exocytosis (65); thus, CCK-induced PKD3 activation may potentially be involved in either basolateral or apical exocytosis. Based on the effect of PKD3 overexpression on pancreatic amylase release, we postulate that PKD3 likely plays a functional role in zymogen granule trafficking and exocrine secretion. Confirming this supposition awaits more definitive knock-out studies using pancreas-specific, PKD3-deficient animals.
PKD isoforms serve different functions depending on their intracellular localization (66). In the pancreatic acini, PKD3 partly distributes to VAMP2-positive structures, which is consistent with findings that in Chinese hamster ovary and HEK293 cells, PKD3 localizes to VAMP2-positive vesicles and promotes vesicle transport by recruiting VAMP2 to the plasma membrane (7). VAMP2 are found on a distinct subset of zymogen granules in pancreatic acinar cells, and these granules are involved in both constitutive and stimulated exocytosis (47). We hypothesized that PKD3 may be involved in zymogen granule trafficking in pancreatic acinar cells; however, whereas CCK stimulation induced translocation of PKD3 to the plasma membrane, adenoviral-mediated overexpression of PKD3 did not promote the recruitment of VAMP2-structures to the plasma membrane (data not shown). Thus, it is likely that, rather than affecting VAMP2-postive granules, PKD3 may target other subsets of zymogen granules for exocytosis. In Panc-1 cells, PKD3 redistributes to the nucleus after catalytic activation (44). In acinar cells, hormone activation of PKD3 re-distributes the protein to the plasma membrane but not the nucleus, which suggests a different mechanism in primary, differentiated cells. Hormone stimulation increases PKD3 kinase activity in acinar cells similar to the 2-4-fold increase in syntide-2 phosphorylation in other cell types (31, 44). Hormone stimulation also generates Ca2+ and DAG (54). Kunkel et al. (67) recently reported that Ca2+ alone was sufficient to activate PKD by increasing DAG-mediated phosphorylation. However, in mouse acinar cells, Ca2+ alone was not sufficient to activate PKD phosphorylation, which is a PKC-dependent process. CCK can activate PKCδ in pancreatic acinar cells (68), and PKCδ can further promote CCK-induced amylase secretion (52). PKCδ is a well known upstream kinase of PKD; however, unlike PKD1 and PKD2, which interact with PKCδ at the tyrosine motif, PKD3 lacks the PKCδ target motif (15), suggesting that it is not PKCδ but another PKC isoform that activates PKD3 in acinar cells.
Our results demonstrate that activation of CCK1/CCK2 receptors can lead to PKD phosphorylation and that PKD3 potentiates mitogen-activated protein kinase (MAPK) signaling in pancreatic acinar cells. CCK is known to activate the three MAPK cascades leading to activation of ERKs, JNKs, and p38 MAPK in acinar cells (54). PKD1 and PKD2 are reported to activate the ERK and JNK pathways in various other cell types (69-73). Here, we report that PKD3 overexpression is associated with activation of the MEK/ERK/RSK cascade, suggesting that PKD3 mediates CCK-induced ERK activation. The PKC/PKD3/ERK pathway may modulate different physiologic effects of CCK such as stimulation of pancreatic enzyme secretion, induction of exocrine protein synthesis, and promotion of acinar cell growth. The potentiation of ERK signaling in pancreatic acinar cells likely modulates these different biological effects depending on the intensity and duration of ERK activation. The precise functional significance of PKD3-mediated ERK activation in acinar cells remains to be fully elucidated.
Another unexpected result is the finding that mouse and human pancreatic islet cells predominantly expressed PKD1 and PKD2 isoforms. This is consistent with our previous data showing that these proteins are the predominant isoforms in the endocrine BON cells (14) and further suggests a role for PKD1 and PKD2 in endocrine cell functions. Supporting this notion are functional data demonstrating that PKD1 and PKD2 regulate exocytic sorting of Kidins220, a neuroendocrine cell-specific membrane protein, in PC12 cells (12, 41), and that PKD1 and PKD2 phosphorylate Kidins220 to regulate neurotensin secretion in BON cells (14). Although our current study shows that PKD3 contributes to exocrine cell secretion, these recent studies show that PKD1 and PKD2 critically regulate endocrine cell secretion. In the pancreas, the relative importance of each PKD isoform in mediating endocrine versus exocrine secretion remains to be determined by using effective knockdowns of specific PKD isoforms in primary pancreatic cells.
In summary, we have identify PKD3 as the predominant PKD isoform in mouse and human exocrine pancreas and demonstrate that PKD3 can be activated by GI hormone stimulation via a DAG/PKC-dependent pathway. Furthermore, we show that PKD3 activation is associated with enhanced amylase secretion in mouse pancreatic acinar cells, indicating that PKD3 promotes enzyme secretion in response to GI hormone signaling. Our findings reveal a unique distribution pattern for PKD isoforms in the pancreas and implicate the PKD3 isoform in hormone-stimulated exocrine secretion.
Supplementary Material
Acknowledgments
We thank Karen Martin for manuscript preparation, Dr. John Williams laboratory for technical suggestions, and Drs. Kathleen O'Connor and Tianyan Gao for thoughtful suggestions and review of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants PO1 DK35608, RO1 DK48489, and T32 DK007639. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables 1 and 2 and Figs. 1 and 2.
Footnotes
The abbreviations used are: PKD, protein kinase D; PKC, protein kinase C; BBS, bombesin; CCK, cholecystokinin; DAG, diacylglycerol; GI, gastrointestinal; IHC, immunohistochemistry; NT, neurotensin; MEK, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase; FBS, fetal bovine serum; KHB, Krebs-Henseleit buffer; MAPK, mitogen-activated protein kinase; RT, reverse transcription; DMEM, Dulbecco's modified Eagle's medium; GST, glutathione S-transferase; WT, wild type; Bis-Tris, 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol; ERK, extracellular signal-regulated kinase; JNK, c-Jun NH2-terminal kinase; RSK, ribosomal S6 kinase.
References
- 1.Baron, C. L., and Malhotra, V. (2002) Science 295 325-328 [DOI] [PubMed] [Google Scholar]
- 2.Wang, Q. J. (2006) Trends Pharmacol. Sci. 27 317-323 [DOI] [PubMed] [Google Scholar]
- 3.Oster, H., Abraham, D., and Leitges, M. (2006) Gene Expr. Patterns 6 400-408 [DOI] [PubMed] [Google Scholar]
- 4.Li, J., O'Connor, K. L., Hellmich, M. R., Greeley, G. H., Jr., Townsend, C. M., Jr., and Evers, B. M. (2004) J. Biol. Chem. 279 28466-28474 [DOI] [PubMed] [Google Scholar]
- 5.Maier, D., Hausser, A., Nagel, A. C., Link, G., Kugler, S. J., Wech, I., Pfizenmaier, K., and Preiss, A. (2006) Gene Expr. Patterns 6 849-856 [DOI] [PubMed] [Google Scholar]
- 6.Papazyan, R., Rozengurt, E., and Rey, O. (2006) Biochem. Biophys. Res. Commun. 342 685-689 [DOI] [PubMed] [Google Scholar]
- 7.Lu, G., Chen, J., Espinoza, L. A., Garfield, S., Toshiyuki, S., Akiko, H., Huppler, A., and Wang, Q. J. (2007) Cell. Signal. 19 867-879 [DOI] [PubMed] [Google Scholar]
- 8.Marklund, U., Lightfoot, K., and Cantrell, D. (2003) Immunity 19 491-501 [DOI] [PubMed] [Google Scholar]
- 9.Hausser, A., Storz, P., Martens, S., Link, G., Toker, A., and Pfizenmaier, K. (2005) Nat. Cell Biol. 7 880-886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews, S., Iglesias, T., Cantrell, D., and Rozengurt, E. (1999) FEBS Lett. 457 515-521 [DOI] [PubMed] [Google Scholar]
- 11.Rozengurt, E., Rey, O., and Waldron, R. T. (2005) J. Biol. Chem. 280 13205-13208 [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Ruiloba, L., Cabrera-Poch, N., Rodriguez-Martinez, M., Lopez-Menendez, C., Jean-Mairet, R. M., Higuero, A. M., and Iglesias, T. (2006) J. Biol. Chem. 281 18888-18900 [DOI] [PubMed] [Google Scholar]
- 13.Yeaman, C., Ayala, M. I., Wright, J. R., Bard, F., Bossard, C., Ang, A., Maeda, Y., Seufferlein, T., Mellman, I., Nelson, W. J., and Malhotra, V. (2004) Nat. Cell Biol. 6 106-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, J., Chen, L. A., Townsend, C. M., Jr., and Evers, B. M. (2008) J. Biol. Chem. 283 2614-2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doppler, H., and Storz, P. (2007) J. Biol. Chem. 282 31873-31881 [DOI] [PubMed] [Google Scholar]
- 16.Rey, O., Papazyan, R., Waldron, R. T., Young, S. H., Lippincott-Schwartz, J., Jacamo, R., and Rozengurt, E. (2006) J. Biol. Chem. 281 5149-5157 [DOI] [PubMed] [Google Scholar]
- 17.Anderson, G., Chen, J., and Wang, Q. J. (2005) Cell. Signal. 17 1397-1411 [DOI] [PubMed] [Google Scholar]
- 18.Matthews, S. A., Liu, P., Spitaler, M., Olson, E. N., McKinsey, T. A., Cantrell, D. A., and Scharenberg, A. M. (2006) Mol. Cell. Biol. 26 1569-1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen, J., Lu, G., and Wang, Q. J. (2005) Mol. Pharmacol. 67 152-162 [DOI] [PubMed] [Google Scholar]
- 20.Case, R. M. (1978) Biol. Rev. Camb. Philos. Soc. 53 211-354 [DOI] [PubMed] [Google Scholar]
- 21.Scheele, G. A., Palade, G. E., and Tartakoff, A. M. (1978) J. Cell Biol. 78 110-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan, A., Burgoyne, R. D., Barclay, J. W., Craig, T. J., Prescott, G. R., Ciufo, L. F., Evans, G. J., and Graham, M. E. (2005) Biochem. Soc. Trans. 33 1341-1344 [DOI] [PubMed] [Google Scholar]
- 23.Werner, M. H., Bielawska, A. E., and Hannun, Y. A. (1992) Mol. Pharmacol. 41 382-386 [PubMed] [Google Scholar]
- 24.McKay, C., and Miller, A. (1996) Endocrinology 137 2473-2479 [DOI] [PubMed] [Google Scholar]
- 25.Litvak, V., Dahan, N., Ramachandran, S., Sabanay, H., and Lev, S. (2005) Nat. Cell Biol. 7 225-234 [DOI] [PubMed] [Google Scholar]
- 26.Maeda, Y., Beznoussenko, G. V., Van Lint, J., Mironov, A. A., and Malhotra, V. (2001) EMBO J. 20 5982-5990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas, R. P., Hellmich, M. R., Townsend, C. M., Jr., and Evers, B. M. (2003) Endocr. Rev. 24 571-599 [DOI] [PubMed] [Google Scholar]
- 28.Williams, J. A. (2006) Curr. Opin. Gastroenterol. 22 498-504 [DOI] [PubMed] [Google Scholar]
- 29.Chiu, T., and Rozengurt, E. (2001) Am. J. Physiol. 280 C929-C942 [DOI] [PubMed] [Google Scholar]
- 30.Guha, S., Rey, O., and Rozengurt, E. (2002) Cancer Res. 62 1632-1640 [PubMed] [Google Scholar]
- 31.Chiu, T., and Rozengurt, E. (2001) FEBS Lett. 489 101-106 [DOI] [PubMed] [Google Scholar]
- 32.Jackson, L. N., Li, J., Chen, L. A., Townsend, C. M., and Evers, B. M. (2006) Biochem. Biophys. Res. Commun. 348 945-949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellwanger, K., Pfizenmaier, K., Lutz, S., and Hausser, A. (2008) BMC Dev. Biol. 8 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe, H., Saito, H., Rychahou, P. G., Uchida, T., and Evers, B. M. (2005) Gastroenterology 128 1391-1404 [DOI] [PubMed] [Google Scholar]
- 35.Kang, J. H., Rychahou, P. G., Ishola, T. A., Qiao, J., Evers, B. M., and Chung, D. H. (2006) Biochem. Biophys. Res. Commun. 351 192-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olszewska-Pazdrak, B., Townsend, C. M., Jr., and Hellmich, M. R. (2004) J. Biol. Chem. 279 40400-40404 [DOI] [PubMed] [Google Scholar]
- 37.Wiznerowicz, M., and Trono, D. (2003) J. Virol. 77 8957-8961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabbatini, M. E., Rodriguez, M., di Carlo, M. B., Davio, C. A., Vatta, M. S., and Bianciotti, L. G. (2007) Am. J. Physiol. Gastrointest. Liver Physiol. 293 G987-G994 [DOI] [PubMed] [Google Scholar]
- 39.Ethridge, R. T., Chung, D. H., Slogoff, M., Ehlers, R. A., Hellmich, M. R., Rajaraman, S., Saito, H., Uchida, T., and Evers, B. M. (2002) Gastroenterology 123 1311-1322 [DOI] [PubMed] [Google Scholar]
- 40.Tsien, R. Y., and Harootunian, A. T. (1990) Cell Calcium 11 93-109 [DOI] [PubMed] [Google Scholar]
- 41.Iglesias, T., Cabrera-Poch, N., Mitchell, M. P., Naven, T. J., Rozengurt, E., and Schiavo, G. (2000) J. Biol. Chem. 275 40048-40056 [DOI] [PubMed] [Google Scholar]
- 42.Song, S. Y., Gannon, M., Washington, M. K., Scoggins, C. R., Meszoely, I. M., Goldenring, J. R., Marino, C. R., Sandgren, E. P., Coffey, R. J., Jr., Wright, C. V., and Leach, S. D. (1999) Gastroenterology 117 1416-1426 [DOI] [PubMed] [Google Scholar]
- 43.Berna, M. J., Hoffmann, K. M., Tapia, J. A., Thill, M., Pace, A., Mantey, S. A., and Jensen, R. T. (2007) Biochim. Biophys. Acta 1773 483-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rey, O., Yuan, J., Young, S. H., and Rozengurt, E. (2003) J. Biol. Chem. 278 23773-23785 [DOI] [PubMed] [Google Scholar]
- 45.Pickett, J. A., Thorn, P., and Edwardson, J. M. (2005) J. Biol. Chem. 280 1506-1511 [DOI] [PubMed] [Google Scholar]
- 46.Burgoyne, R. D., and Morgan, A. (1993) Biochem. J. 293 305-316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weng, N., Thomas, D. D., and Groblewski, G. E. (2007) J. Biol. Chem. 282 9635-9645 [DOI] [PubMed] [Google Scholar]
- 48.Yuan, J., Rey, O., and Rozengurt, E. (2006) Cell. Signal. 18 1051-106216198087 [Google Scholar]
- 49.Ji, B., Bi, Y., Simeone, D., Mortensen, R. M., and Logsdon, C. D. (2001) Gastroenterology 121 1380-1390 [DOI] [PubMed] [Google Scholar]
- 50.Owyang, C., and Logsdon, C. D. (2004) Gastroenterology 127 957-969 [DOI] [PubMed] [Google Scholar]
- 51.Wang, B. J., and Cui, Z. J. (2007) Am. J. Physiol. Regul. Integr. Comp. Physiol. 292 666-678 [DOI] [PubMed] [Google Scholar]
- 52.Li, C., Chen, X., and Williams, J. A. (2004) Am. J. Physiol. Gastrointest. Liver Physiol. 287 764-771 [DOI] [PubMed] [Google Scholar]
- 53.Gautam, D., Han, S. J., Heard, T. S., Cui, Y., Miller, G., Bloodworth, L., and Wess, J. (2005) J. Pharmacol. Exp. Ther. 313 995-1002 [DOI] [PubMed] [Google Scholar]
- 54.Williams, J. A. (2001) Annu. Rev. Physiol. 63 77-97 [DOI] [PubMed] [Google Scholar]
- 55.Wank, S. A. (1995) Am. J. Physiol. 269 G628-G646 [DOI] [PubMed] [Google Scholar]
- 56.Toullec, D., Pianetti, P., Coste, H., Bellevergue, P., Grand-Perret, T., Ajakane, M., Baudet, V., Boissin, P., Boursier, E., Loriolle, F., et al. (1991) J. Biol. Chem. 266 15771-15781 [PubMed] [Google Scholar]
- 57.Gschwendt, M., Dieterich, S., Rennecke, J., Kittstein, W., Mueller, H. J., and Johannes, F. J. (1996) FEBS Lett. 392 77-80 [DOI] [PubMed] [Google Scholar]
- 58.Zugaza, J. L., Waldron, R. T., Sinnett-Smith, J., and Rozengurt, E. (1997) J. Biol. Chem. 272 23952-23960 [DOI] [PubMed] [Google Scholar]
- 59.Lemonnier, J., Ghayor, C., Guicheux, J., and Caverzasio, J. (2004) J. Biol. Chem. 279 259-264 [DOI] [PubMed] [Google Scholar]
- 60.Storz, P., and Toker, A. (2003) EMBO J. 22 109-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Endo, K., Oki, E., Biedermann, V., Kojima, H., Yoshida, K., Johannes, F. J., Kufe, D., and Datta, R. (2000) J. Biol. Chem. 275 18476-18481 [DOI] [PubMed] [Google Scholar]
- 62.Prigozhina, N. L., and Waterman-Storer, C. M. (2004) Curr. Biol. 14 88-98 [DOI] [PubMed] [Google Scholar]
- 63.Jaggi, M., Rao, P. S., Smith, D. J., Wheelock, M. J., Johnson, K. R., Hemstreet, G. P., and Balaji, K. C. (2005) Cancer Res. 65 483-492 [PubMed] [Google Scholar]
- 64.Liljedahl, M., Maeda, Y., Colanzi, A., Ayala, I., Van Lint, J., and Malhotra, V. (2001) Cell 104 409-420 [DOI] [PubMed] [Google Scholar]
- 65.Cosen-Binker, L. I., Lam, P. P., Binker, M. G., Reeve, J., Pandol, S., and Gaisano, H. Y. (2007) J. Biol. Chem. 282 13047-13058 [DOI] [PubMed] [Google Scholar]
- 66.Van Lint, J., Rykx, A., Maeda, Y., Vantus, T., Sturany, S., Malhotra, V., Vandenheede, J. R., and Seufferlein, T. (2002) Trends Cell Biol. 12 193-200 [DOI] [PubMed] [Google Scholar]
- 67.Kunkel, M. T., Toker, A., Tsien, R. Y., and Newton, A. C. (2007) J. Biol. Chem. 282 6733-6742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tapia, J. A., Garcia-Marin, L. J., and Jensen, R. T. (2003) J. Biol. Chem. 278 35220-35230 [DOI] [PubMed] [Google Scholar]
- 69.Sinnett-Smith, J., Zhukova, E., Hsieh, N., Jiang, X., and Rozengurt, E. (2004) J. Biol. Chem. 279 16883-16893 [DOI] [PubMed] [Google Scholar]
- 70.Wong, C., and Jin, Z. G. (2005) J. Biol. Chem. 280 33262-33269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hausser, A., Storz, P., Hubner, S., Braendlin, I., Martinez-Moya, M., Link, G., and Johannes, F. J. (2001) FEBS Lett. 492 39-44 [DOI] [PubMed] [Google Scholar]
- 72.Brandlin, I., Eiseler, T., Salowsky, R., and Johannes, F. J. (2002) J. Biol. Chem. 277 45451-45457 [DOI] [PubMed] [Google Scholar]
- 73.Sinnett-Smith, J., Zhukova, E., Rey, O., and Rozengurt, E. (2007) J. Cell. Physiol. 211 781-790 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.