Abstract
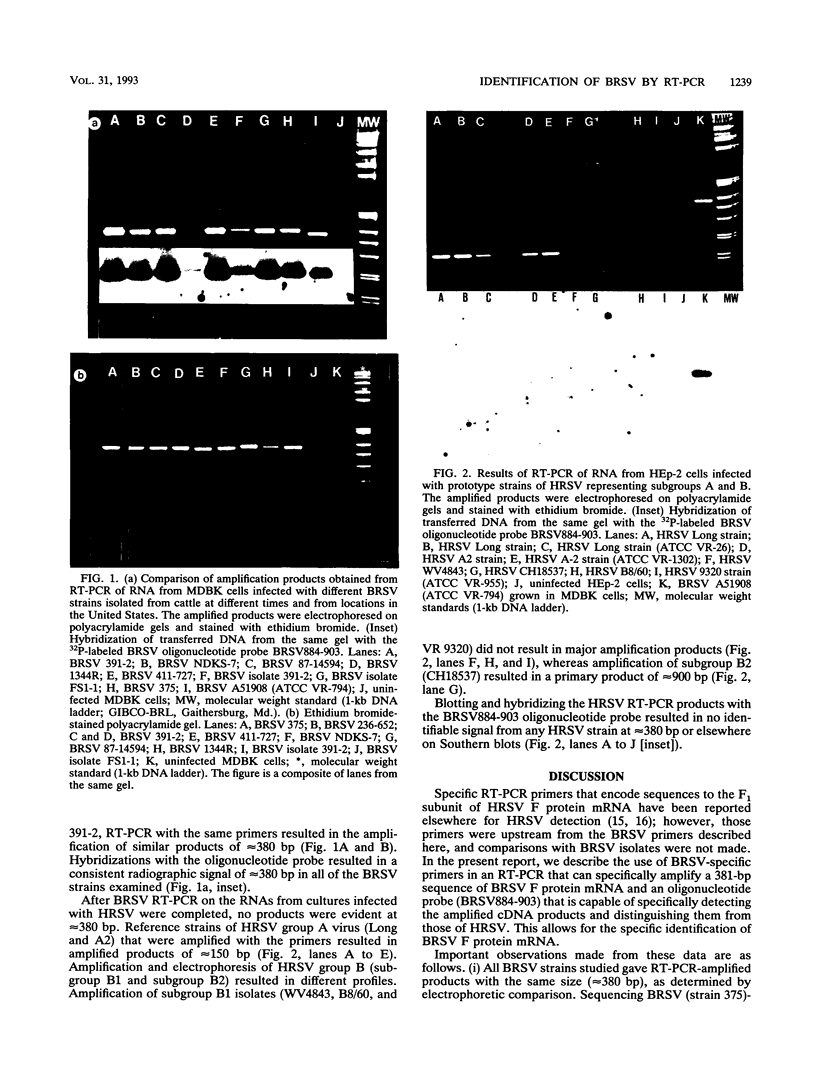
An assay to identify tissue culture cells infected with bovine respiratory syncytial virus (BRSV) that utilizes reverse transcription (RT), the polymerase chain reaction (PCR), and a synthetic oligonucleotide hybridization probe has been developed. The RT-PCR assay uses a BRSV-specific negative-sense oligonucleotide primer to synthesize cDNA from a BRSV fusion protein mRNA template and another BRSV-specific oligonucleotide primer (positive sense) upstream from the negative-sense primer for PCR amplification. In the presence of mRNA templates of BRSV isolates originating from locations throughout the United States, the BRSV RT-PCR assay resulted in amplified products (381 bp) that were specific to BRSV, as demonstrated in hybridizations with a positive-sense oligonucleotide probe complementary to internal sequences and in sequence comparisons with the F protein of BRSV 391-2. In analysis of the BRSV RT-PCR assay with prototype strains of human RSV subgroups A and B, amplification of a similar 381-bp RT-PCR product was not evident, and no RT-PCR product hybridized with the internal probe. We conclude that the specific ability to amplify DNA sequences of BRSV F protein mRNA by RT-PCR and then to demonstrate the presence of the amplified product with a BRSV-specific oligonucleotide probe will greatly add to the speed, sensitivity, and specificity of BRSV diagnostics.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. C., Ames T. R., Markham R. J. Serologic studies of bovine respiratory syncytial virus in Minnesota cattle. Am J Vet Res. 1985 Apr;46(4):891–892. [PubMed] [Google Scholar]
- Baker J. C., Ames T. R., Werdin R. E. Seroepizootiologic study of bovine respiratory syncytial virus in a beef herd. Am J Vet Res. 1986 Feb;47(2):246–253. [PubMed] [Google Scholar]
- Baker J. C., Werdin R. E., Ames T. R., Markham R. J., Larson V. L. Study on the etiologic role of bovine respiratory syncytial virus in pneumonia of dairy calves. J Am Vet Med Assoc. 1986 Jul 1;189(1):66–70. [PubMed] [Google Scholar]
- Bilofsky H. S., Burks C. The GenBank genetic sequence data bank. Nucleic Acids Res. 1988 Mar 11;16(5):1861–1863. doi: 10.1093/nar/16.5.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins J. K., Teegarden R. M., MacVean D. W., Salman, Smith G. H., Frank G. R. Prevalence and specificity of antibodies to bovine respiratory syncytial virus in sera from feedlot and range cattle. Am J Vet Res. 1988 Aug;49(8):1316–1319. [PubMed] [Google Scholar]
- Cubie H. A., Inglis J. M., McGowan A. M. Detection of respiratory syncytial virus antigen and nucleic acid in clinical specimens using synthetic oligonucleotides. J Virol Methods. 1991 Sep;34(1):27–35. doi: 10.1016/0166-0934(91)90118-j. [DOI] [PubMed] [Google Scholar]
- Edwards S., Newman R. H., Stanley M. Respiratory syncytial virus diagnosis. Vet Rec. 1984 Jan 28;114(4):101–101. doi: 10.1136/vr.114.4.101. [DOI] [PubMed] [Google Scholar]
- Himes S. R., Gershwin L. J. Bovine respiratory syncytial virus fusion protein gene: sequence analysis of cDNA and expression using a baculovirus vector. J Gen Virol. 1992 Jun;73(Pt 6):1563–1567. doi: 10.1099/0022-1317-73-6-1563. [DOI] [PubMed] [Google Scholar]
- Holzhauer C. Pinkengriep: het bovine respiratory syncytial virus als oorzaak van een atypische interstitiële pneumonie bij jonge runderen. Tijdschr Diergeneeskd. 1979 Sep 1;104(17):679–684. [PubMed] [Google Scholar]
- Kimman T. G., Straver P. J., Zimmer G. M. Pathogenesis of naturally acquired bovine respiratory syncytial virus infection in calves: morphologic and serologic findings. Am J Vet Res. 1989 May;50(5):684–693. [PubMed] [Google Scholar]
- Lehmkuhl H. D., Gough P. M., Reed D. E. Characterization and identification of a bovine respiratory syncytial virus isolated from young calves. Am J Vet Res. 1979 Jan;40(1):124–126. [PubMed] [Google Scholar]
- Lerch R. A., Anderson K., Amann V. L., Wertz G. W. Nucleotide sequence analysis of the bovine respiratory syncytial virus fusion protein mRNA and expression from a recombinant vaccinia virus. Virology. 1991 Mar;181(1):118–131. doi: 10.1016/0042-6822(91)90476-r. [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Shirotori K., Kudo K., Ito E., Togawa K., Saito I., Moro I., Ogra P. L. Genomic sequences of respiratory syncytial virus in otitis media with effusion. Lancet. 1991 Oct 19;338(8773):1025–1026. doi: 10.1016/0140-6736(91)91894-z. [DOI] [PubMed] [Google Scholar]
- Paton A. W., Paton J. C., Lawrence A. J., Goldwater P. N., Harris R. J. Rapid detection of respiratory syncytial virus in nasopharyngeal aspirates by reverse transcription and polymerase chain reaction amplification. J Clin Microbiol. 1992 Apr;30(4):901–904. doi: 10.1128/jcm.30.4.901-904.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbarth P. H., Hermus M. C., Schrijnemakers P. Reliability of two new test kits for rapid diagnosis of respiratory syncytial virus infection. J Clin Microbiol. 1991 Apr;29(4):824–826. doi: 10.1128/jcm.29.4.824-826.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. H., Frey M. L., Dierks R. E. Isolation, characterization, and pathogenicity studies of a bovine respiratory syncytial virus. Arch Virol. 1975;47(3):237–247. doi: 10.1007/BF01317811. [DOI] [PubMed] [Google Scholar]
- Sullender W. M., Mufson M. A., Anderson L. J., Wertz G. W. Genetic diversity of the attachment protein of subgroup B respiratory syncytial viruses. J Virol. 1991 Oct;65(10):5425–5434. doi: 10.1128/jvi.65.10.5425-5434.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullender W. M., Wertz G. W. Synthetic oligonucleotide probes differentiate respiratory syncytial virus subgroups in a nucleic acid hybridization assay. J Clin Microbiol. 1991 Jun;29(6):1255–1257. doi: 10.1128/jcm.29.6.1255-1257.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto S., Grandien M., Ishida M. A., Pereira M. S., Paiva T. M., Ishimaru T., Makita E. M., Martinez C. H. Comparison of enzyme-linked immunosorbent assay, indirect immunofluorescence assay, and virus isolation for detection of respiratory viruses in nasopharyngeal secretions. J Clin Microbiol. 1991 Mar;29(3):470–474. doi: 10.1128/jcm.29.3.470-474.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walravens K., Kettmann R., Collard A., Coppe P., Burny A. Sequence comparison between the fusion protein of human and bovine respiratory syncytial viruses. J Gen Virol. 1990 Dec;71(Pt 12):3009–3014. doi: 10.1099/0022-1317-71-12-3009. [DOI] [PubMed] [Google Scholar]