Abstract
Mycobacteria produce two sets of unusual polymethylated polysaccharides, the 3-O-methylmannose polysaccharides and the 6-O-methylglucose lipopolysaccharides. Both polysaccharides localize to the cytoplasm, where they have been postulated to regulate fatty acid metabolism due to their ability to form stable 1:1 complexes with fatty acyl chains. Physiological evidence for this assumption is lacking, however. Recent advances in our knowledge of the processes underlying sugar transfer in mycobacteria, together with the availability of genome sequences and tools for the genetic manipulation of these microorganisms, have opened the way to the elucidation of the biosynthetic pathways and biological functions of these unique carbohydrates.
Mycobacterium spp. are a source of unique carbohydrates (1) whose structural oddities have stimulated the interest of many scientists both from a fundamental perspective and as a source of potential antigens and targets for new vaccines, diagnostics, and drugs for tuberculosis. Despite this, relatively little is known about the physiological functions and regulation of most of these compounds, and only in recent years have the genetics of their biosynthesis started to be explored.
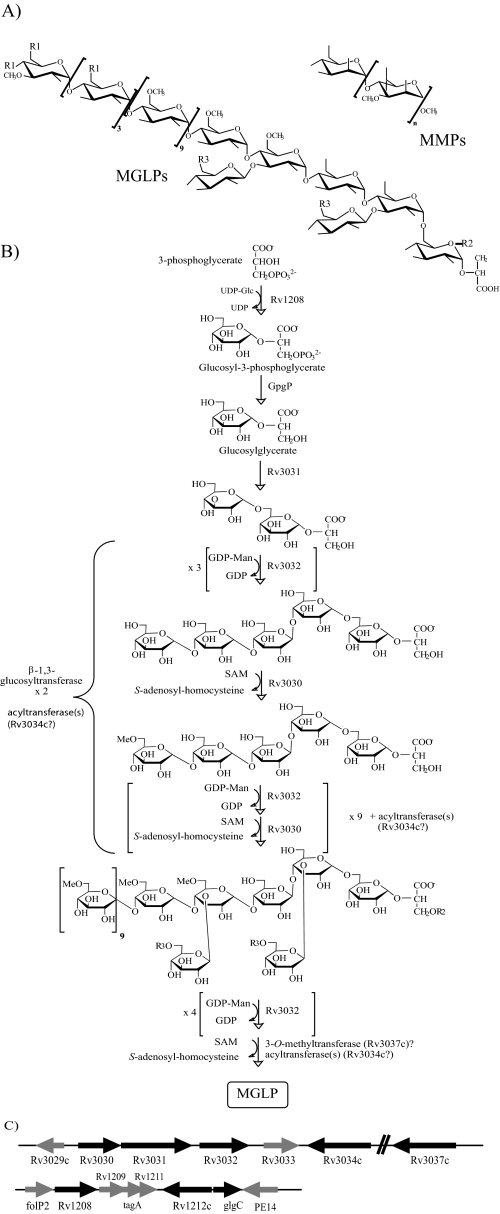
One such family of unusual carbohydrates unique to the order Actinomycetales is the polymethylated polysaccharides (PMPS).2 PMPS are cytoplasmic (lipo)polysaccharides of intermediate size composed of 10–20 sugar units, many of which are partially O-methylated, thus conferring on the molecules a slight hydrophobicity. Mycobacteria produce two such classes. One class is known as the 3-O-methylmannose polysaccharides (MMPs) and the second as the 6-O-methylglucose lipopolysaccharides (MGLPs) (Fig. 1). A remarkable property associated with MGLPs and MMPs is their ability to form stable 1:1 complexes with long-chain fatty acids and acyl-coenzyme A derivatives in vitro, resulting in the suggestion that they may be important regulators of lipid metabolism in mycobacteria.
FIGURE 1.
Structure and genetics of MGLPs and MMPs. A, structures of the MGLPs from M. bovis BCG and MMPs from M. smegmatis (n = 10–13). R1, R2, and R3 are acyl groups: R1, acetate, propionate, or isobutyrate; R2, octanoate; and R3, succinate. B, proposed biosynthetic model of MGLPs (20) with the names of the genes thought to be involved in the different steps of their elongation and modifications now indicated. Acylation and methylation are thought to occur concurrently; the precise stage at which the two β-(1→3)-linked Glc residues are attached is not known, but the definition of early MGLP precursors (20) suggests that they are added early during the elongation process. GpgP is an unknown phosphatase. C, organization and proposed functions of the two gene clusters involved in the biosynthesis of MGLPs in M. tuberculosis H37Rv (see text for details). The genes likely to be involved in MGLP synthesis are in black; genes of unknown function or involved in other pathways are in gray. In M. tuberculosis H37Rv, the start codon of Rv3031 overlaps the stop codon of Rv3030, and Rv3031 and Rv3032 are separated by an intergenic region of 32 bp. Nothing is known of the genetics of MMP synthesis.
PMPS were first isolated from Mycobacterium phlei, Mycobacterium smegmatis, and Mycobacterium tuberculosis in Dr. Clinton Ballou's laboratory (2–4), and much of our present information on these molecules comes from the early work of this group. Indeed, from the mid-1960s until the end of the 1980s, Ballou and associates spent more than 20 years characterizing the structures, exploring the biosynthetic pathways, and, in parallel with Konrad Bloch's laboratory, studying the biological activities of these unusual polysaccharides. Even though others later revised the structure of MGLPs and extended the analysis of these molecules to other mycobacterial species (5–7), since the end of the 1980s, no further work had been undertaken on the biogenesis of PMPS, and physiological evidence for a role of these molecules in the regulation of fatty acid metabolism is still lacking. The availability of a growing number of mycobacterial genome sequences combined with efficient tools for the genetic manipulation of these bacteria has prompted new research on the biosynthesis, genetics, and functions of PMPS.
Structure and Distribution of PMPS
The structures of MGLPs and MMPs are shown in Fig. 1A. The MMPs from M. smegmatis have been characterized in detail (8). They are composed of 10–13 α-(1→4)-linked 3-O-methyl-d-mannoses terminated at the nonreducing end by a single α-(1→4)-linked unmethylated d-mannose and at the reducing end by an α-methyl aglycon. MMPs occur in the cells as a mixture of at least four isomers owing to differences in size and degree of O-methylation. The MGLPs from Mycobacterium bovis BCG are composed of 10 α-(1→4)-linked 6-O-methylglucosyl residues with a nonreducing end made of the tetrasaccharide 3-O-methyl-d-Glcp-(α-(1→4)-d-Glcp)3-α-(1→. The tetrasaccharide →4)-(α-(1→4)-d-Glcp)3-α-(1→6)-d-Glcp-α-(1→ linked to position 2 of d-glyceric acid constitutes the reducing end of the molecule (7). Position 3 of the second and that of fourth α-d-Glcp residues (closest to the reducing end) are substituted by single α-d-Glcp residues. The nonreducing end of the polymer is acylated by a combination of acetate, propionate, and isobutyrate, whereas octanoate esterifies position 1 of glyceric acid, and zero to three succinate groups esterify the glucosyl residues of the reducing end. MGLPs occur as a mixture of four main components that differ in their content of esterified succinate.
MGLPs have been isolated from several Nocardia species as well as M. phlei, M. smegmatis, M. bovis BCG, M. tuberculosis, Mycobacterium leprae, and Mycobacterium xenopi (2, 3, 5–7, 9–11). MMPs have been found in different nonpathogenic fast-growing species of mycobacteria (12), and closely related acetylated forms of these compounds have also been reported in Streptomyces griseus (13). There has been, however, no report of the production of these polysaccharides in slow-growing mycobacteria, and our inability to detect any forms of MMPs in two different strains of M. tuberculosis using different analytical techniques (14) raises the possibility that they may be restricted only to the fast-growing mycobacterial species.
Biosynthesis and Genetics of PMPS
Biosynthetic Models—Current knowledge of the biosynthesis of MMPs is limited to the early work carried out in Ballou's laboratory in the 1970s–1980s. The isolation of precursors of MMPs and the characterization of an α-(1→4)-mannosyltransferase and 3-O-methyltransferase from cell-free extracts of M. smegmatis (8, 12, 15, 16) led to the proposal of a biosynthetic model in which MMPs are elongated by a linear alternating process of mannosylation followed by methylation (supplemental Material 1). GDP-Man serves as the sugar donor for the mannosyltransferase, and S-adenosylmethionine (SAM) is the source of methyl groups for the methyltransferase. Termination of the elongation reaction occurs when the chain reaches a length compatible with fatty acid-binding properties (11–13 3-O-methylmannoses). At this stage, the chain ends with an unmethylated mannose because the Km of the 3-O-methyltransferase for the polysaccharide-fatty acid complex becomes much higher than that of the α-(1→4)-mannosyltransferase. None of the genes involved in the biosynthesis of MMPs have yet been identified.
The model that emerged from Ballou's early work on the biogenesis of MGLPs resembles that proposed for MMPs. Using similar biochemical approaches for both sets of polysaccharides, Ballou and co-workers (17, 18) first reported the existence of a soluble protein fraction from M. phlei capable of transferring methyl groups from SAM to positions 6 and 3 of some glucosyl units in MGLPs and partially acetylated α-(1→4)-d-gluco-oligosaccharides. Because the position of methylation on the oligosaccharide acceptor was dependent on its degree of acetylation, it was suggested that acylation and methylation occurred together, the former process exerting a control on the latter. A membrane-associated acyltransferase activity catalyzing the transfer of acetyl, propionyl, isobutyryl, octanoyl, and succinyl groups from their respective acyl-CoA derivatives onto purified MGLPs and partially acetylated α-(1→4)-linked d-gluco-oligosaccharides was later reported (19). Finally, the characterization of weakly acidic and partially methylated methylglucose polysaccharide (MGP) precursors from M. smegmatis allowed Kamisango et al. (20) to propose a model for the biosynthesis of MGLPs in which the elongation of the chain proceeds stepwise, from the reducing toward the nonreducing end, through a sequential glucosylation-methylation reaction (Fig. 1B). Nothing is known of the termination reaction, and until our recent work on mycobacterial glycosyltransferases (GTs) (1, 14), none of the glucosyltransferase activities involved in the elongation of the glucan backbone of these lipopolysaccharides had been characterized.
Genetics of MGLP Synthesis—We recently reported the existence of two clusters of genes dedicated to the biosynthesis of MGLPs in the genomes of mycobacteria (Fig. 1C and supplemental Material 2) and the partial functional characterization of three of these genes: Rv3032, Rv1208, and the M. smegmatis ortholog of Rv3030, MSMEG2349 (14).3 Rv3032 carries the conserved motif (D/E)X7E characteristic of the CAZy GT-4 family of NDP-sugar-dependent retaining GTs (afmb.cnrsmrs.fr/CAZY/). The disruption of Rv3032 by homologous recombination in M. tuberculosis H37Rv resulted in a dramatic decrease in all forms of MGLPs, whereas the overexpression of this gene in M. smegmatis mc2155 stimulated the production of mature forms of MGLPs. Our results also indicated that Rv3032 participates in the biosynthesis of glycogen (14) and that partially purified recombinant Rv3032 has the ability to elongate short-chain α-(1→4)-d-gluco-oligosaccharides and glycogen in vitro using UDP-d-Glc as the glucosyl donor.4 Altogether, results suggest that Rv3032 is the main α-(1→4)-glucosyltransferase responsible for the elongation of MGLPs, even though this enzyme also participates in the elongation of other α-(1→4)-glucans. Adjacent or in close vicinity to this glucosyltransferase gene lies a putative acetyltransferase gene (Rv3034c), two putative SAM-dependent methyltransferase genes (Rv3030 and Rv3037c), and a putative α-amylase/glucoside hydrolase/GH-57 family branching enzyme gene (Rv3031) (Fig. 1C). The disruption of the ortholog of Rv3030 in M. smegmatis resulted in a dramatic reduction in the amounts of MGLPs synthesized and in the accumulation of precursors of these molecules, suggesting that Rv3030 encodes the main O-methyltransferase of the pathway, i.e. the one responsible for the 6-O-methylation of the lipopolysaccharides (Fig. 1B) (14). Important information derived from the analysis of this M. smegmatis mutant is that a defect in O-methylation abolishes MGLP synthesis. Thus, despite both Rv3032 and the 6-O-methyltransferase being active on unmethylated α-(1→4)-d-gluco-oligosaccharides in vitro (17, 18),4 the elongation of MGLPs in whole bacterial cells seems to proceed with glucosylation and O-methylation occurring hand in hand. This observation is consistent with the biosynthetic model proposed by Kamisango et al. (20) based on the structural analysis of MGLP precursors (Fig. 1B). Whether Rv3037c encodes the putative SAM-dependent 3-O-methyltransferase responsible for the 3-O-methylation of the glucosyl units of the reducing end of MGLPs (Fig. 1A) remains to be determined. Likewise, the sequence similarities between the product of Rv3031 and the GH-57 family branching enzyme from Thermococcus kodak-araensis (21) suggest that it is responsible for generating the α-(1→6)-glycosidic bond linking the first and second d-Glcp residues at the reducing end of the molecule in a mechanism similar to the one involved in the branching of glycogen.
As the disruption of Rv3032 in M. tuberculosis and that of the ortholog of Rv3030 in M. smegmatis did not totally abolish MGLP production, we had to assume on the existence of compensatory glucosyltransferase and methyltransferase activities in these species. A search for the enzyme compensating for Rv3032 in M. tuberculosis H37Rv led us to identify Rv1212c as a possible α-(1→4)-glucosyltransferase candidate; Rv1212c is the ortholog of the glycogen synthase gene from Corynebacterium glutamicum (22). Disruption of Rv1212c in M. tuberculosis H37Rv resulted in a significant reduction in the amount of capsular α-d-glucan produced by this bacterium but did not appreciably affect its MGLP and glycogen contents (23). However, capsular α-d-glucan production in the Rv1212c mutant was restored upon overexpression of Rv3032, confirming a partial redundancy of the two enzymes even though, under physiological conditions, Rv3032 seems to be involved primarily in the production of MGLP and glycogen and Rv1212c in the elongation of the capsular α-d-glucan (23). Failure to disrupt both the Rv3032 and Rv1212c genes in the same M. tuberculosis H37Rv strain further indicated that bacterial growth requires at least one of these two genes to be functional (23). In this regard, it is interesting to note that although not all mycobacterial species display functional orthologs of Rv1212c and Rv3032, they all have retained at least one of the two genes. For instance, M. leprae, which produces MGLPs (5), carries an ortholog of Rv3032 in its genome, but Rv1212c is a pseudogene (supplemental Material 2). Conversely, a frameshift mutation at the 5′-end of the Rv3032 gene of M. smegmatis apparently abolishes the activity of this gene.4 M. smegmatis does, however, carry an ortholog of Rv1212c, which most likely accounts for the production of MGLPs in this species.
As is the case for Rv3032, Rv1212c is located in a cluster of genes encoding putative sugar-modifying enzymes (Fig. 1C). We have undertaken the characterization of some of the genes from this cluster and found that disruption of the putative ADP-glucose pyrophosphorylase gene glgC in M. tuberculosis H37Rv led to a 40–50% reduction in glycogen and capsular glucan contents (23). The product of Rv1208 was predicted to be a CAZy family 2 sugar-nucleotide-utilizing GT of unknown function. Weak sequence similarities to the glucosyl-3-phosphoglycerate synthase (GpgS) from Persephonella marina (∼24% amino acid identity) suggested, however, that this enzyme might be responsible for the transfer of the first Glcp residue onto d-3-phosphoglycerate or d-glycerate (24) to form the glucosyl-(1→2)-glycerate found at the reducing end of MGLP (Fig. 1A). Supporting this hypothesis, recombinant forms of the M. smegmatis and M. bovis BCG Rv1208 enzymes produced in Escherichia coli were shown to display GpgS activity in vitro (25). Moreover, our recent evidence indicated that the disruption of the ortholog of Rv1208 in M. smegmatis (MSMEG_5084) reduced by ∼80% the de novo production of MGLP in this species.3 The ability of the mutant to synthesize residual amounts of the lipopolysaccharides was apparently attributable to the existence of compensatory GpgS activity in M. smegmatis. The crystal structure of the ortholog of Rv1208 in Mycobacterium avium spp. paratuberculosis (MAP2569c) has been solved (26, 27), and diffraction-quality crystals of Rv1208 from M. tuberculosis were obtained.5
Recent studies thus revealed the participation of at least two clusters of genes in the biosynthesis of MGLPs and other α-(1→4)-glucans in M. tuberculosis and a partial redundancy in the α-(1→4)-glucosyltransferase activities responsible for their elongation. The gene(s) encoding the GT(s) responsible for the glucosylation of position 3 of the second and fourth α-d-Glcp residues closest to the reducing end of MGLPs are not known.
Biological Activities
Postulated Roles of PMPS in Regulation of Fatty Acid Metabolism—Studies from the Ballou and Bloch laboratories have highlighted the unique regulatory roles of MMPs and MGLPs in mycobacterial fatty acid synthase I (FAS-I) in cell-free assays. In vitro, both PMPS raise the overall rate of synthesis of fatty acids by FAS-I and shift the neo-synthesized chain length pattern from long (C20–C24) to short (C14–C18) (28). These effects are thought to result from the ability of the (lipo)polysaccharides to form complexes with fatty acyl chains and acyl-CoAs. In sequestering the products of FAS-I, PMPS are thought to facilitate the release of the neo-synthesized chains from the enzyme, thereby not only reopening active sites essential for enzyme turnover but also terminating their elongation. In addition, in complexing the acyl-CoAs released in the reaction mixture, MGLPs and MMPs are thought to relieve product inhibition of the synthetase. With an intracellular concentration of long-chain acyl-CoAs in M. smegmatis of ∼0.3 mm, the concentration of PMPS approaching 1 mm, and the dissociation constant of the polysaccharide-lipid complex estimated to 0.1 μm, all of the long-chain fatty acids of the cytosol may form complexes with PMPS (29). Under these circumstances, PMPS could be regarded as general fatty acyl carriers in mycobacteria, protecting long-chain fatty acyl-CoAs from attack by degradative enzymes and regulating their further processing for the synthesis of more complex fatty acids and lipids, including mycolates (28–30).
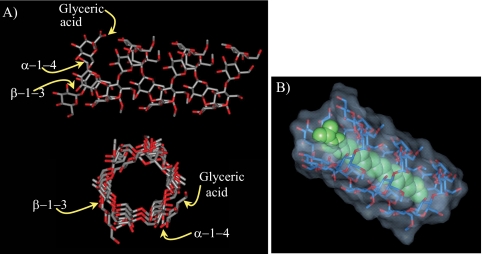
Evidence for the formation of complexes between PMPS and fatty acyl/fatty acyl-CoAs was supported by multiple experimental studies using a variety of techniques (29, 31–35). The fact that both MMPs and MGLPs are composed of hexose units predominantly or exclusively in α-(1→4)-linkage confers on these molecules a proclivity to assume the helical conformation characteristic of amylose (Fig. 2A). Within the complexes, the fatty acyl chain is included in the nonpolar cavity of the coiled polysaccharide chain (Fig. 2B). NMR studies have shown that MMP, a random coil in its free form, undergoes a major conformational transition upon enclosing long-chain acyl-CoAs. This polysaccharide, probably in helical conformation in the complexed form, interacts with both the paraffinic chain and the CoA moieties of the included fatty acyl thioester (29, 33). Similar conclusions apply to MGLPs (34). However, the presence of acetate, propionate, isobutyrate, octanoate, and succinate groups acylating the polysaccharide backbone and glyceric acid of MGP may confer upon the native MGLP a coiled conformation even in the absence of added long-chain fatty acyls. It was proposed that the acylation of MGP may alter its fatty acid-binding specificity and serve to fine-tune its regulatory activity in the cell (34). As expected, the number of sugar units of PMPS was shown to greatly impact their fatty acid-binding properties (32).
FIGURE 2.
A, top and side views of a structural model of MGP containing 19 glucosyl units of which 12 are O-methylated (MGP19,12); B, structural model of stearic acid (in green) bound to MGP20,12.
Thus far, physiological evidence for a role of PMPS in the regulation of mycobacterial lipid metabolism has been lacking. In the only published study on the role of PMPS in mycobacterial fatty acid metabolism in vivo (36), a M. smegmatis spontaneous mutant was described that contained only ∼50 and 7% of the wild-type levels of MGLPs and MMPs, respectively. Although this mutant accumulated slightly more short-chain and less long-chain unsaturated fatty acids than the wild-type strain, fatty acid synthesis was not dramatically altered. Further doubts regarding the prevailing hypothesis that MGLPs are involved in the regulation of fatty acid metabolism were cast by the observation that Rv3032 and MSMEG_5084 knock-out mutants of M. tuberculosis H37Rv and M. smegmatis, known to be impaired in MGLP synthesis, displayed wild-type fatty acid contents (14).3 The question of the regulatory roles of these unique PMPS in fatty acid metabolism thus remains open, as is the reason for the existence of two types of PMPS with apparent redundant functions in some Mycobacterium spp.
Other Possible Functions—Although the main environmental factor(s) governing the synthesis of MGLPs remain to be identified, studies from Ballou's laboratory (15, 20) and our own observations suggest that the MGLP content of mycobacteria greatly varies with the culture medium and/or oxygen tension. We found that the greatest amounts of these compounds were recovered from M. tuberculosis or M. smegmatis grown as surface pellicles in minimal Sauton's medium.4 Observations made on the growth characteristics of Rv3032 or Rv3030 mutants of M. tuberculosis and M. smegmatis displaying reduced MGLP contents indicated that a diminution in the concentration of these polysaccharides dramatically affected growth at high temperatures (14). Finally, the occurrence of glucosylglycerate at the reducing end of MGLPs may suggest a role for these lipopolysaccharides in osmoprotection (24).
Are PMPS Essential for Mycobacterial Growth?—Our results indicate that at least one of the two α-1,4-glucosyltransferase genes of M. tuberculosis, Rv1212c or Rv3032, must be functional for sustained growth in vitro (23), implying that the production of glycogen, glucan, and/or MGLPs is an essential physiological requirement under axenic conditions. Whether this requirement for α-1,4-glucosyltransferase activity is a consequence of the essentiality of MGLPs will have to await the construction of defined (conditional) mutants totally deficient in the production of these molecules. In vivo, partial loss of MGLPs in the M. tuberculosis Rv3032 mutant did not compromise the growth and pathogenicity of this strain in BALB/c mice (23).
Conclusions
Despite a wealth of in vitro and in vivo data pointing to a role of MGLPs and MMPs in fatty acid metabolism, the questions of the essentiality, regulation, and physiological role(s) of these molecules remain open. Answers to these questions will require the construction of mutants totally deficient in their synthesis. In the case of MGLPs, this goal is likely to be achievable in M. tuberculosis through the individual or combined inactivation of the glucosyl-3-phosphoglycerate synthase, branching enzyme, α-(1→4)-glucosyltransferase, methyltransferase, and the acetyltransferase genes (Rv1208, Rv3032, Rv3031, Rv3030, Rv3037c, and Rv3034c). In contrast, the construction of knock-out mutants deficient in the production of MMPs will have to await the identification of the genes involved in their synthesis in fast-growing mycobacteria. The construction of defined mutants, together with the development of cell-free assays for each identified enzyme, will also shed light on the sequential reactions leading to the formation of these unique polysaccharides.
Supplementary Material
Acknowledgments
We thank Drs. D. Kaur and M. Guerin (Colorado State University) and Dr. G. Stadthagen (Biotech Research & Innovation Centre, Copenhagen, Denmark) for sharing unpublished information. Fig. 2 was generously provided by Dr. M. Rivière (Institut de Pharmacologie et de Biologie Structurale, CNRS, Toulouse, France).
This work was supported, in whole or in part, by National Institutes of Health Grant AI064798 from NIAID. This work was also supported by European Commission Contract LSHP-CT-2005-018923 (NM4TB). This minireview will be reprinted in the 2009 Minireview Compendium, which will be available in January, 2010.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Material 1 and Material 2.
Footnotes
The abbreviations used are: PMPS, polymethylated polysaccharides; MMPs, 3-O-methylmannose polysaccharides; MGLPs, 6-O-methylglucose lipopolysaccharides; BCG, bacille Calmette-Guérin; SAM, S-adenosylmethionine; MGP, methylglucose polysaccharide; GTs, glycosyltransferases; FAS-I, fatty acid synthase I.
D. Kaur et al., unpublished data.
G. Stadthagen and M. Jackson, unpublished data.
Gest, P., Kaur, D., Pham, H. T., van der Woerd, M., Hansen, E., Brennan, P. J., Jackson, M., and Guerin, M. E. (2008) Acta Crystallogr. F Struct. Biol. Crystalliz. Comm., in press.
References
- 1.Berg, S., Kaur, D., Jackson, M., and Brennan, P. J. (2007) Glycobiology 17 35R–56R [DOI] [PubMed] [Google Scholar]
- 2.Lee, Y. C., and Ballou, C. E. (1964) J. Biol. Chem. 239 PC3602–PC3603 [PubMed] [Google Scholar]
- 3.Lee, Y. C. (1966) J. Biol. Chem. 241 1899–1908 [PubMed] [Google Scholar]
- 4.Gray, G. R., and Ballou, C. E. (1971) J. Biol. Chem. 246 6835–6842 [PubMed] [Google Scholar]
- 5.Hunter, S. W., Gaylord, H., and Brennan, P. J. (1986) J. Biol. Chem. 261 12345–12351 [PubMed] [Google Scholar]
- 6.Tuffal, G., Ponthus, C., Picard, C., Rivière, M., and Puzo, G. (1995) Eur. J. Biochem. 233 377–383 [DOI] [PubMed] [Google Scholar]
- 7.Tuffal, G., Albigot, R., Rivière, M., and Puzo, G. (1998) Glycobiology 8 675–684 [DOI] [PubMed] [Google Scholar]
- 8.Maitra, S. K., and Ballou, C. E. (1977) J. Biol. Chem. 252 2459–2469 [PubMed] [Google Scholar]
- 9.Keller, J. M., and Ballou, C. E. (1968) J. Biol. Chem. 243 2905–2910 [PubMed] [Google Scholar]
- 10.Lornitzo, F. A., and Goldman, D. S. (1968) Biochim. Biophys. Acta 158 329–335 [DOI] [PubMed] [Google Scholar]
- 11.Pommier, M. T., and Michel, G. (1986) J. Gen. Microbiol. 132 2433–2441 [DOI] [PubMed] [Google Scholar]
- 12.Weisman, L. S., and Ballou, C. E. (1984) J. Biol. Chem. 259 3457–3463 [PubMed] [Google Scholar]
- 13.Harris, L. S., and Gray, G. R. (1977) J. Biol. Chem. 252 2470–2477 [PubMed] [Google Scholar]
- 14.Stadthagen, G., Sambou, T., Guerin, M., Barilone, N., Boudou, F., Korduláková, J., Charles, P., Alzari, P. M., Lemassu, A., Daffé, M., Puzo, G., Gicquel, B., Rivière, M., and Jackson, M. (2007) J. Biol. Chem. 282 27270–27276 [DOI] [PubMed] [Google Scholar]
- 15.Yamada, H., Cohen, R. E., and Ballou, C. E. (1979) J. Biol. Chem. 254 1972–1979 [PubMed] [Google Scholar]
- 16.Weisman, L. S., and Ballou, C. E. (1984) J. Biol. Chem. 259 3464–3469 [PubMed] [Google Scholar]
- 17.Ferguson, J. A., and Ballou, C. E. (1970) J. Biol. Chem. 245 4213–4223 [PubMed] [Google Scholar]
- 18.Grellert, E., and Ballou, C. E. (1972) J. Biol. Chem. 247 3236–3241 [PubMed] [Google Scholar]
- 19.Tung, K.-K., and Ballou, C. E. (1973) J. Biol. Chem. 248 7126–7133 [PubMed] [Google Scholar]
- 20.Kamisango, K., Dell, A., and Ballou, C. E. (1987) J. Biol. Chem. 262 4580–4586 [PubMed] [Google Scholar]
- 21.Murakami, T., Kanai, T., Takata, H., Kuriki, T., and Imanaka, T. (2006) J. Bacteriol. 188 5915–5924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzvetkov, M., Klopprogge, C., Zelder, O., and Liebl, W. (2003) Microbiology (Read.) 149 1659–1673 [DOI] [PubMed] [Google Scholar]
- 23.Sambou, T., Dinadayala, P., Stadthagen, G., Barilone, N., Bordat, Y., Constant, P., Levillain, F., Neyrolles, O., Gicquel, B., Lemassu, A., Daffé, M., and Jackson, M. (2008) Mol. Microbiol. 70 762–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes, C., Empadinhas, N., and da Costa, M. S. (2007) J. Bacteriol. 189 4014–4019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Empadinhas, N., Albuquerque, L., Mendes, V., Macedo-Ribeiro, S., and da Costa, M. S. (2008) FEMS Microbiol. Lett. 280 195–202 [DOI] [PubMed] [Google Scholar]
- 26.Fulton, Z., Crellin, P. K., Brammananth, R., Zaker-Tabrizi, L., Coppel, R. L., Rossjohn, J., and Beddoe, T. (2008) Acta Crystallogr. F Struct. Biol. Crystalliz. Comm. 64, 428–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fulton, Z., McAlister, A., Wilce, M. C. J., Brammananth, R., Zaker-Tabrizi, L., Perugini, M. A., Bottomley, S. P., Coppel, R. L., Crellin, P. K., Rossjohn, J., and Beddoe, T. (2008) J. Biol. Chem. 283 27881–27890 [DOI] [PubMed] [Google Scholar]
- 28.Bloch, K. (1977) Adv. Enzymol. Relat. Areas Mol. Biol. 45 1–84 [DOI] [PubMed] [Google Scholar]
- 29.Yabusaki, K. K., and Ballou, C. E. (1979) J. Biol. Chem. 254 12314–12317 [PubMed] [Google Scholar]
- 30.Forsberg, L. S., Dell, A., Walton, D. J., and Ballou, C. E. (1982) J. Biol. Chem. 257 3555–3563 [PubMed] [Google Scholar]
- 31.Machida, Y., and Bloch, K. (1973) Proc. Natl. Acad. Sci. U. S. A. 70 1146–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yabusaki, K. K., and Ballou, C. E. (1978) Proc. Natl. Acad. Sci. U. S. A. 75 691–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maggio, J. E. (1980) Proc. Natl. Acad. Sci. U. S. A. 77 2582–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hindsgaul, O., and Ballou, C. E. (1984) Biochemistry 23 577–584 [DOI] [PubMed] [Google Scholar]
- 35.Tuffal, G., Tuong, A., Dhers, C., Uzabiaga, F., Rivière, M., Picard, C., and Puzo, G. (1998) Anal. Chem. 70 1853–1858 [DOI] [PubMed] [Google Scholar]
- 36.Maloney, D. H., and Ballou, C. E. (1980) J. Bacteriol. 141 1217–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.