Abstract
Cathepsin K is responsible for the degradation of type I collagen in osteoclast-mediated bone resorption. Collagen fragments are known to be biologically active in a number of cell types. Here, we investigate their potential to regulate osteoclast activity. Mature murine osteoclasts were seeded on type I collagen for actin ring assays or dentine discs for resorption assays. Cells were treated with cathepsins K-, L-, or MMP-1-predigested type I collagen or soluble bone fragments for 24 h. The presence of actin rings was determined fluorescently by staining for actin. We found that the percentage of osteoclasts displaying actin rings and the area of resorbed dentine decreased significantly on addition of cathepsin K-digested type I collagen or bone fragments, but not with cathepsin L or MMP-1 digests. Counterintuitively, actin ring formation was found to decrease in the presence of the cysteine proteinase inhibitor LHVS and in cathepsin K-deficient osteoclasts. However, cathepsin L deficiency or the general MMP inhibitor GM6001 had no effect on the presence of actin rings. Predigestion of the collagen matrix with cathepsin K, but not by cathepsin L or MMP-1 resulted in an increased actin ring presence in cathepsin K-deficient osteoclasts. These studies suggest that cathepsin K interaction with type I collagen is required for 1) the release of cryptic Arg-Gly-Asp motifs during the initial attachment of osteoclasts and 2) termination of resorption via the creation of autocrine signals originating from type I collagen degradation.
Osteoclasts are monocyte-macrophage lineage-derived, large multinucleated cells. They are the major bone resorbing cells, essential for bone turnover and development. Active osteoclasts display characteristic membranes, including the ruffled border, attachment zone, and the basolateral secretory membrane. After attachment to bone, the ruffled border secretes enzymes and protons enabling the solubilization and digestion of the bone matrix. Osteoclasts express many proteases including cathepsins and matrix metalloproteases (MMPs)2 (for review see Refs. 1-3). However, it is the general consensus that cathepsin K (catK) is the major bone-degrading enzyme (4-7).
Rapid cytoskeletal reorganization is essential for osteoclast function and formation of the specialized membranes. Bone resorption occurs within the sealing zone, which is formed by an actin ring structure. This can be identified as a solid circular belt like formation and consists of an actin filament core surrounded by actin-binding proteins such as talin, α-actinin, and vinculin, which link matrix-recognizing integrins to the cytoskeleton (8). The ruffled border is contained within this structure. The actin ring is initiated by the formation of podosomes, which represent dot-like actin structures of small F-actin containing columns surrounded by proteins also found in focal adhesion such as vinculin and paxillin (9). It was previously thought that the sealing zone was formed by the fusion of podosomes after the osteoclast becomes activated (10, 11), but it has since been demonstrated that podosomes and the sealing zone are distinct structures (12, 13). It should be noted that bone resorption only occurs when the sealing zone is formed and the actin ring is present (14).
Osteoclasts bind and interact with the bone surface through specific integrin receptors. The most abundant integrin present in osteoclasts is the αvß3 receptor also known as the vitronectin receptor (15, 16). This receptor attaches to RGD sequence containing components of the bone matrix, e.g. vitronectin, osteopontin, and type I collagen (17-19). This interaction enables the formation and regulation of the actin ring and therefore osteoclast activity (20-22). It has previously been shown that soluble RGD containing peptides added to cell supernatant are capable of inhibiting osteoclast binding and bone resorption (18, 22-24).
This study investigates the effect of collagen degradation fragments on osteoclast activity. Soluble type I collagen and the bone powder of murine long bones were subjected to digestion reactions by the cysteine proteases, catK and catL, and the interstitial collagenase, MMP-1. The effect of these degradation products on osteoclasts was investigated by monitoring actin ring and resorption pit formation. We further investigated the role of cathepsins using catK- and catL-deficient mice. Finally, we looked in more detail at the effect of collagen, as a cell adhesion matrix, on osteoclast activity.
EXPERIMENTAL PROCEDURES
Mouse Models—In these studies, the mouse models used were WT C57BL/6 (The Jackson Laboratories), catK knock-out mice 129:C57BL/6 (7), and catL knock-out mice 129:C57BL/6 (25).
Osteoclast Isolation from Neonatal Murine Long Bones—Mature osteoclasts were isolated from 6-day-old mouse long bones (26). Long bones were isolated and collected in α-modified minimal essential medium (α-MEM, Invitrogen) supplemented with 10% fetal bovine serum, 2 mm l-glutamate (Invitrogen), they were then diced into small pieces, and bone cells were released by gentle pipetting. The resulting cell suspension (without bone pieces) was then plated onto collagen-coated slides in a 24-well plate and incubated at 37 °C (95% air and 5% CO2). After 2 h, nonattached cells were washed away, and cells were treated with 10 μg/ml lipopolysaccharide (Sigma-Aldrich) and additional treatment as specified below. Cells were then cultured at 37 °C (5% CO2) for 24 h. To confirm the presence of osteoclasts, cells were stained for tartrate-resistant acid phosphatase activity (TRAP), an osteoclast marker, according to the manufacturers' instructions (Sigma-Aldrich). The number of positive TRAP-stained cells was about 50% of the total number of cells in preparations from wild type, catL-/- and catK-/- cells.
Cell Treatments—GRGDS and SDGRG (Sigma-Aldrich) were used at a final concentration of 100 μg/ml. For repeat RGD dose experiments, the media was changed every 2 h for 8 h to fresh media containing GRGDS (5-50 μg/ml). The irreversible (potent, non-selective) vinyl sulfone cathepsin inhibitor, LHVS, (27, 28) was used at a final concentration of 5 μm. The broad spectrum MMP inhibitor GM6001 (Chemicon, Temecula, CA) was used at a final concentration of 5 μm. All cell treatments were for 24 h unless otherwise stated.
Proteases—Recombinant human cathepsins K and L were expressed using the Pichia pastoris expression system (29, 30). Recombinant human MMP-1 was a generous gift from Dr. Chris Overall (Centre for Blood Research, University of British Columbia, Canada).
Fluorescent Staining, Actin Ring Formation of Osteoclasts—Cells were washed with PBS, fixed with 3.7% formaldehyde in PBS, and permeabilized with 0.2% Triton X-100 for 10 min. Osteoclast actin rings were visualized using FITC-phalloidin (Sigma-Aldrich) (1:50 dilution) staining as previously described (31). After staining, cells were washed with PBS, and the nuclei were stained with bisbenzimide (Sigma-Aldrich) (2 μg/ml) for 5 min and then rinsed with water. Cells were mounted with Fluoromount (Sigma-Aldrich). Actin rings were visualized fluorescently using a Leica DMI 6000B microscope (Leica Microsystems, Inc, Richmond Hill, ON) and the total number per slide was counted. Osteoclasts were identified by the presence of at least 2 nuclei. Typically osteoclasts contained between 3-7 nuclei, no distinction was made between large and small osteoclasts. If an osteoclast displayed one or more actin rings it was denoted as actin ring positive (AR+), osteoclasts without or disrupted actin rings were actin ring negative (AR-). The ratio of normal versus disrupted actin rings was calculated. An actin ring was considered disrupted if less than half of it exhibited typical actin ring morphology (10). Osteoclasts were also visualized by confocal microscopy using a Nikon confocal C1 microscope with EZ-C1 software (Nikon Instruments Inc, Mississauga, ON).
Resorption Assay—Cells were plated out onto dentine discs (Osteosite Dentine Discs, Immunodiagnostic Systems Inc, Fountain Hills, AR) in 96-well plates as previously described (26). After 2 h, discs were removed and placed in 6-well plates containing media (α-MEM at around pH 7.0) and test substance. There were typically 4 dentine discs per group. After 24 h, slices then stained for TRAP, which allowed the identification of osteoclasts (TRAP+ with over 2 nuclei). Once osteoclasts were counted, cells were removed with 5% sodium hypochlorite for 10 min. Discs were rinsed with water and stained with 1% (w/v) toluidine blue in 0.5% sodium borate for 30 s and then washed with water. The number and the area of resorption pits were then measured by light microscopy using Openlab 4.0.3 software. Results are expressed as the number of resorption pits, and total area resorbed per dentine disc.
Collagen Digests—Soluble type I collagen (5 mg/ml calf skin) (USB, Cleveland, OH) was incubated with human catK (200 nm) or human catL (200 nm) in sodium acetate buffer, pH 5.5, containing 2.5 mm dithiothreitol and EDTA or with p-aminophenylmercuric acetate (APMA) activated MMP-1 (50 nm) in 5 mm CaCl2, 50 mm HEPES, 100 mm NaCl, pH 7.2. Total volume of each reaction was 100 μl. Collagen digestions were performed at 28 °C in the absence and presence of 400 nm chondroitin 4-sulfate (Sigma-Aldrich) for 8 h (chondroitin 4-sulfate concentration based on an average molecular mass of 30 kDa). In both collagen and bone digests enzymes were inactivated by raising the pH of the reaction to 7.2 and then heating at 50 °C for 2 min. In cell treatments where collagen degradation products were added to media, 20 μl of pH-adjusted collagen digest was added to 1 ml of media. CatK-digested collagen was also subject to degradation by trypsin (Sigma-Aldrich), the pH was altered to 7.6 with a Tris buffer (100 mm final concentration) trypsin was added at 1 μm for 4 h at 30 °C.
Bone Digests—Long bones from 4-6-week-old wild-type mice were isolated, cleaned, and the epiphysis and bone marrow removed. Lipids were removed by incubating long bones overnight in xylene, bones were then frozen and crushed with a pestle and mortar to obtain bone powder. Bone powder was washed three times with sodium acetate buffer before enzyme was added. Human catK or catL were added to bone powder (15 mg with 100-μl reaction volume) at a concentration of 400 nm and incubated at 28 °C for 24 h. Samples were then spun down, the soluble fraction was removed and after its pH was neutralized 20 μl was added to 1 ml of media for cell treatments.
Collagen-coated Coverslips—Thin collagen coatings were generated as described by R&D systems (R&D systems, Inc. Minneapolis, MN) on glass coverslips. Soluble type I collagen (final concentration 50 μg/ml) was diluted in 500 μl of 0.02 mm acetic acid and 200 μl added per coverslip. Coverslips were incubated at room temperature for 1 h, residual volume was removed, and coverslips were washed with PBS before cells were added. Type I collagen was also pre-degraded with 200 nm catL or 200 nm catK in 100 mm sodium acetate buffer, pH 5.5 at 28 °C. Digested collagen was then added at the same concentration as undigested collagen to coverslips.
Gel Electrophoresis—Collagen degradation was analyzed by SDS-PAGE. Samples (1.5 μg per well) were boiled for 5 min with 2× reducing SDS-PAGE sample buffer and separated using 4-20% gradient gels (1.5 h at 125 V) (Invitrogen), 5 μl of prestained protein ladder (PAGE, Invitrogen) was included for orientation. Bands were visualized using Coomassie Brilliant Blue R 250 (0.5 mg/l, in 40% methanol and 10% acetic acid) and were then destained (40% methanol, 10% acetic acid).
Statistical Analysis—Experiments were performed in duplicate three times using osteoclast cultures from 3 different mice. Data are expressed as mean ± S.D. The statistical significance of the difference between the control and the experimental group was determined by Student's t test. In bone resorption assays, experiments were performed four times and comparisons between control and each treatment group was made using the Mann Whitney U test. Effects were considered statistically significant when p ≤ 0.05.
RESULTS
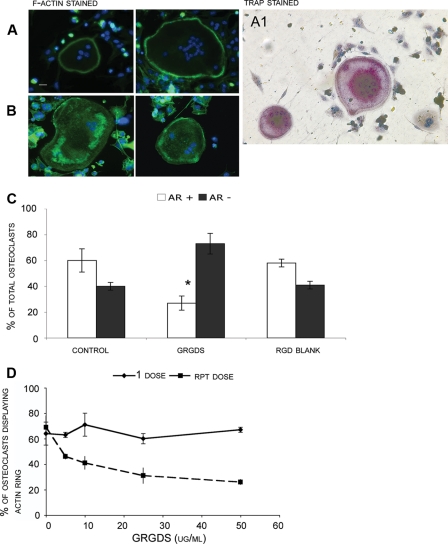
Effect of Proteolytically Degraded Soluble Collagens and Bone Powder on Actin Ring Presence in Wild-type Osteoclasts—This study investigated the effect of collagen fragments on osteoclast actin ring presence using neonatal murine osteoclasts seeded onto type I collagen. As mature osteoclasts were used in experiments a differentiation stage was not required. Type I collagen was used as a matrix as it is a well-defined catK substrate, and this substrate also decreases the matrix variability often found with bone slices. After attachment to collagen slides the osteoclasts were incubated for 24 h before the presence of actin rings was investigated. Under our conditions, 24-h incubations resulted in the highest incidence of actin rings and so this time point was used for all subsequent experiments. Wild-type osteoclasts were first treated with a synthetic peptide containing the RGD sequence. The majority of untreated osteoclasts revealed well-formed actin rings (Fig. 1A), whereas, as expected, cells treated with GRGDS peptide displayed mainly disrupted actin rings (Fig. 1B). The quantification of intact and disrupted actin rings in wild-type osteoclasts is shown in Fig. 1C. The addition of 100 μg/ml GRGDS peptide to wild type osteoclasts resulted in a 50% decrease in actin ring formation (Fig. 1C); in contrast the reverse sequence peptide SDGRG showed no effect on actin rings as it does not affect integrin function. Similar effects on osteoclast activity have previously been described for RGD containing proteins and peptides (32-34). We found that the GRGDS peptide had an accumulative inhibitory effect over time. A low concentration of RGD (10-50 μg/ml) left in contact with cells over 8 h had minimal effect on osteoclast actin rings. However when the same low doses were repeatedly administered to the cells every 2 h accompanied by a media exchange, the percentage of actin rings present was strongly reduced (Fig. 1D).
FIGURE 1.
Wild-type osteoclasts cultures obtained from murine long bones were seeded on type I collagen substrate, after 24 h of incubation cultures were stained for actin with FITC-phalloidin. Osteoclasts displaying a full actin ring or disrupted actin rings with more than 50% intact were identified as active (A). Osteoclasts displaying a disrupted actin ring (diffuse ring or less than 50% intact ring) or complete lack of actin structure were identified as inactive (B). Bar is 20 μm. Cultured wild-type osteoclasts were fixed and stained for the presence of TRAP, an osteoclast marker displayed as purple color. Cells were counterstained with H&E (A1). Panel A1 shows two TRAP-stained multinucleated osteoclasts in the presence of TRAP-negative cells. C, long bone osteoclasts isolated and cultured on collagen substrate from wild-type mice were analyzed for the presence of actin rings. After 24 h of incubation, cultures were stained for actin, and the total number of osteoclasts with actin rings were counted and compared with the total number of osteoclasts present (determined by cells containing 2 or more nuclei). Bar chart shows comparison between percentage of osteoclasts displaying an actin ring (positive for Actin Ring +) or without (negative for Actin Ring -) under different experimental conditions. Data are expressed as percentage. Osteoclasts were incubated with and without 100 μg/ml GRGDS (positive RGD control) or SDGRG peptide (blank). The presence of the GRGDS peptide decreased the percentage of active osteoclasts by half. The percentage of actin rings without treatment was compared with the actin rings with GRGDS treatment and was found to be statistically significant (p < 0.05). D, osteoclast cultures were also treated with one time and repeated low doses of GRGDS (5-50 μg/ml). Repeat doses were given every 2 h for 8 h, the previous dose was removed and cells were washed once between treatments. Data are expressed as mean ± S.D. of triplicate cultures. *, significantly different from the control, p ≤ 0.05.
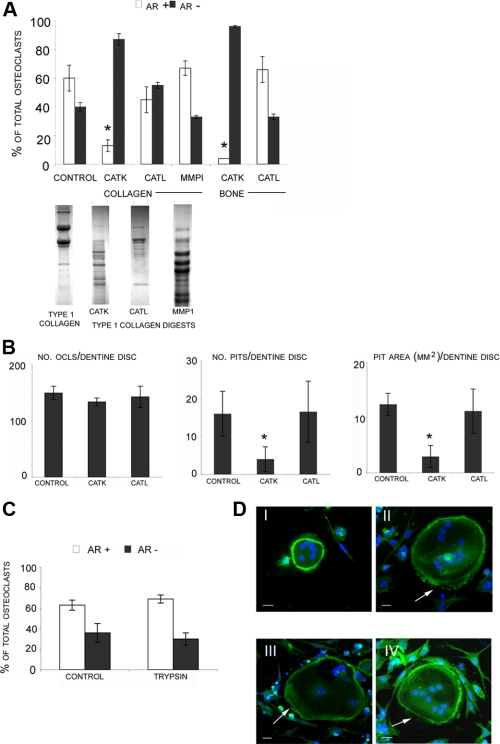
Next, we subjected triple-helical type I collagen which cumulatively contains 7 cryptic RGD motifs to degradation by catK, catL, and MMP-1 in the presence of chondroitin sulfate to mimic the presence of glycosaminoglycans in bone. We have previously demonstrated that glycosaminoglycans specifically modulate the collagenolytic activities of cathepsins (35). As described, CatK digestion resulted in a complete degradation of type I collagen, whereas the catL-mediated digest was minimal and the MMP-1 mediated digest revealed typical 3/4 and 1/4 fragments (35, 36) (Fig. 2A). F-actin staining of murine wild-type osteoclasts treated with catK-degraded type I collagen (final concentration 100 ng/ml) for 24 h showed a 75-80% inhibition of actin ring formation in wild-type cells (Fig. 2). No statistically significant inhibition was found after treatments with catL or MMP-1-digested collagen although catL-predigested type I collagen revealed a trend toward actin ring inhibition (Fig. 2A). There was also no effect on the percentage of osteoclasts with actin rings when undegraded collagen or vehicle (sodium acetate buffer) alone was added (results not shown). The inhibitory effect of catK-digested collagen was lost when it was subjected to further degradation by trypsin (Fig. 2C), known to cleave after arginine or lysine residues and thus likely to destroy the RGD moiety (37). Prior to the addition of the tryptic digest to the cells, trypsin was heat-inactivated.
FIGURE 2.
A, long bone osteoclasts obtained from wild-type mice were analyzed for activity after the addition of collagen and bone enzymatic degradations. Collagen type I and bone powder were degraded by catK, catL, or MMP-1 for 24 h. Cathepsin digestion reactions were in the presence of chondroitin 4-sulfate (C4-S) and were performed at 28 °C. MMP-1 reactions were performed at 28 °C in the absence of C4-S. Collagen degradations were analysed on 4-20% gradient gels and are shown below A for comparison. After 24-h incubations, cultures were stained for actin, and the total number of osteoclasts with actin rings were counted and compared with the total number of osteoclasts. Only cathepsin K-degraded substrates were able to inhibit actin ring formation. Actin ring percentage of osteoclasts in the presence or absence of protease-generated degradation products were compared. B, WT osteoclasts were cultured on dentine discs with or without catK- or catL-degraded soluble bone products for 24 h. The number of TRAP+ osteoclasts and the number and area of resorption pits were analyzed. Values are the means plus S.D. of 4 bone slices. C, wt cells were treated for 24 h with type I collagen digested by cathepsin K as before, and then further degraded by trypsin for 4 h at 30 °C. For comparison treatment of cells treated with type I collagen digested by cathepsin K alone is shown in Fig. 2A. Percentages of osteoclasts with actin rings are shown as AR+.*, p ≤ 0.05 when compared with wt control. D, confocal microscope images of wild-type osteoclasts stained for F-actin seeded on type I collagen substrate with no treatment (I), treated with GRGDS peptide (II), and treated with cathepsin K-degraded type I collagen (III/IV). Arrows indicate areas of cell retraction. Original magnification ×40, scale bar is 20 μm.
We also incubated the osteoclasts in the presence of the soluble products of protease-pretreated murine bone powder. Similar to the observation made with predigested soluble collagen, catK-digested bone powder revealed the strongest actin ring reduction. Up to 95% of the cells displayed disrupted actin ring structures suggesting a complete inhibition of the osteoclast activity (Fig. 2A). In contrast no effect was observed when cells were exposed to bone powder pretreated with catL. These results were also reflected in bone resorption assays performed on dentine discs. Both the number of resorption pits and the area resorbed per disc decreased on addition of catK-digested bone powder with no change in osteoclast number (Fig. 2B). As expected, catL-pretreated bone powder had no effect on bone resorption.
Actin formations observed in osteoclasts treated with catK degraded collagen showed podosome-like structures, often with actin forming clumps in a similar manner to cells treated with GRGDS (see Fig. 1B). Confocal microscopy showed that osteoclasts treated with catK-digested collagen fragments also displayed signs of cell retraction (Fig. 2D) similar to that observed when osteoclasts are treated with GRGDS peptide (23). The observed reduction in actin ring numbers was not thought to be due to a decrease in attached cells as all experiments had comparable osteoclast numbers. It has been previously shown that actin ring formation can be disrupted with no effect on osteoclast differentiation, survival, and attachment (38).
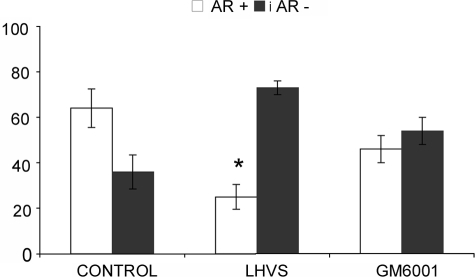
Effect of Cysteine and Metalloprotease Inhibitors on Actin Ring Formation in Wild-type Osteoclasts—After demonstrating that catK-predigested RGD peptide containing substrates affect the formation of actin rings, we analyzed the effect of protease inhibition in osteoclasts. Considering that cathepsin K activity generates active RGD peptides, we expected that the inhibition of cathepsin K and thus eliminating the generation of soluble RGD fragments would stabilize or increase the presence of actin rings. However, the addition of 5 μm of the cell-permeable broad spectrum cathepsin inhibitor, LHVS, to wild-type osteoclasts resulted in a 50% reduction of actin ring formation when compared with untreated osteoclasts (Fig. 3A). This suggests that cathepsin activities are required for the initial formation and/or maintenance of actin rings. At 5 μm concentration, LHVS is a potent inhibitor of cathepsins K, L, and S (27). In contrast, the broad spectrum metalloprotease inhibitor GM6001 had only a weak and statistically non-significant effect on the reduction of actin rings.
FIGURE 3.
Long bone osteoclasts obtained from wild-type mice were analyzed for actin ring presence after the addition of protease inhibitors. Wild-type osteoclasts were treated with either 5 μm of the broad spectrum cathepsin inhibitor, LHVS, or the broad spectrum MMP inhibitor, GM6001. LHVS acts in a dose-dependent manner on osteoclasts and at 5 μm, LHVS inhibits cathepsins K, L, S, and B (27, 55). After 2 h of incubation, cultures were stained for actin, and the total number of osteoclasts with actin rings were counted and compared with the total number of osteoclasts. Cathepsin inhibition reduced the percentage of actin rings by 50%, MMP inhibition showed no effect. Actin ring percentage of osteoclasts without treatment was compared with actin ring percentage in the presence of inhibitors. *, p ≤ 0.05 when compared with control.
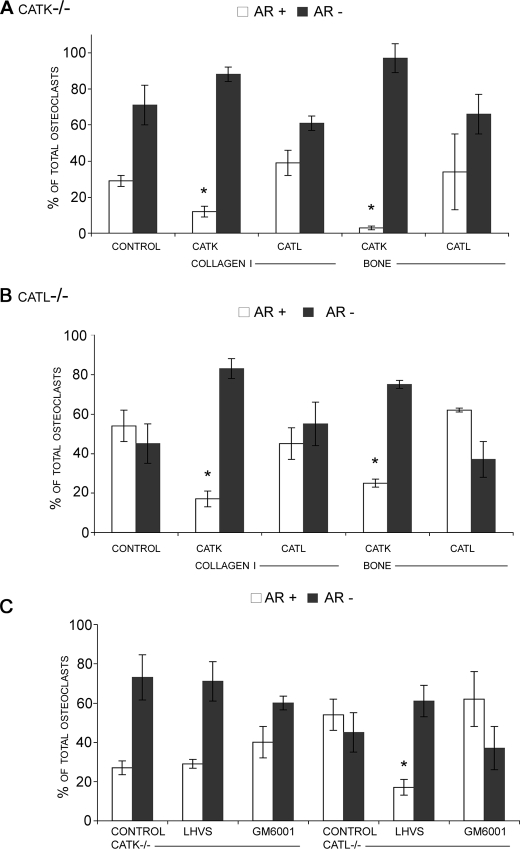
Effect of catK and -L Deficiency on Actin Ring Formation—As the broad spectrum cathepsin inhibitor, LHVS, was able to decrease actin ring formation, we next aimed to identify the individual cathepsin responsible for this inhibition by analyzing catK- and catL-deficient osteoclasts. CatK-deficient cells showed a similar suppression of actin rings as wild-type osteoclasts treated with LHVS. Both conditions displayed less than 50% of actin rings compared with untreated wild-type osteoclasts. The addition of catK-predigested soluble type I collagen and bone powder to catK-deficient osteoclasts further suppressed the percentage of cells exhibiting intact actin rings (Fig. 4A). On the other hand and similar to wild type osteoclasts, catL-pretreated collagen or bone powder did not affect the number of actin rings (Fig. 4A). On the contrary, catL-deficient osteoclasts were statistically undistinguishable from wild-type cells in the absence or presence of collagen/bone digest mixtures (Figs. 4B and 2A) suggesting that the loss of catL activity is not critical to osteoclast activity.
FIGURE 4.
Long bone osteoclasts obtained from catK-deficient (A) and catL-deficient (B) mice were analyzed for actin ring presence after the addition of collagen and bone enzymatic degradations. Collagen type I and bone powder were degraded by catK or catL in the presence of C4-S for 24 h at 28 °C. After 24 h, cultures were stained for actin, and the percentage of osteoclasts displaying an actin ring (AR+) is shown compared with the percentage without (AR-). As with wild-type osteoclasts only catK-mediated substrate digests showed an inhibitory effect on actin ring percentage. Note the low activity of untreated catK-/- osteoclasts compared with wild-type and catL-/- osteoclasts. The effect of cathepsin inhibition and MMP inhibition on actin ring percentage in catK- and catL-deficient osteoclasts was also investigated (C). Both cultures were incubated with 5 μm LHVS or GM6001 for 24 h. Results showed actin ring inhibition with cathepsin inhibition only in catL-/- osteoclasts. No inhibitory effect was observed in catK-/- osteoclasts. Actin ring percentage of osteoclasts without treatment was compared with actin ring percentage in the presence of degradation or inhibitory treatments and was statistically analyzed. *, p ≤ 0.05 when compared with catK-/- or catL-/- control.
Treatment of cathepsin-deficient cells with LHVS had no effect on catK-deficient cells whereas catL-deficient cells revealed a strong reduction in intact actin rings (60%) similar to that observed in wild-type cells in the presence of LHVS (Fig. 3) and of catK-deficient cells in the presence and absence of the cysteine protease inhibitor (Fig. 4C). In each case the percentage of osteoclasts displaying actin rings was reduced to between 15 and 30% indicating that catK alone is critically involved in actin ring formation. On the other hand, the metalloprotease inhibitor GM6001 had no significant effect on both cathepsin-deficient cell types (Fig. 4C).
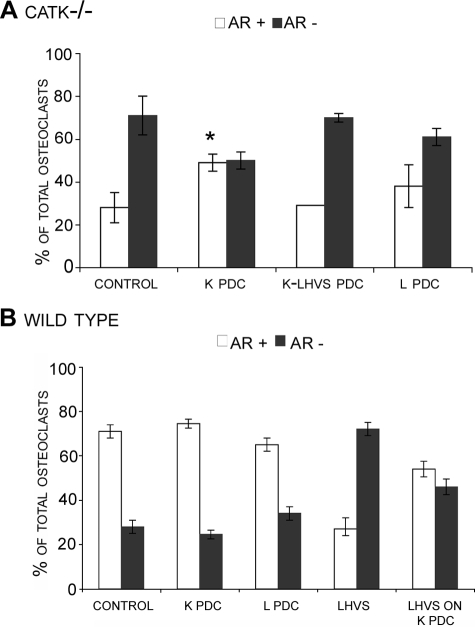
Effect of catK-predigested Type I Collagen Matrix on CatK-/- Osteoclasts and LHVS-treated Wild-type Osteoclasts—Both the addition of extracellular RGD-containing peptides or proteolytic fragments and the inhibition of catK inhibit actin ring formation and thus indicate the involvement of cathepsins, in particular catK, in the generation as well as the dissolution of actin rings following at least two pathways. To investigate whether cathepsin activities are required for the initial exposure of cryptic integrin binding sites in the collagen matrix and subsequently allowing actin ring formation, we predigested type I collagen with catK or catL and seeded catK-deficient osteoclasts on the predigested substrate matrix. CatK-deficient osteoclasts revealed a significant increase in actin ring numbers when grown on catK-predigested but not on catL-predigested collagen matrix (Fig. 5A). In contrast, there was no difference observed for wild-type cells on untreated, catK-, or catL-predigested matrices. However, similar to catK-deficient cells, LHVS treated wild-type cells seeded on catK-predigested matrix increased the actin ring content compared with wild-type cells treated with LHVS seeded on intact matrix (Fig. 5B). Although there was an increase in the number of actin rings, osteoclasts did not reach the levels of actin rings observed when untreated osteoclasts are seeded on intact collagen. These results suggest that catK activity is required to expose cryptic RGD motifs in type I collagen and to form actin rings.
FIGURE 5.
Long bone osteoclasts obtained from catK-/- (A) or wild type (B) mice were seeded onto intact type I collagen matrix (control) or type I collagen predegraded by catK (K PDC) or catL (L PDC) and analyzed for actin ring presence. Collagen type I was predegraded by 200 nm catK or catL in the presence of C4-S for 24 h at 28 °C. CatK collagen digestions occurred with and without the presence of 10 μm LHVS (K-LHVS PDC). After 24 h, cultures were stained for actin, and the percentage of osteoclasts displaying an actin ring (AR+) is shown compared with the percentage without (AR-). CatK-degraded type I collagen matrix was found to increase the percentage of actin rings in catK-/- osteoclasts. CatK-degraded collagen matrix had no effect on actin ring formation in wild-type osteoclasts except when cells were treated with 5 μm LHVS. Here the degraded substrate was able to reverse the inhibition and increased the percentage of actin rings present to levels similar to non-treated osteoclasts (B). Actin ring percentage of osteoclasts seeded on intact collagen was compared with actin ring percentage of osteoclasts on degraded matrix. *, p ≤ 0.05 when compared with catK-/- control.
DISCUSSION
The regulation of individual osteoclast activity remains unclear. This study aimed to investigate whether catK, responsible for bone degradation and highly expressed in osteoclasts, is capable of directly regulating osteoclast action by its extracellular matrix-degrading activity. To do this we exploited a cell based actin ring assay and minimized the effects of the extracellular matrix by using a single component (collagen type I) system representing the major and biologically relevant cathepsin K substrate (90% of the organic bone matrix is type I collagen). Mature murine osteoclasts were seeded on the collagen matrix and treated with catK, catL, or MMP1 predegraded as well as undegraded soluble type I collagen or bone powder and the percentage of osteoclasts displaying actin rings were determined. Only soluble type I collagen or type I collagen containing bone powder degraded by catK was capable of decreasing the percentage of active osteoclasts from wild-type mice (Fig. 1), which was in a similar range to the inhibition found after the addition of the synthetic control GRGDS peptide. Triple helical type I collagen which contains in its α1 and α2 chains a total of 7 RGD motifs, is cleaved by catK at multiple sites and generates low molecular weight fragments (39, 40), which may contain small soluble RGD containing peptides. The presence of soluble RGD-motif containing peptides was further supported by the loss of inhibitory effect when catK-digested collagen fragments were further degraded by trypsin before addition to cells (Fig. 2C). As trypsin preferentially cleaves after arginine and lysine its activity has likely degraded the soluble RGD sequences created by catK and thus explaining the loss of effect. In dentine resorption assays, catK-created soluble bone fragments were also able to decrease the number of pits and the area of bone resorbed (Fig. 2B). In this study collagen or bone degraded by catL or MMP-1 had no significant effect on the number of cells bearing an intact actin ring or dentine resorption. The substrate degradation by the two later enzymes was limited, suggesting that the cryptic RGD motifs residing within type I collagen are not successfully released from the protein and thus were not available to act as soluble ligands for the αvβ3 integrin receptor-RGD binding sites.
The experiments performed with catK-digested collagen/bone are indicative that RGD-containing fragments generated by catK activity during in vivo bone resorption have the potential to inhibit osteoclast resorption once sufficiently high concentrations of the RGD peptides are generated. Our studies have found that repeated exposure of low concentrations of RGD have an inhibitory effect greater than a single high dose. Thus a continual release of small amounts of collagen from the basal membrane or ruffled boarder may allow the RGD bound integrin receptors to build up over time until they reach such a concentration where they inhibit osteoclast activity. Therefore depending on the concentration of collagen fragments released over time the effect could be additive, allowing bone resorption to take place and be naturally inhibited once a certain amount of collagen has been processed and critical soluble RGD peptide concentrations are reached. We calculated that the concentration of type I collagen released from an average resorption pit would be ∼5 mm (radius 25 μm, depth 15 μm, ∼1/3 of bone volume is type I collagen and a volume of 530 nm3 per triple helical collagen molecule). In our studies, we added 333 μm type I collagen to the media. As the amount of type I collagen we added is more than 10× less of that potentially being solubilized by the osteoclast, we can speculate that the amount of collagen released in vivo may be enough to have an inhibitory effect although future research must consider the intracellular transport routes of collagen fragments and their diffusion rates from the osteoclasts and within the resorption lacuna.
These studies suggest a specific role for catK in the autocrine regulation of osteoclast activity through the release of type I collagen fragments able to disrupt the actin ring and therefore osteoclast-mediated bone resorption. This type of regulatory role has not previously been shown for catK activity in osteoclasts. In other cell types, proteolytically derived collagen fragments have varying effects including anti-angiogenesis, apoptosis, and tumor growth inhibition (41-45).
During bone resorption the release of calcium from the matrix is also thought to regulate osteoclast activity. Released calcium is thought to interact with osteoclast calcium receptors to inhibit bone resorption (46, 47). However in our experimental system when wild type osteoclasts plated on type I collagen were treated with 20 mm Ca2+ we found no effect on actin ring presence (data not shown).
The potential role of catK in actin ring dissolution may lead to the conclusion that the absence of catK (inhibition or catK deficiency) will result in the permanent presence of actin rings and potentially to an extended resorption/demineralization phase to compensate for the absence of the major bone degrading protease. However, our data obtained with catK-deficient cells or wild-type cells exposed to a potent and cell permeable cysteine protease inhibitor revealed a significant reduction of actin rings in the osteoclast population. This counterintuitive finding indicates that catK activity is also required for the initial formation of actin rings and thus for the activation of the osteoclasts. Secreted catK may participate in the release of cryptic RGD motifs present in an intact triple helical collagen matrix prior to the main resorption process. Partial digests may be sufficient to induce conformational changes in type I collagen, which expose the RGD motifs and allow their binding to αvβ3 integrin receptors on osteoclasts. Actively resorbing osteoclasts are associated with the presence of actin rings and the actin ring formation is enabled by the interaction of the osteoclast αvβ3 integrin receptor with RGD ligands in the matrix (18, 22-24).
The critical involvement of catK in the processes initiating actin formation is revealed by the finding that the low percentage of actin rings in catK-deficient osteoclasts is not further decreased by the presence of a broad spectrum cysteine protease inhibitor LHVS. CatL and surprisingly MMP1 seem to contribute little to the activation of the osteoclasts as neither catL-deficient osteoclasts nor the presence of the broad spectrum MMP inhibitor, GM6001, affected the formation of actin rings. It has been suggested that interstitial collagenase activity is involved in osteoclast activation (48). However in our system the use of a broad spectrum MMP inhibitor at up to 20 μm had no effect on osteoclast actin ring occurrence. On the other hand, LHVS reduced the actin ring percentage in catL-deficient osteoclasts to the level of catK-deficient cells. These results all indicate that the effects produced by LHVS are primarily through the inhibition of catK and not other cathepsins.
The lack of effect demonstrated by MMP inhibition was unexpected and may reflect the greater role for MMPs in migration than resorbing activity of osteoclasts as has previously been suggested (49). MMPs seem to play an important role in osteoclast migration to respective bone resorption sites; however when the cell reaches this site it may rely on cysteine cathepsins, to uncover integrin binding sites in the matrix and permit the formation of actin rings.
The effect of cathepsin inhibitors on osteoclasts, however, may be substrate-dependent. A recent study found no actin ring disruption when rat osteoclasts were cultured on bovine cortical bone slices and treated with E-64 (50). Bone matrices, in contrast to our simplified single component (type I collagen) matrix system, contain multiple RGD-containing proteins such as osteopontin and fibronectin. These may not require a proteolytic modification to reveal their RGD motifs and will thus be available to interact with the osteoclast resident αvβ3 integrin receptors to form actin rings.
Further investigation into the critical role of catK in osteoclast activation resulted in the finding that catK but not catL pretreatment of the collagen matrix increased the number of actin ring containing catK-deficient cells and LHVS-treated wild-type cells. At this point, it should be noted that the absence of catK activity does not completely shut down the formation of actin rings and thus potentially bone resorption. CatK-deficient osteoclasts still revealed a significant ability to form actin rings (reduction from 60 to 30% when compared with wild-type cells). It has been demonstrated that catK-deficient osteoclasts upregulate other proteases (7, 51, 52) and that these proteases may partially substitute catK in bone resorption (7, 53). There are also some differences between human and murine catK-deficient osteoclasts, in human catK knock-out osteoclasts there is a build up of undegraded type I collagen, this was not found to be the case in murine catK knock-out osteoclasts (54) and so is unlikely to be involved in our studies.
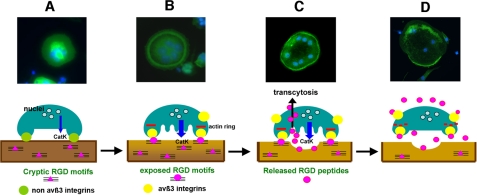
Based on the findings presented in this report, we propose the following model of protease (catK)-mediated osteoclast adhesion and detachment (Scheme 1). In this model, prior to the transformation into a resorbing cell, the osteoclast secretes catK and potentially other cysteine proteases to uncover cryptic RGD motifs within the collagen matrix. These can then interact with αvβ3 integrin receptors initiating cell signaling leading to the formation of actin rings. Upon initiating active bone resorption, large quantities of catK are released from lysosomes into the resorption lacunae where catK extensively degrades type I collagen. Some of this collagen is taken into the cell for further degradation and released from the cell via the basolateral membrane. Therefore, osteoclasts could be exposed to collagen fragments at both the ruffled border and basolateral/apical membranes. Once a critical RGD peptide concentration is reached, the integrin-matrix RGD binding is competitively disrupted and the actin rings dissolve. As the binding of RGD peptides to the integrins appears to be accumulative, maximum RGD concentrations do not need to be sustained to lead to a saturation of the integrin binding sites as long as sufficient concentrations are present over time. This will finally lead to cell retraction and termination of the active bone resorption phase of the individual osteoclast.
SCHEME 1.
Fluorescence microscopy (upper panel) and schematic (lower panel) presentation of actin ring formation and dissolution in osteoclasts. A, migration of multinucleated osteoclasts on bone surface, initial cell attachment via non αvß3 integrins (green circles). Osteoclast recognition of resorption site is leading to initial cathepsin K secretion. RGD attachment sites residing within collagen structure are not initially available (pink triangles). B, limited proteolytic matrix degradation mediated by cathepsin K and potentially MMPs results in the exposure of cryptic RGD motifs (pink circles) in the bone matrix (from collagen and other matrix proteins). Osteoclasts bind to newly exposed RGD sequences in the matrix via αvβ3 integrins (yellow circles) resulting in the formation of the sealing zone and actin ring (red bar). C, formation of the sealing zone initiates the demineralization and resorption phases. Abundant secretion of active cathepsin K into the resorption lacuna leads to extensively degraded collagen matrix. Degradation of collagen by cathepsin K liberates large amounts of soluble small RGD-containing digestion products (free pink circles), which are subsequently removed with other digestion products by the osteoclast through transcytosis. D, RGD peptides from the degradation of collagen are released from the osteoclast into the extracellular space where they saturate αvβ3 integrins in an autocrine or paracrine pathway leading to the disintegration/disappearance of the actin ring (dotted red line)/sealing zones and subsequent termination of bone resorption by the osteoclast.
In summary, this study elaborates on a novel role of catK in bone resorption demonstrating its ability to regulate the initiation and termination of the resorption process in an autocrine manner. In addition, the study lends weight to the investigation of cryptic signaling motifs in ECM molecules potentially released by catK activity.
Acknowledgments
We thank Andre Wong for technical assistance with confocal microscopy.
This work was supported, in whole or in part, by National Institutes of Health Grants AR48669 and DK072070. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: MMP, matrix metalloprotease; catK, cathepsin K; catL, cathepsin L; TRAP, tartrate-resistant acid phosphatase; AR, actin ring; PBS, phosphate-buffered saline; wt, wild type.
References
- 1.Goto, T., Yamaza, T., and Tanaka, T. (2003) J. Electron. Microsc. (Tokyo) 52 551-558 [DOI] [PubMed] [Google Scholar]
- 2.Delaisse, J. M., Engsig, M. T., Everts, V., del Carmen Ovejero, M., Ferreras, M., Lund, L., Vu, T. H., Werb, Z., Winding, B., Lochter, A., Karsdal, M. A., Troen, T., Kirkegaard, T., Lenhard, T., Heegaard, A. M., Neff, L., Baron, R., and Foged, N. T. (2000) Clin. Chim. Acta 291 223-234 [DOI] [PubMed] [Google Scholar]
- 3.Delaisse, J. M., Andersen, T. L., Engsig, M. T., Henriksen, K., Troen, T., and Blavier, L. (2003) Microsc. Res. Tech. 61 504-513 [DOI] [PubMed] [Google Scholar]
- 4.Inaoka, T., Bilbe, G., Ishibashi, O., Tezuka, K. I., Kumegawa, M., and Kokubo, T. (1995) Biochem. Biophys. Res. Commun. 206 89-96 [DOI] [PubMed] [Google Scholar]
- 5.Brömme, D., Okamoto, K., Wang, B. B., and Biroc, S. (1996) J. Biol. Chem. 271 2126-2132 [DOI] [PubMed] [Google Scholar]
- 6.Inui, T., Ishibashi, O., Inaoka, T., Origane, Y., Kumegawa, M., Kokubo, T., and Yamamura, T. (1997) J. Biol. Chem. 272 8109-8112 [DOI] [PubMed] [Google Scholar]
- 7.Saftig, P., Wehmeyer, O., Hunziker, E., Jones, S., Boyde, A., Rommerskirch, W., and von Figura, K. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 13453-13458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchisio, P. C., Cirillo, D., Naldini, L., Primavera, M. V., Teti, A., and Zambonin-Zallone, A. (1984) J. Cell Biol. 99 1696-1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfaff, M., and Jurdic, P. (2001) J. Cell Sci. 114 2775-2786 [DOI] [PubMed] [Google Scholar]
- 10.Lakkakorpi, P. T., and Vaananen, H. K. (1991) J. Bone Miner. Res. 8 817-826 [DOI] [PubMed] [Google Scholar]
- 11.Lakkakorpi, P. T., and Vaananen, H. K. (1996) Microsc. Res. Tech. 33 171-181 [DOI] [PubMed] [Google Scholar]
- 12.Jurdic, P., Saltel, F., Chabadel, A., and Destaing, O. (2006) Eur. J. Cell Biol. 85 195-202 [DOI] [PubMed] [Google Scholar]
- 13.Saltel, F., Destaing, O., Bard, F., Eichert, D., and Jurdic, P. (2004) Mol. Biol. Cell 15 5231-5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi, N., Ejiri, S., Yanagisawa, S., and Ozawa, H. (2007) Odontology 95 1-9 [DOI] [PubMed] [Google Scholar]
- 15.Davies, J., Warwick, J., Totty, N., Philp, R., Helfrich, M., and Horton, M. (1989) J. Cell Biol. 109 1817-1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nesbitt, S., Nesbit, A., Helfrich, M., and Horton, M. (1993) J. Biol. Chem. 268 16737-16745 [PubMed] [Google Scholar]
- 17.Helfrich, M. H., Nesbitt, S. A., Lakkakorpi, P. T., Barnes, M. J., Bodary, S. C., Shankar, G., Mason, W. T., Mendrick, D. L., Vaananen, H. K., and Horton, M. A. (1996) Bone 19 317-328 [DOI] [PubMed] [Google Scholar]
- 18.Flores, M. E., Norgard, M., Heinegard, D., Reinholt, F. P., and Andersson, G. (1992) Exp. Cell Res. 201 526-530 [DOI] [PubMed] [Google Scholar]
- 19.Flores, M. E., Heinegard, D., Reinholt, F. P., and Andersson, G. (1996) Exp. Cell Res. 227 40-46 [DOI] [PubMed] [Google Scholar]
- 20.Hynes, R. O., George, E. L., Georges, E. N., Guan, J. L., Rayburn, H., and Yang, J. T. (1992) Cold Spring Harb. Symp. Quant. Biol. 57 249-258 [DOI] [PubMed] [Google Scholar]
- 21.Chellaiah, M. A. (2006) Eur. J. Cell Biol. 85 311-317 [DOI] [PubMed] [Google Scholar]
- 22.van der Pluijm, G., Mouthaan, H., Baas, C., de Groot, H., Papapoulos, S., and Lowik, C. (1994) J. Bone Miner. Res. 9 1021-1028 [DOI] [PubMed] [Google Scholar]
- 23.Horton, M. A., Taylor, M. L., Arnett, T. R., and Helfrich, M. H. (1991) Exp. Cell Res. 195 368-375 [DOI] [PubMed] [Google Scholar]
- 24.King, K. L., D'Anza, J. J., Bodary, S., Pitti, R., Siegel, M., Lazarus, R. A., Dennis, M. S., Hammonds, R. G., Jr., and Kukreja, S. C. (1994) J. Bone Miner. Res. 9 381-387 [DOI] [PubMed] [Google Scholar]
- 25.Roth, W., Deussing, J., Botchkarev, V. A., Pauly-Evers, M., Saftig, P., Hafner, A., Schmidt, P., Schmahl, W., Scherer, J., Anton-Lamprecht, I., Von Figura, K., Paus, R., and Peters, C. (2000) Faseb. J. 14 2075-2086 [DOI] [PubMed] [Google Scholar]
- 26.Hoebertz, A., and Arnett, T. R. (2003) in Bone Research Protocols (Helfrich, M. H., and Ralston, S. H., eds) Vol. 80, pp. 53-64, Humana Press, Totowa, NJ [Google Scholar]
- 27.Palmer, J. T., Rasnick, D., Klaus, J. L., and Brömme, D. (1995) J. Med. Chem. 38 3193-3196 [DOI] [PubMed] [Google Scholar]
- 28.Riese, R. J., Wolf, P., Bromme, D., Natkin, L. R., Villadangos, J. A., Ploegh, H. L., and Chapman, H. A. (1996) Immunity 4 357-366 [DOI] [PubMed] [Google Scholar]
- 29.Linnevers, C. J., McGrath, M. E., Armstrong, R., Mistry, F. R., Barnes, M., Klaus, J. L., Palmer, J. T., Katz, B. A., and Brömme, D. (1997) Protein Sci. 6 919-921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bromme, D., Nallaseth, F. S., and Turk, B. (2004) Methods 32 199-206 [DOI] [PubMed] [Google Scholar]
- 31.Graebert, K. S., Bauch, H., Neumuller, W., Brix, K., and Herzog, V. (1997) Exp. Cell Res. 231 214-225 [DOI] [PubMed] [Google Scholar]
- 32.Masarachia, P., Yamamoto, M., Leu, C. T., Rodan, G., and Duong, L. (1998) Endocrinology 139 1401-1410 [DOI] [PubMed] [Google Scholar]
- 33.Nakamura, I., Tanaka, H., Rodan, G. A., and Duong, L. T. (1998) Endocrinology 139 5182-5193 [DOI] [PubMed] [Google Scholar]
- 34.Nakamura, I., Pilkington, M. F., Lakkakorpi, P. T., Lipfert, L., Sims, S. M., Dixon, S. J., Rodan, G. A., and Duong, L. T. (1999) J. Cell Sci. 112 3985-3993 [DOI] [PubMed] [Google Scholar]
- 35.Li, Z., Yasuda, Y., Li, W., Bogyo, M., Katz, N., Gordon, R. E., Fields, G. B., and Bromme, D. (2004) J. Biol. Chem. 279 5470-5479 [DOI] [PubMed] [Google Scholar]
- 36.Li, Z., Hou, W. S., and Bromme, D. (2000) Biochemistry 39 529-536 [DOI] [PubMed] [Google Scholar]
- 37.Perona, J. J., and Craik, C. S. (1995) Protein Sci. 4 337-360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakagawa, H., Takami, M., Udagawa, N., Sawae, Y., Suda, K., Sasaki, T., Takahashi, N., Wachi, M., Nagai, K., and Woo, J. T. (2003) Bone 33 443-455 [DOI] [PubMed] [Google Scholar]
- 39.Garnero, P., Borel, O., Byrjalsen, I., Ferreras, M., Drake, F. H., McQueney, M. S., Foged, N. T., Delmas, P. D., and Delaisse, J. M. (1998) J. Biol. Chem. 273 32347-32352 [DOI] [PubMed] [Google Scholar]
- 40.Kafienah, W., Bromme, D., Buttle, D. J., Croucher, L. J., and Hollander, A. P. (1998) Biochem. J. 331 727-732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marneros, A. G., and Olsen, B. R. (2001) Matrix Biol. 20 337-345 [DOI] [PubMed] [Google Scholar]
- 42.Schenk, S., and Quaranta, V. (2003) Trends Cell Biol. 13 366-375 [DOI] [PubMed] [Google Scholar]
- 43.Albini, A., and Adelmann-Grill, B. C. (1985) Eur. J. Cell Biol. 36 104-107 [PubMed] [Google Scholar]
- 44.Malone, J. D., Richards, M., and Jeffrey, J. J. (1991) Matrix 11 289-295 [DOI] [PubMed] [Google Scholar]
- 45.Fitzsimmons, C. M., Cawston, T. E., and Barnes, M. J. (1986) Thromb. Haemost. 56 95-99 [PubMed] [Google Scholar]
- 46.Moonga, B. S., Moss, D. W., Patchell, A., and Zaidi, M. (1990) J. Physiol. 429 29-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharan, K., Siddiqui, J. A., Swarnkar, G., and Chattopadhyay, N. (2008) Indian J. Med. Res. 127 274-286 [PubMed] [Google Scholar]
- 48.Holliday, L. S., Welgus, H. G., Hanna, J., Lee, B. S., Lu, M., Jeffrey, J. J., and Gluck, S. L. (2003) Calcif. Tissue Int. 72 206-214 [DOI] [PubMed] [Google Scholar]
- 49.Sato, T., Foged, N. T., and Delaisse, J. M. (1998) J. Bone Miner. Res. 13 59-66 [DOI] [PubMed] [Google Scholar]
- 50.Laitala-Leinonen, T., Rinne, R., Saukko, P., Vaananen, H. K., and Rinne, A. (2006) Matrix Biol. 25 149-157 [DOI] [PubMed] [Google Scholar]
- 51.Kiviranta, R., Morko, J., Alatalo, S. L., NicAmhlaoibh, R., Risteli, J., Laitala-Leinonen, T., and Vuorio, E. (2005) Bone 36 159-172 [DOI] [PubMed] [Google Scholar]
- 52.Gowen, M., Lazner, F., Dodds, R., Kapadia, R., Feild, J., Tavaria, M., Bertoncello, I., Drake, F., Zavarselk, S., Tellis, I., Hertzog, P. H., Debouck, C., and Kola, I. (1999) J. Bone Miner Res. 14 1654-1663 [DOI] [PubMed] [Google Scholar]
- 53.Everts, V., Korper, W., Hoeben, K. A., Jansen, I. D., Bromme, D., Cleutjens, K. B., Heeneman, S., Peters, C., Reinheckel, T., Saftig, P., and Beertsen, W. (2006) J. Bone Miner Res. 21 1399-1408 [DOI] [PubMed] [Google Scholar]
- 54.Everts, V., Hou, W. S., Rialland, X., Tigchelaar, W., Saftig, P., Bromme, D., Gelb, B. D., and Beertsen, W. (2003) Calcif. Tissue Int. 73 380-386 [DOI] [PubMed] [Google Scholar]
- 55.Xia, L., Kilb, J., Wex, H., Lipyansky, A., Breuil, V., Stein, L., Palmer, J. T., Dempster, D. W., and Brömme, D. (1999) Biol. Chem. 380 679-687 [DOI] [PubMed] [Google Scholar]