Abstract
The insulin receptor substrate (IRS) proteins are cytoplasmic adaptor molecules that function as signaling intermediates downstream of activated cell surface receptors. Based on data implicating IRS-2 but not IRS-1 in breast cancer invasion, survival, and metastasis, we assessed the contribution of IRS-1 and IRS-2 to aerobic glycolysis, which is known to impact tumor growth and progression. For this purpose, we used tumor cell lines derived from transgenic mice that express the polyoma virus middle T antigen (PyV-MT) in the mammary gland and that are wild-type (WT) or null for either Irs-1 (Irs-1–/–) or Irs-2 (Irs-2–/–). Aerobic glycolysis, as assessed by the rate of lactic acid production and glucose consumption, was diminished significantly in Irs-2–/– cells when compared with WT and Irs-1–/– cells. Expression of exogenous Irs-2 in Irs-2–/– cells restored the rate of glycolysis to that observed in WT cells. The transcription factor FoxO1 does not appear to be involved in Irs-2-mediated glycolysis. However, Irs-2 does regulate the surface expression of glucose transporter 1 (Glut1) as assessed by flow cytometry using a Glut1-specific ligand. Suppression of Glut1 expression inhibits Irs-2-dependent invasion, which links glycolysis to mammary tumor progression. Irs-2 was shown to be important for mammalian target of rapamycin (mTor) activation, and Irs-2-dependent regulation of Glut1 surface expression is rapamycin-sensitive. Collectively, our data indicate that Irs-2, but not Irs-1, promotes invasion by sustaining the aerobic glycolysis of mouse mammary tumor cells and that it does so by regulating the mTor-dependent surface expression of Glut1.
Glucose metabolism in cancer cells differs significantly from that of normal cells as observed initially by Warburg in the 1920s (1). Specifically, cancer cells depend more on glycolysis than oxidative phosphorylation to generate ATP, even in high oxygen tensions. Subsequent studies have affirmed the importance of aerobic glycolysis in tumor progression and have shown that it provides tumor cells with a selective advantage in their ability to progress toward invasive and metastatic disease (2–4). Significantly, the “Warburg effect” has become a powerful and standard imaging technique for detecting tumors and their metastases by positron emission tomography using [18F]deoxyglucose (5). Aerobic glycolysis is also an ideal target for therapeutic intervention because apoptosis results if this process is perturbed (2, 6). To exploit aerobic glycolysis for the clinical management of cancer, however, much more needs to be learned about how this form of metabolism is induced and sustained in tumors. Our interest is in understanding how aerobic glycolysis is regulated in breast cancer.
The insulin receptor substrate (IRS)3 proteins are cytoplasmic adaptor molecules that function as signaling intermediates downstream of activated cell surface receptors (7). The role of the IRS-2 family member is of particular significance in breast cancer. IRS-2 is predominantly expressed in estrogen receptor-negative breast carcinoma cell lines, which are invasive and metastatic (8). Moreover, insulin-like growth factor-1 (IGF-1) promotes cell motility and invasion and metastasis in human breast carcinoma cell lines that signal preferentially through IRS-2 (8–12). In contrast, signaling through IRS-1 promotes breast carcinoma cell proliferation (13). Our own studies using a transgenic mouse model of breast cancer have revealed a striking role for Irs-2 in promoting tumor survival and metastasis that cannot be compensated for by Irs-1 (14). Given that both IRS-2 signaling and aerobic glycolysis enhance the invasive and metastatic behavior of breast carcinomas, we sought to establish a role for IRS-2 in regulating this essential process in breast cancer. We report here that Irs-2 but not Irs-1 promotes invasion by sustaining aerobic glycolysis in mouse mammary tumor cells and that it does so by regulating the mTor-dependent surface expression of glucose transporter 1 (Glut1).
EXPERIMENTAL PROCEDURES
Cell Lines, Antibodies, and Reagents—Mammary tumor cell lines were isolated from PyV-MT-derived wild-type (WT), Irs-1–/–, and Irs-2–/– tumors as described previously (14). Cell lines were maintained in low glucose DMEM (Invitrogen) supplemented with 10% fetal bovine serum (Sigma). The murine Irs-2 construct (pCMVHis-Irs-2) was provided by Morris White (Children's Hospital, Boston, MA). Cells were transfected using Oligofectamine (Invitrogen) according to the manufacturer's recommendations and then selected for stable expression of the constructs with histidinol (Sigma). LY294002 was obtained from Cell Signaling, Inc. (Beverly, MA), and rapamycin was obtained from Sigma.
The following antibodies were used for immunoprecipitation or immunoblotting: IRS-1 (Bethyl Laboratories); IRS-2 (immunoblot, Calbiochem, EMD Biosciences, Inc., Darmstadt, Germany; immunoprecipitation, Bethyl Laboratories); phosphotyrosine (PY99; Santa Cruz Biotechnology); FoxO1 (CeMines); Glut1 (AbCam); and actin and tubulin (Sigma). All other antibodies were purchased from Cell Signaling, Inc.
Glucose Uptake and Lactic Acid Production Assays—Mammary tumor cells were grown to near confluence in 24-well plates, washed with phosphate-buffered saline, and then incubated for 6–24 h with either fresh serum-containing medium or DMEM containing 0.1% heat-inactivated BSA (DMEM/BSA) in the presence or absence of IGF-1 (50 ng/ml) and inhibitors. Glucose and lactic acid were subsequently assayed in the conditioned medium using a glucose assay kit (Sigma) and lactic acid assay kit (Trinity Biotech). The amount of cellular protein per well was quantified by the Bradford assay, and the metabolism of glucose and lactic acid was expressed as a rate measurement (mm/mg of total protein/h).
Immunoprecipitation and Immunoblotting—Cells were deprived of serum overnight in DMEM/BSA and then stimulated with IGF-1 (100 ng/ml) for the time periods indicated in Fig. 5. Cells were solubilized at 4 °C for 10 min in a 50 mm Tris buffer, pH 7.6, containing 0.15 m NaCl, 1% Nonidet P-40, 0.1% SDS, 1% sodium deoxycholate, 1 mm sodium orthovanadate, 1 mm NaF, and protease inhibitors (Complete mini tab; Roche Applied Science). Nuclei were removed by centrifugation at 12,000 × g for 10 min. Aliquots of cell extracts containing equivalent amounts of protein were incubated for 3 h at 4 °C with antibodies and protein-A-Sepharose (Amersham Biosciences) with constant agitation. Frozen tumors were homogenized at 4 °C in T-PER tissue protein extraction reagent (Pierce Biotechnology, Inc.), containing 1 mm sodium orthovanadate, 10 mm NaF, and protease inhibitors (Complete mini; Roche Applied Science).
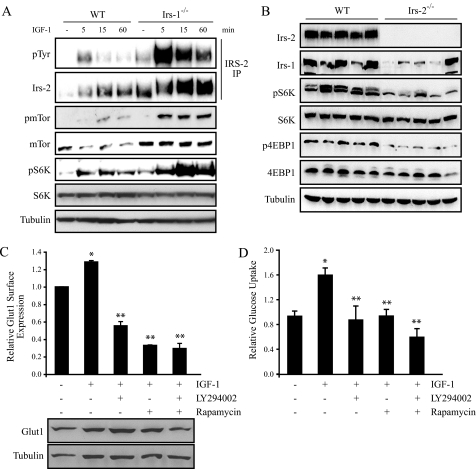
FIGURE 5.
Irs-2 regulates Glut1 surface expression in a mTor-dependent manner. A, cells were stimulated for the indicated time periods with IGF-1 (100 ng/ml). Aliquots of the cell extracts containing equivalent amounts of total protein were immunoprecipitated (IP) with an Irs-2-specific antisera and immunoblotted with phosphotyrosine-specific antibodies (pTyr). The immunoblots were subsequently stripped and reprobed with Irs-2-specific antibodies. Total cell extracts were also immunoblotted with antibodies specific for phosphoserine-2448 of mTor (pmTor), total mTor, phosphothreonine-389 of S6 Kinase (pS6K), total S6-kinase, and tubulin. B, aliquots of tumor extracts from PyV-MT:WT and PyV-MT:Irs-2–/– mammary tumors containing equivalent amounts of total protein were immunoblotted with antibodies specific for Irs-2, Irs-1, phosphothreonine-389 of S6 Kinase (pS6K), total S6 kinase, phosphothreonine-70 of 4EBP1 (p4EBP1), total 4EBP1, and tubulin. C, Irs-1–/– cells were treated in the presence or absence of IGF-1 (50 ng/ml), LY294002, or rapamycin for 24 h and then incubated with EGFP-HTLV peptide to determine the surface expression of Glut1 using flow cytometry. The data are expressed as the level of surface expression relative to untreated Irs-1–/– cells. The data shown represent the mean (± S.D.) of two independent experiments. Aliquots of cell extracts that contained equivalent amounts of total protein were resolved by SDS-PAGE and then immunoblotted with antibodies that recognize either Glut1 or actin (lower panels). D, Irs-1–/– cells were treated in the presence or absence of IGF-1 (50 ng/ml), LY294002, or rapamycin for 24 h and then assayed for glucose metabolism. The data are expressed as the ratio of the rate of glucose depletion from the culture medium (mm/mg of total protein/h) relative to untreated Irs-1–/– cells. The data shown represent the mean (± S.E.) of three independent experiments performed in triplicate. *, p ≤ 0.003 relative to untreated Irs-1–/– cells; **, p ≤ 0.01 relative to Irs-1–/– cells treated with IGF-1.
Immune complexes, as well as aliquots of cell and tumor extracts containing equivalent amounts of total protein, were resolved by SDS-PAGE and transferred to nitrocellulose filters. Membranes were blocked for an hour using a 50 mm Tris buffer, pH 7.5, containing 0.15 m NaCl, 0.05% Tween 20, and 5% (w/v) Carnation dry milk. Membranes were incubated overnight at 4 °C in the same buffer containing primary antibodies. Proteins were detected by enhanced chemiluminescence (Pierce) after a 1-h incubation with horseradish peroxidase-conjugated secondary antibodies. For phospho-immunoblots, the blocking buffer for the primary antibodies contained 5% (w/v) BSA.
FoxO1 Experiments—Cytoplasmic and nuclear fractions were prepared using the NE-PER extraction kit (Pierce) according to the manufacturer's instructions. To suppress FoxO1 expression, cells were infected with a pLKO lentivirus containing either a FoxO1-specific or a GFP-specific shRNA (Open Biosystems), and stable subclones were selected with puromycin.
Glut1 Surface Expression—Mammary tumor cells were grown overnight, washed with phosphate-buffered saline, and then incubated for 24 h with fresh DMEM/BSA in the presence or absence of IGF-1 (50 ng/ml) and inhibitors. Cells were trypsinized, washed twice in phosphate-buffered saline containing 2% fetal bovine serum and 0.01% sodium azide (PBA), and incubated for 30 min at 37 °C in PBA in the presence or absence of EGFP-Glut1 ligand (1:10 dilution; AbCys (15, 16)). Cells were washed once in PBA and analyzed by flow cytometry.
Invasion Assay—To suppress Glut1 expression, cells were infected with pLKO lentiviruses containing either Glut1-specific or GFP-specific shRNA (Open Biosystems). Infected cells were incubated for 48 h, and then Matrigel invasion assays were performed as described previously using 6.5-mm Transwell chambers (8-μm pore size) (17). After 5 h, the cells that had invaded to the lower surface of the filters were fixed in methanol for 10 min. The fixed membranes were mounted on glass slides using Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). Invasion was quantified by counting the number of stained nuclei in five independent fields in each Transwell.
Statistics—All statistical analyses were performed using a two-tailed Student's t test for unpaired data. A p value of 0.05 was considered statistically significant. Corresponding significance levels are indicated in the figure legends.
RESULTS
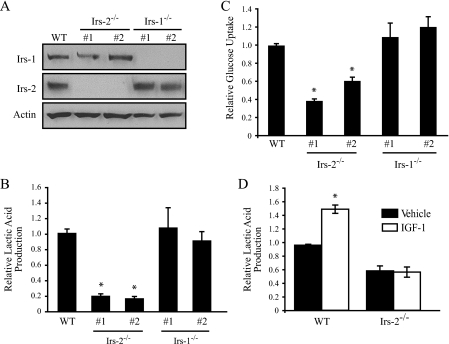
Irs-2 Is Required for the Aerobic Glycolysis of Metastatic Mammary Carcinoma Cells in Vitro—To assess the importance of Irs-1 and Irs-2 in mammary carcinoma aerobic glycolysis, we examined the rate of lactic acid production and glucose consumption in tumor cell lines established from WT, Irs-1–/–, and Irs-2–/– PyV-MT-derived mammary tumors (Fig. 1A). Generation of these cell lines has been reported previously, and Irs-2–/– cells have been shown to be poorly invasive and more apoptotic in response to growth factor deprivation than their WT or Irs-1–/– counterparts (14). As shown in Fig. 1B, mammary tumor cells lacking Irs-2 had a dramatically reduced (∼80%) basal rate of lactic acid production when compared with WT cells. The effect of Irs-2 on aerobic glycolysis was also assessed by measuring the rate of glucose consumption. A 50–70% decrease in glucose consumption was observed in Irs-2–/– cells when compared with WT cells (Fig. 1C). Importantly, glucose uptake and lactic acid production were not diminished in Irs-1–/– cell lines that express only Irs-2 (Fig. 1, B and C). To assess the contribution of Irs-2 to glycolysis directly, WT and Irs-2–/– cells were stimulated with IGF-1, which signals through the Irs proteins (7). WT cells responded to IGF-1 with an ∼2-fold increase in lactic acid production. In contrast, no IGF-1-dependent increase in lactic acid production was observed in cells lacking Irs-2 expression (Fig. 1D).
FIGURE 1.
Contribution of Irs-1 and Irs-2 to aerobic glycolysis in mouse mammary tumor cells. A, mammary tumor cells that were isolated from PyV-MT wild-type (WT), PyV-MT:Irs-2–/–, and PyV-MT:Irs-1–/– mice were assayed for lactic acid production (B) or glucose metabolism (C). Two individual subclones of Irs-2–/– and Irs-1–/– cells are shown, and the data are expressed as the ratio of the rate of lactic acid production or glucose depletion from the culture medium (mm/mg of total protein/h) relative to WT. B, the data shown represent the mean (± S.E.) of four (Irs-2–/–) or six (WT and Irs-1–/–) independent experiments performed in triplicate. C, the data shown represent the mean (± S.E.) of four independent experiments performed in triplicate. D, PyV-MT:WT and PyV-MT:Irs-2–/– cells were assayed for their rate of lactic acid production in the presence or absence of IGF-1 stimulation. The data shown represent the mean (± S.E.) of three independent experiments performed in triplicate. *, p < 0.001.
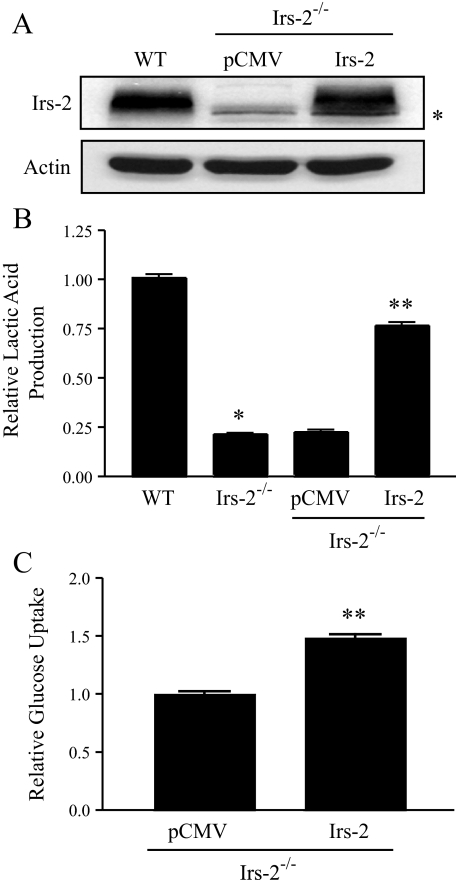
We next confirmed the specificity of Irs-2 in regulating aerobic glycolysis in mammary carcinoma cells. Irs-2 expression was restored in Irs-2–/– cells following stable transfection with pCMVHis-Irs-2. The level of Irs-2 protein expression in stable transfectants was found to be similar to Irs-2 protein levels in WT cells, as determined by immunoblotting (Fig. 2A). As shown in Fig. 2B, restoration of Irs-2 in Irs-2–/– cells increased their rate of lactic acid production by 3.5-fold when compared with vector control cells. The rate of lactic acid production in the Irs-2-rescued cells was ∼75% of WT cells. We also observed a corresponding and significant increase (∼50%) in glucose consumption when Irs-2 expression was restored in Irs-2–/– cells (Fig. 2C). We conclude from these data that Irs-2 expression in mouse mammary tumor cells is required to sustain aerobic glycolysis.
FIGURE 2.
Irs-2 rescues aerobic glycolysis in Irs-2–/– mammary tumor cells. A, Irs-2–/– cells were transfected with either a control plasmid (pCMVHis) or murine Irs-2 (pCMVHis-Irs-2), and stable cell lines were generated following histidinol selection. Irs-2 expression was restored to approximately WT levels. *, nonspecific band. pCMV, Irs-2–/– cells transfected with vector; Irs-2, Irs-2–/– cells transfected with Irs-2. B, cells were assayed for lactic acid production, and the data are expressed as the ratio of the rate of lactic acid production (mm/mg of total protein/h) relative to WT. The data shown represent the mean (± S.E.) of three independent experiments performed in triplicate. *, p < 0.01 relative to WT cells; **, p < 0.01 relative to pCMVHis-vector control cells. C, cells were assayed for glucose metabolism, and the data are expressed as the ratio of the rate of glucose depletion (mm/mg of total protein/h) from the culture medium relative to the vector control Irs-2–/– cells (pCMV). The data shown represent the mean (± S.E.) of three independent experiments performed in triplicate. **, p < 0.01 relative to pCMVHis-vector control cells.
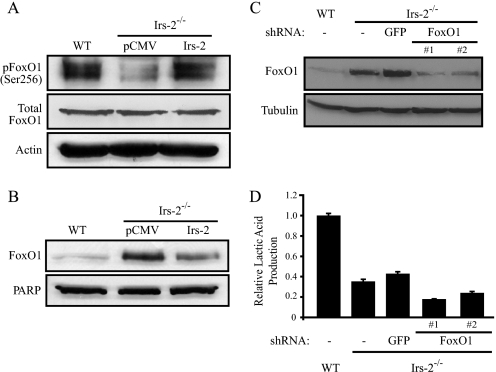
Irs-2 Promotes Glycolysis by Stimulating Glut1 Surface Expression—We next explored the molecular mechanism through which Irs-2 regulates aerobic glycolysis. The Akt-FoxO1 pathway has been linked specifically to IRS-2 signaling (18). It is known that the intracellular distribution of the transcription factor FoxO1 is primarily determined by its phosphorylation status (19). Specifically, Akt initially phosphorylates FoxO1 on Ser256, causing its exclusion from the nucleus and subsequent retention in the cytoplasm. Conversely, a decrease in the phosphorylation of FoxO1 allows FoxO1 to remain in the nucleus, where it represses transcription of FoxO1 target genes. FoxO1 signaling has been linked to the repression of glycolysis and stimulation of gluconeogenesis (20, 21). On the basis of these reports and our previous demonstration that Irs-2 preferentially activates Akt signaling in tumors (22), we initially postulated that Irs-2 stimulates aerobic glycolysis in mouse mammary tumor cells by signaling through Akt-FoxO1. In Irs-2–/– cells, FoxO1 was hypophosphorylated at Ser256 and localized at higher levels in the nucleus when compared with both WT cells and Irs-2-restored Irs-2–/– cells (Fig. 3, A and B). Importantly, however, suppression of FoxO1 expression by shRNA targeting did not rescue glycolysis in the Irs-2–/– cells (Fig. 3, C and D). These observations discount a major role for FoxO1 in mediating Irs-2-regulated aerobic glycolysis.
FIGURE 3.
Involvement of FoxO1 in the Irs-2-dependent regulation of aerobic glycolysis. A, aliquots of cell extracts that contained equivalent amounts of total protein were resolved by SDS-PAGE and then immunoblotted with antibodies that recognize phosphoSer256 of FoxO1, total FoxO1, or actin. pCMV, Irs-2–/– cells transfected with vector; Irs-2, Irs-2–/– cells transfected with Irs-2. B, aliquots of nuclear extracts containing equivalent amounts of total protein were resolved by SDS-PAGE and then immunoblotted with a FoxO1-specific antibody. Poly(ADP-ribose) polymerase-2 (PARP) was used as a nuclear loading control. C, FoxO1 expression was suppressed in the PyV-MT:Irs-2–/– cells by lentiviral infection with a FoxO1-specific shRNA. Total cell extracts from WT and Irs-2–/– parental cell lines and the Irs-2–/– cells expressing either a GFP-shRNA or a FoxO1-shRNA (two independent subclones) were immunoblotted with either FoxO1-specific or tubulin-specific antibodies. D, cells were assayed for lactic acid production, and the data are expressed as the ratio of the rate of lactic acid production (mm/mg of total protein/h) relative to WT cells. The data shown represent the mean (± S.E.) of a representative experiment performed in triplicate.
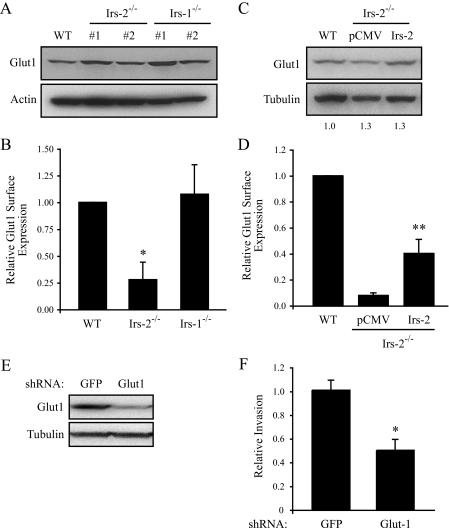
Another hypothesis to account for our data is that Irs-2 regulates glucose transporter expression to enhance glucose uptake, a rate-limiting step in the glycolysis pathway. Glut1, in particular, plays an important role in tumor glycolysis (2) and appears to be the predominant glucose transporter in breast cancer (23, 24), and its expression has been shown to correlate with tumor aggressiveness and reduced survival time in women with breast cancer (23). As shown in Fig. 4A, total expression of Glut1 is similar in WT, Irs-1–/–, and Irs-2–/– cells, indicating that Irs-2 does not regulate Glut1 expression. Next, we assessed the surface expression of Glut1 by flow cytometry using a GFP-tagged human T cell leukemia virus peptide (EGFP-Glut1) that specifically recognizes extracellular Glut1 (15, 16). EGFP-Glut1 binding was decreased significantly in Irs-2–/– cells when compared with WT or Irs-1–/– cells, indicating that Irs-2 regulates Glut1 surface expression (Fig. 4B). Moreover, expression of Irs-2 in the null cells restored EGFP-Glut1 binding without affecting total Glut1 protein expression (Fig. 4, C and D).
FIGURE 4.
Irs-2 regulates the surface expression of Glut1, which is required for mammary tumor cell invasion. A, aliquots of cell extracts that contained equivalent amounts of total protein were resolved by SDS-PAGE and then immunoblotted with antibodies that recognize either Glut1 or actin. Two individual subclones of Irs-2–/– and Irs-1–/– cells are shown. B, cells were incubated with EGFP-HTLV peptide and analyzed by flow cytometry to determine the surface expression of Glut1. The data are expressed as the level of surface expression relative to WT cells. The data shown represent the mean (± S.E.) of three independent experiments. *, p < 0.0002 relative to WT cells. C, aliquots of cell extracts that contained equivalent amounts of total protein were immunoblotted with antibodies that recognize either Glut1 or tubulin. pCMV, Irs-2–/– cells transfected with vector; Irs-2, Irs-2–/– cells transfected with Irs-2. D, cells were incubated with EGFP-HTLV peptide and analyzed by flow cytometry to determine the surface expression of Glut1. The data are expressed as the level of surface expression relative to WT cells. The data shown represent the mean (± S.E.) of four independent experiments. **, p < 0.03 relative to pCMV vector control cells. E, Glut1 expression was suppressed in the PyV-MT:Irs-1–/– cells by combined lentiviral infection with two independent Glut1-specific shRNAs. Total cell extracts from Irs-1–/– cells expressing either a GFP-shRNA or the Glut1-shRNAs were immunoblotted with either Glut1-specific or tubulin-specific antibodies. F, cells were assayed for their invasive potential using a Matrigel transwell assay. Five independent fields/well were counted. The data shown represent the mean (± S.E.) of four independent experiments performed in triplicate. *, p < 0.004 relative to WT cells.
Glut1-dependent Glycolysis Contributes to Mammary Tumor Cell Invasion—To investigate the functional link between our observation that Irs-2 regulates aerobic glycolysis and the preferential ability of Irs-2 to promote breast carcinoma cell invasion and metastasis, Glut1 expression was suppressed in Irs-1–/– mammary tumor cells, which express only Irs-2, by shRNA targeting. Suppression of Glut1 expression in these cells by ∼80% resulted in a significant decrease in both glucose uptake (data not shown) and mammary tumor cell invasion (Fig. 4, E and F).
Irs-2 Regulates Glut 1 Surface Expression through a PI3K/mTor-dependent Pathway—The Irs proteins function by organizing signaling complexes at activated surface receptors to initiate downstream signaling cascades (7). To investigate the mechanism by which Irs-2 selectively regulates Glut1 surface expression, we evaluated the activation of signaling pathways downstream of this adaptor protein. WT cells, which express both Irs-1 and Irs-2, and Irs-1–/– cells, which express only Irs-2, were stimulated with IGF-1 to activate Irs-dependent signaling pathways. As shown in Fig. 5A, IGF-1 treatment of WT cells resulted in a modest increase in mTor activation and subsequent activation of its downstream effector S6-kinase. In contrast, stimulation of Irs-1–/– cells resulted in a marked increase in activation of the mTor pathway downstream of Irs-2. The basal level of mTor activation was also higher in the Irs-1–/– cells, which supports the enhanced Irs-2-dependent glycolysis observed in the absence of IGF-1 stimulation (Fig. 1). These findings agree with our previous observation that activation of the PI3K/mTor pathway is enhanced in Irs-1–/– tumors (22). To examine further whether Irs-2 is required for mTor signaling, we assessed the status of this pathway in Irs-2–/– tumors that express only Irs-1. When compared with WT tumors, activation of the mTor effectors S6-kinase and 4EBP1 was significantly lower in tumors that lack Irs-2 expression, indicating that Irs-1 cannot compensate for the activation of this pathway in vivo (Fig. 5B).
To assess the involvement of mTor signaling in the Irs-2-mediated enhancement of glycolysis, Irs-1–/– cells were stimulated with IGF-1 in the presence of inhibitors of this signaling pathway. IGF-1 stimulation increased Glut1 surface expression, and this increase was prevented when PI3K and mTor signaling was inhibited using LY29004 and rapamycin, respectively (Fig. 5C). IGF-1 also stimulated a corresponding increase in glucose uptake in the Irs-1–/– cells, which was also prevented by inhibition of PI3K/mTor signaling (Fig. 5D). Given that the total levels of Glut1 expression were not affected significantly by IGF-1 stimulation or inhibition of mTor signaling (Fig. 5C, lower panels), our data indicate that Irs-2 regulates the surface expression of Glut1 in an mTor-dependent manner to enhance tumor glycolysis.
DISCUSSION
This study establishes an important link among insulin receptor substrate signaling, aerobic glycolysis, and the aggressive behavior of mammary carcinoma cells. Specifically, we demonstrate that aerobic glycolysis in mammary carcinoma cells is dependent on Irs-2 but not on Irs-1 and that Irs-2 functions to maintain the surface expression of Glut1 by an mTor-dependent mechanism. Irs-2-dependent regulation of glycolysis is linked to the ability of this adaptor protein to promote mammary tumor cell invasion. Collectively, our data provide a mechanism to substantiate the finding derived from transgenic mouse models that Irs-2 has a causal role in breast cancer progression (14, 22), and they support the hypothesis that the ability of tumor cells to sustain aerobic glycolysis is an essential component of the metastatic odyssey (2).
The ability of IRS-2 to sustain aerobic glycolysis provides a mechanistic basis for the necessity of this signaling adaptor in breast cancer progression. As mentioned, this study was predicated on the previous finding that mammary tumors deficient in Irs-2 are significantly more apoptotic, less invasive, and unable to metastasize when compared with either wild-type tumors or tumors deficient in Irs-1 (14). As discussed comprehensively in a recent review (4), there are several reasons why the ability to sustain aerobic glycolysis is advantageous for tumors including the ability to sustain fluctuations in oxygen tension that would be toxic to cells that depend on oxidative phosphorylation. Thus, IRS-2 could provide a degree of autonomy that enables cells to survive within a hypoxic tumor microenvironment. In this direction, an important consideration from our data is that mammary carcinoma cells are able to sustain aerobic glycolysis in the absence of exogenous growth factor stimulation (i.e. serum-deprived conditions) and that this glycolysis is dependent on Irs-2. Although we observed that growth factor (IGF-1) stimulation can enhance Irs-2-mediated glycolysis, our findings imply that these cells possess intrinsic mechanisms such as autocrine growth factor stimulation that maintain Irs-2-mediated signaling and glycolysis. The acids (lactic and bicarbonic) that are generated by aerobic glycolysis can facilitate tumor invasion by degrading extracellular matrix (4, 25). This function is consistent with our previous finding that Irs-2–/– cells are significantly less invasive than wild-type or Irs-1–/– cells (14) and our current results demonstrating that suppression of glycolysis inhibits Irs-2-dependent mammary tumor cell invasion. It is worth noting in this context that the rate of aerobic glycolysis correlates with the aggressiveness of human breast carcinoma cell lines (2) and that IRS-2 expression correlates with their rate of glycolysis (data not shown). These observations support that the ability of Irs-2 to regulate aerobic glycolysis contributes to tumor progression.
Although aerobic glycolysis appears to be essential for tumor progression, data that elucidate the mechanisms that promote glycolysis in tumor cells are only beginning to emerge, especially for solid tumors (4). In principle, aerobic glycolysis in tumors can be regulated at the level of glycolytic enzymes or glucose transporters. The recent, seminal finding that tumor cells express an embryonic M2 isoform of pyruvate kinase and that this isoform, but not the M1 isoform expressed in normal adult cells, promotes aerobic glycolysis (26) substantiates the hypothesis that glycolysis can be regulated at the level of glycolytic enzymes in tumor cells. In this direction, it will be interesting to assess the impact of IRS-2 expression on M2 isoform-mediated glycolysis in mammary tumor cells, especially because such cells were shown to express the M2 isoform (26). The data we present here, however, center on the surface expression of glucose transporters as a mechanism to regulate glycolysis. We conclude from our data that Irs-2 regulates the surface expression of Glut1 in an mTor-dependent manner. Multiple mechanisms have been proposed for the regulation of Glut1 by mTor signaling, including at the level of protein expression, transporter activation, and trafficking (27–31). In one study that examined interleukin-3-dependent regulation of glycolysis using a hematopoietic/lymphoid cell line, Glut1 surface expression was not affected by rapamycin treatment (30). Rapamycin did, however, inhibit glucose uptake, suggesting that mTor signaling may regulate Glut1 transporter activity (30). In contrast, a recent study using Tsc2–/– fibroblasts reported that mTor signaling regulates the surface localization of several glucose transporters, including Glut1, through phosphorylation of the microtubule linker protein CLIP170 (31). In this study, constitutive mTor signaling inhibited Glut1 expression on the cell surface. We observed a negative effect of rapamycin on Glut1 surface expression in our studies, which supports a positive role for mTor signaling in Glut1 trafficking and suggests that dynamic regulation of this pathway may be necessary for normal trafficking. However, the possibility that rapamycin also affects Glut1 activity in our model system cannot be excluded.
Ours is the first study to implicate the Irs proteins in regulating glucose uptake in tumor cells, and our data reveal a novel mechanism for regulating tumor cell glycolysis. The involvement of the Irs proteins in regulating glucose transport in insulin-responsive tissues has been investigated previously, and most of these studies have focused on their involvement in regulating Glut4, the major insulin-stimulated glucose transporter. These studies have revealed that the contribution of Irs-1 and Irs-2 to glucose transporter regulation is cell type-dependent. In brown adipocytes, Irs-2 regulates the insulin-dependent translocation of Glut4 to the plasma membrane (32), whereas Irs-1 appears to play the primary role in regulating Glut4 trafficking in other adipocyte model systems and in myotubes (33–35). Our data indicate that the regulation of glucose transporter trafficking is distinct in breast cancer cells from these previously published studies because GLUT1 appears to be the major glucose transporter that is expressed in breast cancer (23, 24) and its surface expression is dependent on IRS-2. A novel conclusion from our data is that increased expression of GLUT1 alone may not be sufficient to confer enhanced glycolysis in human tumors because factors such as IRS-2 may be required for GLUT1 to localize to the cell surface where it can facilitate glucose uptake.
A major finding in this study is that Irs-1 and Irs-2 differ markedly in their ability to activate mTor and sustain aerobic glycolysis in mammary carcinoma cells. These data are consistent with our previous finding that mammary tumor progression is significantly diminished in the absence of Irs-2 and that Irs-1 cannot compensate for loss of Irs-2 (14). Although both Irs-1 and Irs-2 can activate PI3K, which is an upstream regulator of mTor, Irs-2 activates mTor preferentially in mammary tumor cells, suggesting that there are mechanisms that control the signaling outcomes of these adaptor proteins. One potential mechanism that could selectively restrict downstream signaling from Irs-1 or Irs-2 is a differential sensitivity to negative feedback regulation. Many of the kinases that are activated downstream of the IRS proteins including Akt, S6-kinase, mTor, and MAPKs can phosphorylate the IRS proteins on serine/threonine residues to disrupt signaling either through inactivation or through degradation (reviewed in Ref. 36). Differences in the subcellular localization of IRS-1 and IRS-2 could also impact their downstream signaling pathways and consequent ability to regulate aerobic glycolysis. In breast carcinomas, IRS-1, but not IRS-2, is expressed in the nucleus, where it has been reported to contribute to both gene regulation and DNA repair (37–39). In adipocytes, IRS-1 is found predominantly in an intracellular membrane compartment, whereas IRS-2 is more concentrated in the cytosol (40). Differential compartmentalization of IRS-1 and IRS-2 would influence access to distinct substrates and regulatory proteins. These potential mechanisms need to be evaluated with respect to IRS-2-specific regulation of mTOR and aerobic glycolysis in breast cancer cells.
In summary, the data reported here implicate Irs-2 but not Irs-1 in the regulation of aerobic glycolysis in mammary tumor cells by a mechanism that involves mTor-dependent regulation of Glut1 surface expression. Our data link the regulation of aerobic glycolysis in breast cancer to tumor cell invasion and reveal a novel mechanism by which IRS-2 promotes breast cancer progression.
This work was supported, in whole or in part, by National Institutes of Health Grant CA090583 (to L. M. S.). This work was also supported by Grant BCTR0706837 from the Susan G. Komen Foundation (to A. M. M. and L. M. S.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: IRS, insulin receptor substrate; PyV-MT, polyoma virus middle T antigen; WT, wild type; mTor, mammalian target of rapamycin; IGF-1, insulin-like growth factor-1; Glut1, glucose transporter 1; DMEM, Dulbecco's modified Eagle's medium; BSA, bovine serum albumin; GFP, green fluorescent protein; EGFP, enhanced GFP; shRNA, short hairpin RNA; PI3K, phosphatidylinositol 3-kinase.
References
- 1.Warburg, O. (1956) Science 123 309–314 [DOI] [PubMed] [Google Scholar]
- 2.Gatenby, R. A., and Gillies, R. J. (2004) Nat. Rev. Cancer 4 891–899 [DOI] [PubMed] [Google Scholar]
- 3.DeBerardinis, R. J., Lum, J. J., Hatzivassiliou, G., and Thompson, C. B. (2008) Cell Metab. 7 11–20 [DOI] [PubMed] [Google Scholar]
- 4.Kroemer, G., and Pouyssegur, J. (2008) Cancer Cell 13 472–482 [DOI] [PubMed] [Google Scholar]
- 5.Gambhir, S. S. (2002) Nat. Rev. Cancer 2 683–693 [DOI] [PubMed] [Google Scholar]
- 6.Garber, K. (2006) Science 312 1158–1159 [DOI] [PubMed] [Google Scholar]
- 7.White, M. F. (2006) Can. J. Physiol. Pharmacol. 84 725–737 [DOI] [PubMed] [Google Scholar]
- 8.Jackson, J. G., Zhang, X., Yoneda, T., and Yee, D. (2001) Oncogene 20 7318–7325 [DOI] [PubMed] [Google Scholar]
- 9.Dunn, S. E., Ehrlich, M., Sharp, N. J., Reiss, K., Solomon, G., Hawkins, R., Baserga, R., and Barrett, J. C. (1998) Cancer Res. 58 3353–3361 [PubMed] [Google Scholar]
- 10.Sachdev, D., Hartell, J. S., Lee, A. V., Zhang, X., and Yee, D. (2004) J. Biol. Chem. 279 5017–5024 [DOI] [PubMed] [Google Scholar]
- 11.Zhang, X., Kamaraju, S., Hakuno, F., Kabuta, T., Takahashi, S., Sachdev, D., and Yee, D. (2004) Breast Cancer Res. Treat. 83 161–170 [DOI] [PubMed] [Google Scholar]
- 12.Cui, X., Kim, H. J., Kuiatse, I., Kim, H., Brown, P. H., and Lee, A. V. (2006) Cancer Res. 66 5304–5313 [DOI] [PubMed] [Google Scholar]
- 13.Byron, S. A., Horwitz, K. B., Richer, J. K., Lange, C. A., Zhang, X., and Yee, D. (2006) Br. J. Cancer 95 1220–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagle, J. A., Ma, Z., Byrne, M. A., White, M. F., and Shaw, L. M. (2004) Mol. Cell. Biol. 24 9726–9735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manel, N., Kim, F. J., Kinet, S., Taylor, N., Sitbon, M., and Battini, J. L. (2003) Cell 115 449–459 [DOI] [PubMed] [Google Scholar]
- 16.Kim, F. J., Manel, N., Garrido, E. N., Valle, C., Sitbon, M., and Battini, J. L. (2004) Retrovirology 1 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw, L. M., Rabinovitz, I., Wang, H. H., Toker, A., and Mercurio, A. M. (1997) Cell 91 949–960 [DOI] [PubMed] [Google Scholar]
- 18.Guo, S., Dunn, S. L., and White, M. F. (2006) Mol. Endocrinol. 20 3389–3399 [DOI] [PubMed] [Google Scholar]
- 19.Biggs, W. H., III, Meisenhelder, J., Hunter, T., Cavenee, W. K., and Arden, K. C. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 7421–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang, W., Patil, S., Chauhan, B., Guo, S., Powell, D. R., Le, J., Klotsas, A., Matika, R., Xiao, X., Franks, R., Heidenreich, K. A., Sajan, M. P., Farese, R. V., Stolz, D. B., Tso, P., Koo, S. H., Montminy, M., and Unterman, T. G. (2006) J. Biol. Chem. 281 10105–10117 [DOI] [PubMed] [Google Scholar]
- 21.Buteau, J., Shlien, A., Foisy, S., and Accili, D. (2007) J. Biol. Chem. 282 287–293 [DOI] [PubMed] [Google Scholar]
- 22.Ma, Z., Gibson, S. L., Byrne, M. A., Zhang, J., White, M. F., and Shaw, L. M. (2006) Mol. Cell. Biol. 26 9338–9351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang, S. S., Chun, Y. K., Hur, M. H., Lee, H. K., Kim, Y. J., Hong, S. R., Lee, J. H., Lee, S. G., and Park, Y. K. (2002) Jpn. J. Cancer Res. 93 1123–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moadel, R. M., Weldon, R. H., Katz, E. B., Lu, P., Mani, J., Stahl, M., Blaufox, M. D., Pestell, R. G., Charron, M. J., and Dadachova, E. (2005) Cancer Res. 65 698–702 [PubMed] [Google Scholar]
- 25.Gatenby, R. A., Gawlinski, E. T., Gmitro, A. F., Kaylor, B., and Gillies, R. J. (2006) Cancer Res. 66 5216–5223 [DOI] [PubMed] [Google Scholar]
- 26.Christofk, H. R., Vander Heiden, M. G., Harris, M. H., Ramanathan, A., Gerszten, R. E., Wei, R., Fleming, M. D., Schreiber, S. L., and Cantley, L. C. (2008) Nature 452 230–233 [DOI] [PubMed] [Google Scholar]
- 27.Taha, C., Liu, Z., Jin, J., Al-Hasani, H., Sonenberg, N., and Klip, A. (1999) J. Biol. Chem. 274 33085–33091 [DOI] [PubMed] [Google Scholar]
- 28.Zhou, Q. L., Jiang, Z. Y., Holik, J., Chawla, A., Hagan, G. N., Leszyk, J., and Czech, M. P. (2008) Biochem. J. 411 647–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buller, C. L., Loberg, R. D., Fan, M. H., Zhu, Q., Park, J. L., Vesely, E., Inoki, K., Guan, K. L., and Brosius, F. C., III (2008) Am. J. Physiol. 295 C836–C843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wieman, H. L., Wofford, J. A., and Rathmell, J. C. (2007) Mol. Biol. Cell 18 1437–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang, X., Kenerson, H., Aicher, L., Miyaoka, R., Eary, J., Bissler, J., and Yeung, R. S. (2008) Am. J. Pathol. 172 1748–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fasshauer, M., Klein, J., Ueki, K., Kriauciunas, K. M., Benito, M., White, M. F., and Kahn, C. R. (2000) J. Biol. Chem. 275 25494–25501 [DOI] [PubMed] [Google Scholar]
- 33.Kaburagi, Y., Satoh, S., Tamemoto, H., Yamamoto-Honda, R., Tobe, K., Veki, K., Yamauchi, T., Kono-Sugita, E., Sekihara, H., Aizawa, S., Cushman, S. W., Akanuma, Y., Yazaki, Y., and Kadowaki, T. (1997) J. Biol. Chem. 272 25839–25844 [DOI] [PubMed] [Google Scholar]
- 34.Quon, M. J., Butte, A. J., Zarnowski, M. J., Sesti, G., Cushman, S. W., and Taylor, S. I. (1994) J. Biol. Chem. 269 27920–27924 [PubMed] [Google Scholar]
- 35.Huang, C., Thirone, A. C., Huang, X., and Klip, A. (2005) J. Biol. Chem. 280 19426–19435 [DOI] [PubMed] [Google Scholar]
- 36.Gual, P., Le Marchand-Brustel, Y., and Tanti, J. F. (2005) Biochimie (Paris) 87 99–109 [DOI] [PubMed] [Google Scholar]
- 37.Trojanek, J., Ho, T., Del Valle, L., Nowicki, M., Wang, J. Y., Lassak, A., Peruzzi, F., Khalili, K., Skorski, T., and Reiss, K. (2003) Mol. Cell. Biol. 23 7510–7524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morelli, C., Garofalo, C., Sisci, D., del Rincon, S., Cascio, S., Tu, X., Vecchione, A., Sauter, E. R., Miller, W. H., Jr., and Surmacz, E. (2004) Oncogene 23 7517–7526 [DOI] [PubMed] [Google Scholar]
- 39.Sisci, D., Morelli, C., Garofalo, C., Romeo, F., Morabito, L., Casaburi, F., Middea, E., Cascio, S., Brunelli, E., Ando, S., and Surmacz, E. (2006) J. Clin. Pathol. (Lond.) 60 633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inoue, G., Cheatham, B., Emkey, R., and Kahn, C. R. (1998) J. Biol. Chem. 273 11548–11555 [DOI] [PubMed] [Google Scholar]