Abstract
Many G protein-coupled receptors (GPCRs) recycle after agonist-induced endocytosis by a sequence-dependent mechanism, which is distinct from default membrane flow and remains poorly understood. Efficient recycling of the β2-adrenergic receptor (β2AR) requires a C-terminal PDZ (PSD-95/Discs Large/ZO-1) protein-binding determinant (PDZbd), an intact actin cytoskeleton, and is regulated by the endosomal protein Hrs (hepatocyte growth factor-regulated substrate). The PDZbd is thought to link receptors to actin through a series of protein interaction modules present in NHERF/EBP50 (Na+/H+ exchanger 3 regulatory factor/ezrin-binding phosphoprotein of 50 kDa) family and ERM (ezrin/radixin/moesin) family proteins. It is not known, however, if such actin connectivity is sufficient to recapitulate the natural features of sequence-dependent recycling. We addressed this question using a receptor fusion approach based on the sufficiency of the PDZbd to promote recycling when fused to a distinct GPCR, the δ-opioid receptor, which normally recycles inefficiently in HEK293 cells. Modular domains mediating actin connectivity promoted receptor recycling with similarly high efficiency as the PDZbd itself, and recycling promoted by all of the domains was actin-dependent. Regulation of receptor recycling by Hrs, however, was conferred only by the PDZbd and not by downstream interaction modules. These results suggest that actin connectivity is sufficient to mimic the core recycling activity of a GPCR-linked PDZbd but not its cellular regulation.
G protein-coupled receptors (GPCRs)2 comprise the largest family of transmembrane signaling receptors expressed in animals and transduce a wide variety of physiological and pharmacological information. While these receptors share a common 7-transmembrane-spanning topology, structural differences between individual GPCR family members confer diverse functional and regulatory properties (1-4). A fundamental mechanism of GPCR regulation involves agonist-induced endocytosis of receptors via clathrin-coated pits (4). Regulated endocytosis can have multiple functional consequences, which are determined in part by the specificity with which internalized receptors traffic via divergent downstream membrane pathways (5-7).
Trafficking of internalized GPCRs to lysosomes, a major pathway traversed by the δ-opioid receptor (δOR), contributes to proteolytic down-regulation of receptor number and produces a prolonged attenuation of subsequent cellular responsiveness to agonist (8, 9). Trafficking of internalized GPCRs via a rapid recycling pathway, a major route traversed by the β2-adrenergic receptor (β2AR), restores the complement of functional receptors present on the cell surface and promotes rapid recovery of cellular signaling responsiveness (6, 10, 11). When co-expressed in the same cells, the δOR and β2AR are efficiently sorted between these divergent downstream membrane pathways, highlighting the occurrence of specific molecular sorting of GPCRs after endocytosis (12).
Recycling of various integral membrane proteins can occur by default, essentially by bulk membrane flow in the absence of lysosomal sorting determinants (13). There is increasing evidence that various GPCRs, such as the β2AR, require distinct cytoplasmic determinants to recycle efficiently (14). In addition to requiring a cytoplasmic sorting determinant, sequence-dependent recycling of the β2AR differs from default recycling in its dependence on an intact actin cytoskeleton and its regulation by the conserved endosomal sorting protein Hrs (hepatocyte growth factor receptor substrate) (11, 14). Compared with the present knowledge regarding protein complexes that mediate sorting of GPCRs to lysosomes (15, 16), however, relatively little is known about the biochemical basis of sequence-directed recycling or its regulation.
The β2AR-derived recycling sequence conforms to a canonical PDZ (PSD-95/Discs Large/ZO-1) protein-binding determinant (henceforth called PDZbd), and PDZ-mediated protein association(s) with this sequence appear to be primarily responsible for its endocytic sorting activity (17-20). Fusion of this sequence to the cytoplasmic tail of the δOR effectively re-routes endocytic trafficking of engineered receptors from lysosomal to recycling pathways, establishing the sufficiency of the PDZbd to function as a transplantable sorting determinant (18). The β2AR-derived PDZbd binds with relatively high specificity to the NHERF/EBP50 family of PDZ proteins (21, 22). A well-established biochemical function of NHERF/EBP50 family proteins is to associate integral membrane proteins with actin-associated cytoskeletal elements. This is achieved through a series of protein-interaction modules linking NHERF/EBP50 family proteins to ERM (ezrin-radixin-moesin) family proteins and, in turn, to actin filaments (23-26). Such indirect actin connectivity is known to mediate other effects on plasma membrane organization and function (23), however, and NHERF/EBP50 family proteins can bind to additional proteins potentially important for endocytic trafficking of receptors (23, 25). Thus it remains unclear if actin connectivity is itself sufficient to promote sequence-directed recycling of GPCRs and, if so, if such connectivity recapitulates the normal cellular regulation of sequence-dependent recycling. In the present study, we took advantage of the modular nature of protein connectivity proposed to mediate β2AR recycling (24, 26), and extended the opioid receptor fusion strategy used successfully for identifying diverse recycling sequences in GPCRs (27-29), to address these fundamental questions.
Here we show that the recycling activity of the β2AR-derived PDZbd can be effectively bypassed by linking receptors to ERM family proteins in the absence of the PDZbd itself. Further, we establish that the protein connectivity network can be further simplified by fusing receptors to an interaction module that binds directly to actin filaments. We found that bypassing the PDZ-mediated interaction using either domain is sufficient to mimic the ability of the PDZbd to promote efficient, actin-dependent recycling of receptors. Hrs-dependent regulation, however, which is characteristic of sequence-dependent recycling of wild-type receptors, was recapitulated only by the fused PDZbd and not by the proposed downstream interaction modules. These results support a relatively simple architecture of protein connectivity that is sufficient to mimic the core recycling activity of the β2AR-derived PDZbd, but not its characteristic cellular regulation. Given that an increasing number of GPCRs have been shown to bind PDZ proteins that typically link directly or indirectly to cytoskeletal elements (17, 27, 30-32), the present results also suggest that actin connectivity may represent a common biochemical principle underlying sequence-dependent recycling of various GPCRs.
EXPERIMENTAL PROCEDURES
DNA Constructs—All receptor constructs studied were created from a FLAG-tagged version of the murine δOR cloned into pcDNA3.0 (Invitrogen), described previously (12). A C-terminal fusion of the distal 10 residues derived from the β2AR tail, which contains the PDZbd, was also described previously (18) and is called δOR-PDZbd in the present study. The ERM protein-binding domain (Ebd) was isolated from EBP50 (also called human NHERF1, accession no. O14745, generously provided by A. Bretscher at Cornell University) by PCR-mediated amplification of the sequence encoding the C-terminal 39 residues. The actin-binding domain (Abd) was generated by chemical synthesis of a sequence encoding the 34-residue modular domain defined in a C-terminal portion human ezrin (26). In both cases, appropriate stop codon and linker sequences were added to facilitate ligation to the Srf1/Xba1 sites present in the sequence encoding the distal C-terminal cytoplasmic domain of the FLAG-δOR construct. The δOR-Abdt[6] construct was generated by adding a stop codon at the -6 position in the δOR-ABD construct, using oligonucleotide site-directed mutagenesis (QuikChange, Stratagene), a mutation that disrupts actin binding to the fused Abd (26). FLAG-δOR-GFP was constructed using PCR amplification of the FLAG-δOR coding sequence together with AgeI/HindIII appendages, followed by ligation in-frame into pEGFP-N1 (Clontech). The GFP-Hrs construct was created using PCR amplification of a Myc-tagged construct described previously (34) and generously provided by H. Stenmark (Norwegian Radium Hospital). EcoRI/XbaI appendages were added to facilitate ligation into pEGFP-C2 (Clontech), effectively replacing the N-terminal Myc tag with EGFP. All constructs were verified by dideoxynucleotide sequencing (Elim Biopharmaceuticals, Inc.).
Cell Culture and Transfections—Human embryonic kidney 293 cells (ATCC) were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (University of California, San Francisco Cell Culture Facility). Cells plated in 6-well plates were transfected at ∼50% confluency using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Stably transfected cells were selected in 500 μg/ml Geneticin (Invitrogen), and cell clones expressing FLAG-tagged receptor constructs were chosen at closely similar levels based on average surface immunofluorescence/cell measured through flow cytometry and found to have at least 75% of cells expressing surface immunoreactivity (the minor proportion of cells lacking substantial immunoreactivity was excluded in subsequent analyses). Receptor expression was quantified by whole cell radioligand binding using [3H]diprenorphine (Amersham Biosciences) as described previously (18). Cell clones selected for further study expressed receptors in the range of 0.2-1 pmol/mg cell protein. For immunofluorescence studies of receptor trafficking in transiently transfected cells, cells were transfected as above, plated onto coverslips in a 12-well plate 24 h post-transfection, and experiments were conducted 48-72 h post-transfection. In experiments requiring coexpression of GFP-Hrs, cell clones expressing the indicated receptor construct were transiently transfected at ∼50% confluency, as above, except using the GFP-Hrs construct. Cells were split into 12-well plates 24 h after transfection, and used for the preparation of flow cytometry samples 24 h thereafter.
Fluorescence Microscopy—FLAG-tagged receptors present in the plasma membrane of living cells were labeled with anti-FLAG monoclonal antibody (M1, Sigma) as described previously (18). Endocytosis of labeled receptors was promoted by adding 10 μm [d-Ala2, d-Leu5]Enkephalin (DADLE, Research Biochemicals) to the culture medium and incubating cells at 37 °C for 25 min. Recycling was assessed by carrying out a medium change after DADLE incubation, and incubating cells at 37 °C for an additional 45 min in the presence of 10 μm of the opioid antagonist naloxone (Research Biochemicals, used to block residual agonist activity in the culture medium). Following the indicated incubations, specimens were fixed (4% formaldehyde in PBS) and permeabilized (0.1% Triton X-100 in PBS), then antibody-labeled receptors were visualized by incubation with goat anti-mouse IgG conjugates (Invitrogen) linked to AlexaFluor594 (wide-field microscopy), AlexaFluor555 (scanning confocal microscopy), or AlexaFluor488 (transferrin colocalization). For visualizing internalized transferrin receptors, 10 μg/ml Texas Red-labeled diferric transferrin (Invitrogen) was added to serum-free culture medium together with DADLE. Wide field fluorescence images were collected using a Nikon Diaphot epifluorescence microscope with mercury arc lamp illumination and 60×/NA1.4 objective. Images were captured using a cooled CCD camera (Princeton Instruments) interfaced to a PC running MetaMorph acquisition and analysis software (Molecular Devices). Confocal images were acquired using a Zeiss LSM510 laser scanning microscope with 63×/NA1.3 objective, using instrument settings verified to produce negligible bleedthrough between channels, and an estimated section thickness of 1 μm. Micrographs shown are representative optical sections imaged through the center of the cell.
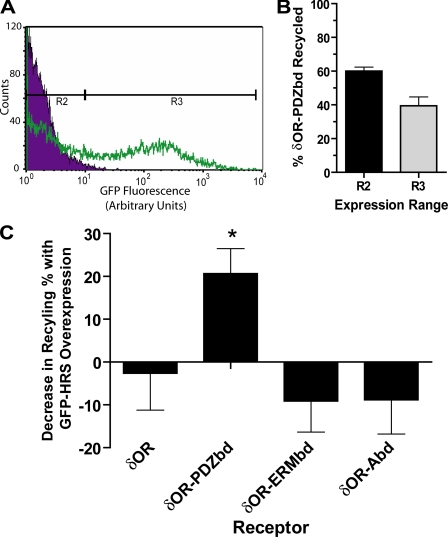
Fluorescence Flow Cytometry—FLAG-tagged receptors present in the plasma membrane were labeled as described previously (18), except that M1 anti-FLAG antibody was conjugated with AlexaFluor647 (Invitrogen) rather than AlexaFluor488 to reduce background autofluorescence in the subsequent analysis. The mean fluorescence of 5,000-20,000 cells/sample was determined using a FACSCalibur instrument and CellQuest Software (BD Biosciences). The mean fluorescence calculated from triplicate samples was normalized to reflect the relative change in surface receptor pools, and these levels were averaged from at least three experiments per cell clone analyzed. The percentage of receptor recycling occurring after agonist washout was then calculated from mean surface receptor fluorescence values (F) as follows: % recycling = (Fwashout - Fagonist-treated)/(Funtreated - Fagonist-treated) × 100. For analysis of recycling under conditions of cytoskeletal disruption, cytochalasin D (Sigma) was administered in culture medium at a 1:500 dilution from a 1 mg/ml stock in DMSO, which was added 20 min prior to incubation with the indicated opioid ligands. Vehicle control was accomplished by adding 1:500 DMSO alone. To evaluate the Hrs-sensitivity of GPCR recycling, 10,000-20,000 cells expressing the indicated receptor construct together with GFP-Hrs (generated as described above) were analyzed. Labeled receptors (AlexaFluor647) and Hrs (EGFP) were quantified simultaneously using separate laser excitations and detection channels in which negligible bleedthrough was verified using singly labeled specimens. This strategy produced a population of cells with uniform levels of receptor expression (based on stable transfection) and variable levels of Hrs overexpression (based on transient transfection). In the analysis, this range of GFP-Hrs expression was divided into two cell populations based on EGFP fluorescence intensity (regions R2 and R3, see Fig. 6A). These two internally controlled groups were then analyzed for AlexaFluor647-labeled receptor fluorescence in triplicates, averaged within each group and drug treatment, and normalized to calculate independent recycling percentages as described above. The average percentage recycling calculated from region R3 was subtracted from that calculated from region R2 and averaged to assess dose-dependent inhibition of recycling by GFP-Hrs expression. Data shown represent mean determinations from 4-5 independent experiments per receptor-expressing cell clone. Graphing and statistical analysis was carried out using Prism (GraphPad, Inc.) software. Error bars represent the standard error of the mean determinations across the experiments.
FIGURE 6.
Hrs overexpression quantitatively inhibits recycling mediated by the PDZbd but not by the ERMbd or Abd. A, dual label fluorescence flow cytometry was used to gate HEK293 cells stably transfected with the indicated FLAG-tagged receptor constructs and then transiently transfected with GFP-Hrs into two populations (regions R2 and R3) differing in GFP fluorescence (indicating relative expression level of GFP-Hrs). Region R2 was defined according to the distribution of mock-transfected cells (purple distribution in the histogram shown), while region R3 was selected to exclude most of these cells and to include cells expressing GFP-Hrs over an ∼100-fold range (green distribution). Data shown for calibration were derived from analysis of 15,000 mock-transfected and 15,000 GFP-Hrs-transfected cells. B, recycling of δOR-PDZbd measured in parallel in the indicated cell populations, confirming diminished surface recovery in the population overexpressing GFP-Hrs. Data represent results from four independent experiments, in which each recycling determination was made in triplicate samples containing 10-20,000 cells each. C, Hrs dose-dependent regulation of recycling was determined by calculating the difference between surface recovery of the indicated receptor constructs measured in parallel in cells expressing relatively low (region R2) and high (region R3) levels of GFP-Hrs. Results are compiled from at least four independent experiments per receptor construct. Error bars represent the standard error of the mean difference in surface receptor recovery across experiments. * denotes p < 0.05 in a paired t test of recycling means between regions R2 and R3 for the indicated receptor.
Cosedimentation of Receptors with Purified F-Actin—Cytoplasmic actin purified from Acanthamoeba castellani, as previously described (35), was generously provided by R. D. Mullins and group (UCSF). Monomeric actin was maintained at 4 °C at a concentration of 50 μm in 0.5 mm TCEP (0.1 mm CaCl2, 0.2 mm ATP, 2 mm Tris pH 8.0) before dilution to 4 μm into KMEH buffer (10 mm HEPES, 50 mm KCl, 1 mm EGTA, and 1 mm MgCl2 pH 7.0) supplemented with 0.04 mg/ml bovine serum albumin. Incubation in this buffer at 25 °C for 1 h allowed polymerization into filaments, which were subsequently stabilized by addition of 4 μg/ml phalloidin (Sigma). Extracts from HEK293 cells transiently transfected with the indicated FLAG-tagged receptor construct (or mock-transfected without added plasmid DNA) were prepared in KMEH supplemented with 1% Triton X-100, 20 mm dithiothreitol, and protease inhibitors (Complete EDTA-free mixture, Roche Applied Science). Extracts were clarified by microcentrifugation for 10 min at 20,000 × g followed by ultracentrifugation in a TLA 100 rotor (BD Biosciences) at 48,000 rpm for an additional 30 min. Total protein concentration in the supernatant was determined by a Bradford assay and adjusted as needed with KMEH lysis buffer to achieve equal concentrations (2-5 μg/ml depending on the individual experiment) of total protein. Extracts (50 μl) were then mixed with 50 μl of F-actin mixture and left on ice for 10 min before sedimenting in a TLA 100 rotor for 30 min at 48K rpm. 20 μl of supernatant was subsequently removed per sample for immunoblot analysis, and the pellet was cleared of remaining supernatant and washed in 100 μl of KMEH lysis buffer before solubilizing by boiling for 5 min in 30 μl of NuPAGE (Invitrogen) LDS sample buffer (500 mm Tris base, 8% lithium dodecyl sulfate, 40% glycerol, 2 mm EDTA) containing 0.25 m 2-mercaptoethanol, separated on 4-12% NuPAGE LDS gels (Invitrogen), transferred to nitrocellulose (Bio-Rad), and blotted for FLAG-tagged receptors using 2.5 μg/ml M1 anti-FLAG antibody (Sigma) followed by secondary antibody incubation using sheep anti-mouse-horseradish peroxidase conjugate (1:3000 dilution, Amersham Biosciences). Detection was carried out using enzyme-linked chemiluminescence (SuperSignal, Pierce) and immunoreactive signals were analyzed using a FluorChem 8000 imaging system (Alpha Innotech Corporation).
Statistical Analysis—Internalization and recycling percentages were calculated for each individual experiment and collected as replicates in a Prism spreadsheet for statistical and graphing analysis (Graphpad, Inc.). Mean percentages were compared between receptors by ANOVA and analyzed post-hoc for pair-wise differences using the Bonferroni Multiple Comparisons test with a significance level of 0.05. Mean recycling was compared between DMSO and cytochalasin D-treated cells, per receptor expressed, by Student's t test at a significance level of 0.05, whereas comparison of recycling means between cells expressing low and high amounts of GFP-Hrs in the same sample were compared for each receptor using a paired t test with a significance level of 0.05.
RESULTS
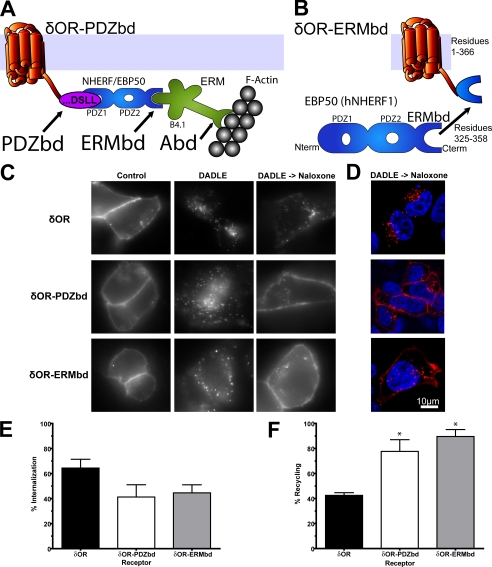
The ERM Protein Binding Domain Conserved in NHERF/EBP50 Family Proteins Is Sufficient to Promote Efficient Recycling When Fused to the δOR—The β2AR-derived PDZbd is sufficient, when fused to the C-terminal cytoplasmic domain of the δOR (δOR-PDZbd mutant receptor), to re-route endocytic trafficking of this distinct GPCR from its usual lysosomal fate to the rapid recycling pathway (18). To test the hypothesis that downstream protein connectivity to actin is sufficient to mediate this recycling activity, we applied the same receptor fusion approach to modular domains proposed to function downstream of the PDZbd (Fig. 1A). We first asked if it is possible to effectively bypass the PDZbd using only a conserved ERMbd. To do so, we fused the C-terminal 39-residues derived from EBP50 (human NHERF1), which fully includes the previously mapped ERMbd (24), to the δOR tail (δOR-ERMbd receptor fusion protein, Fig. 1B).
FIGURE 1.
The conserved ERMbd derived from NHERF/EBP50 proteins is sufficient to promote endocytic recycling of theδOR when fused to the C terminus. A, schematic of the δOR-PDZbd fusion receptor containing the PDZ domain-interacting sequence and its proposed actin connectivity via the ERMbd present in PDZ-linked NHERF/EBP50 proteins and the Abd present in ERM proteins. B, schematic of the δOR-ERMbd fusion receptor. C, FLAG-tagged δOR, δOR-PDZbd, and δOR-ERMbd constructs were transiently expressed in HEK293 cells and surface-labeled with anti-FLAG monoclonal antibody. Cells were incubated in the absence of agonist (control), in the presence of 10 μm DADLE for 25 min (DADLE), or with 10 μm DADLE followed by washout and subsequent incubation for 45 min in the presence of 10 μm naloxone (DADLE->Naloxone). Cells were fixed and stained under permeabilized conditions to track the endocytic fate of surface-labeled receptors. D, representative confocal sections of receptor localization in cellular cross-sections following agonist washout. All images shown are representative of at least four independent experiments. E, agonist-induced internalization of FLAG-tagged receptors was quantified by flow cytometric assay of stably transfected HEK293 cells by calculating the decrease in surface receptor immunoreactivity produced by incubation of cells in the presence of 10 μm DADLE for 25 min, as described under “Experimental Procedures.” F, recycling of FLAG-tagged receptors was quantified using the flow cytometric assay to determine the subsequent recovery of surface receptor immunoreactivity 45 min after agonist washout. Error bars reflect the standard error of the mean of at least three independent experiments. * denotes p < 0.05 in Bonferroni post-hoc analysis relative to the δOR, additional analysis of the δOR-PDZbd relative to the δOR-ERMbd yielded p > 0.05.
We compared the trafficking behavior of the δOR-ERMbd to that of FLAG-tagged versions of the wild-type δOR and δOR-PDZbd constructs described previously (18). In the absence of agonist (control), tagged receptors labeled with anti-FLAG monoclonal antibody were observed in a peripheral pattern indicative of plasma membrane localization, demonstrating that all three tagged receptors were effectively delivered to the plasma membrane (Fig. 1C, left column). Within 25 min after adding the opioid agonist DADLE to the culture medium, labeled receptors redistributed to a punctate intracellular pattern, indicating that each of the constructs was able to undergo rapid agonist-induced endocytosis (Fig. 1C, middle column). 45 min after agonist removal from the culture medium (Fig. 1C, right column), wild-type δOR remained predominantly in intracellular vesicles (top row), consistent with its failure to recycle efficiently. The δOR-PDZbd fusion receptor, as expected, returned to a predominantly plasma membrane localization pattern (middle row). Significantly, the δOR-ERMbd fusion receptor also returned to a predominantly plasma membrane localization pattern after agonist washout (bottom row), which was indistinguishable from efficient recycling of the δOR-PDZbd fusion receptor and visibly different from the endosomal retention observed for the wild-type δOR. This selective return of both the δOR-PDZbd and δOR-ERMbd fusion receptors to the plasma membrane was emphasized in confocal optical sections imaged through the middle of the cell (Fig. 1D). Together, these observations suggest that the ERMbd is indeed sufficient to promote recycling of receptors in the absence of the PDZbd itself.
To quantify the trafficking properties of engineered receptors, a previously established flow cytometric assay was applied that allows evaluation of receptor trafficking in a large population of cells and in the absence of bound antibody (12). Substantial agonist-induced internalization of all receptor constructs tested was confirmed by the pronounced reduction in surface receptor immunoreactivity observed following incubation of cells in the presence of 10 μm DADLE for 25 min (Fig. 1E) (36). Assay of surface receptor recovery after agonist washout established clearly that fusion of the ERMbd promoted recycling of receptors, as indicated by a nearly complete recovery of surface receptors that was indistinguishable in magnitude from that produced by fusion of the PDZbd itself (Fig. 1F). The statistical significance of ERMbd-promoted recycling, and its quantitative similarity to that promoted by the PDZbd, was confirmed by statistical analysis of flow cytometric data across multiple experiments (legend to Fig. 1).
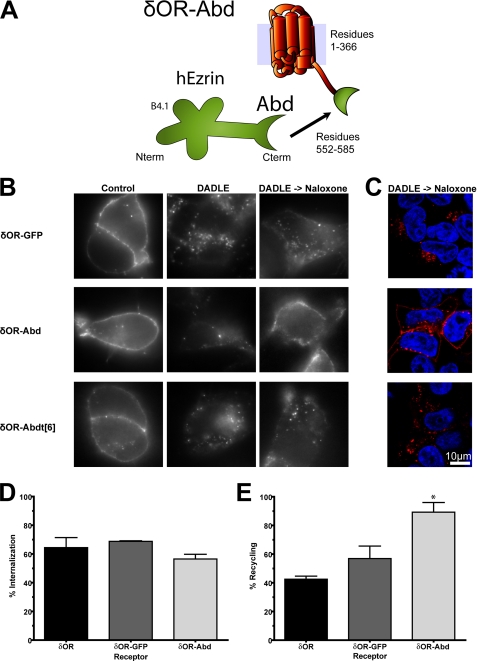
Direct Association of Receptors with the Actin Cytoskeleton Is Sufficient to Promote Efficient Recycling—Having established that the isolated ERMbd promotes recycling of engineered receptors, we next tested the sufficiency of direct receptor connectivity to actin. A conserved F-actin-binding domain (Abd) has been mapped to the carboxyl-terminal 34 residues of human ezrin, and shown to confer specific actin binding in vitro when fused to the C terminus of glutathione S-transferase (26). Accordingly, we fused this sequence to the C terminus of the δOR (δOR-Abd fusion receptor, Fig. 2A) and assessed effects on endocytic trafficking. Visualization of antibody-labeled δOR-Abd fusion receptors by fluorescence microscopy (Fig. 2B, middle row of images) revealed surface targeting and agonist-induced internalization similar to that of wild-type receptors (left and middle panels). Remarkably, the δOR-Abd returned almost completely to the plasma membrane after agonist washout, suggesting that the isolated Abd indeed promotes receptor recycling similar to that mediated by both the PDZbd and ERMbd fusions. This was evident both in wide field micrographs (Fig. 2B, middle, right image) and in confocal optical sections (Fig. 2C, middle row), and stood in marked contrast to the limited recycling observed for the wild-type δOR (Fig. 1C). Two additional observations confirmed the specificity of recycling directed by the Abd. First, fusing a larger protein domain (full-length EGFP) to the δOR tail (δOR-GFP fusion receptor) did not promote detectable recycling (Fig. 2, B and C, top row of images). Second, deleting 6 residues from the extreme C terminus of the Abd (δOR-Abdt[6] mutant receptor), which was shown previously to disrupt binding to actin filaments (26), abrogated the recycling activity of the Abd (Fig. 2, B and C, bottom row).
FIGURE 2.
The conserved Abd derived from ERM proteins is sufficient to promote endocytic recycling. A, schematic of the δOR-Abd fusion receptor. B, FLAG-tagged δOR-GFP, δOR-Abd, and δOR-Abdt[6] constructs were transiently expressed in HEK293 cells and surface-labeled with anti-FLAG monoclonal antibody. Cells were incubated and processed under the same conditions as described in the legend to Fig. 1. C, confocal optical sections showing receptor localization following agonist washout. Images represent mid-focal planes and are representative of at least four independent experiments. Flow cytometric measurement of agonist-induced internalization (D) and recycling after agonist removal (E) were determined using the same procedure as described in Fig. 1 (data for the δOR are re-displayed for comparison). Error bars reflect the standard error of the mean of at least three independent experiments. * denotes p < 0.05 in Bonferroni post-hoc analysis relative to the δOR.
These observations were quantified in stably transfected cells using the fluorescence flow cytometric assays of agonist-induced internalization (Fig. 2D) and recycling after agonist removal (Fig. 2E). Fusion of the Abd produced a pronounced increase in receptor recycling, which was similar in magnitude to that produced by the PDZbd or Ebd, and statistically significant when compared with the wild-type δOR (p < 0.001, Fig. 2E). Together, these results indicate that the Abd is itself sufficient to promote efficient recycling when fused to the δOR.
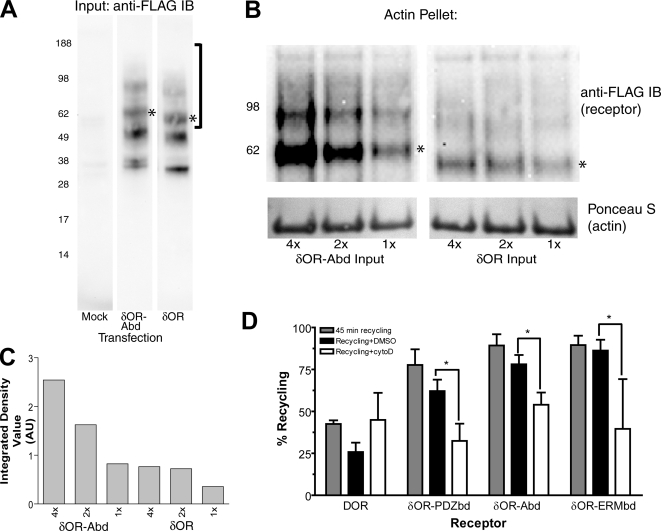
Recycling Promoted by Engineered Protein Connectivity is F-Actin-dependent—To further test the actin connectivity hypothesis, we sought to determine if the δOR-Abd fusion receptor can truly bind actin filaments directly. FLAG-tagged receptor constructs were expressed in HEK293 cells, solubilized using nonionic detergent, and binding of receptors to purified F-actin was determined using an in vitro co-sedimentation assay (see “Experimental Procedures”). Wild-type δOR and δOR-Abd constructs were expressed at similar levels in solubilized extracts, as detected specifically by anti-FLAG immunoblotting (Fig. 3A). The heterogeneous electrophoretic mobility observed for both receptors is consistent with previous studies indicating that the wild-type δOR resolves as a mixture of complex-glycosylated forms (37). After a 10-min incubation with purified F-actin on ice, actin polymers pelleted by ultracentrifugation (detected by Ponceau S staining, lower panel in Fig. 3B) co-sedimented the δOR-Abd (as detected by anti-FLAG immunoblot, upper panel in Fig. 3B). In parallel, samples loaded with identical amounts of receptor (Fig. 3A) and actin (lower panels in Fig. 3B), FLAG-δOR was detected at much lower levels in the actin pellet. The ratio of δOR-Abd compared with δOR co-sedimentation, as estimated across three independent experiments by scanning densitometry, was 3.0 ± 0.71. Titration of receptor input within individual experiments verified concentration-dependent association of δOR-Abd with F-actin (Fig. 3C).
FIGURE 3.
Verification of direct actin connectivity and actin-dependent recycling of engineered receptors. A, anti-FLAG immunoblots of equal amounts (25 μg before clarification) of cell extracts prepared from mock-transfected HEK293 cells or cells transfected with either FLAG-tagged δOR or δOR-Abd fusion receptor. Bracket to the right of the figure indicates the region of the blot shown in B, and the band indicated by * indicates the species corresponding in electrophoretic mobility to the glycosylated receptor monomer. B, anti-FLAG immunoblot showing receptor (top panel) and Ponceau S stain showing actin (bottom panel) from actin co-sedimentation assay comparing δOR-Abd (left) and δOR (right) loaded in equal amount (as shown in A) and processed in parallel. C, background-corrected densitometry of the species corresponding to monomeric receptor. Data shown are representative of three independent experiments. D, flow cytometric analysis assessing the sensitivity of engineered receptor recycling to cytochalasin D. Recycling measured by flow cytometry in normal culture medium (gray bars) is displayed in comparison to that measured in the presence of vehicle (0.2% DMSO, black bars) or 2 μg/ml cytochalasin D (cytoD, white bars). * denotes p < 0.05 by Student's t test comparing vehicle-treated and cytochalasin D-treated cells, n = 4-5 experiments.
We next asked if recycling promoted by the defined interaction domains requires an intact actin cytoskeleton in intact cells. Flow cytometric analysis indicated that the small fraction of wild-type δOR recycling observed after removal of DADLE from the culture medium was not detectably affected by depolymerization of actin filaments by cytochalasin D (Fig. 3D, left set of bars), consistent with actin-independent recycling of a small fraction of the internalized δOR by default (14). The considerably enhanced recycling of receptors mediated by fusion of the PDZ ligand (δOR-PDZbd), in contrast, was markedly inhibited by cytochalasin D. This was indicated by the statistically significant reduction of surface receptor recovery after agonist washout in cytochalasin D-treated cells compared with the vehicle (DMSO)-treated control cells (Fig. 3D, second set of bars). Recycling promoted by both the ERMbd and Abd fusions was similarly sensitive to cytochalasin D (third and fourth set of bars). These results further confirm the importance of F-actin for PDZ-dependent recycling of GPCRs, as established previously in studies of the wild-type β2AR (17), and indicate that bypassing the PDZbd using either of the proposed downstream protein interaction domains preserves this actin-dependence in intact cells.
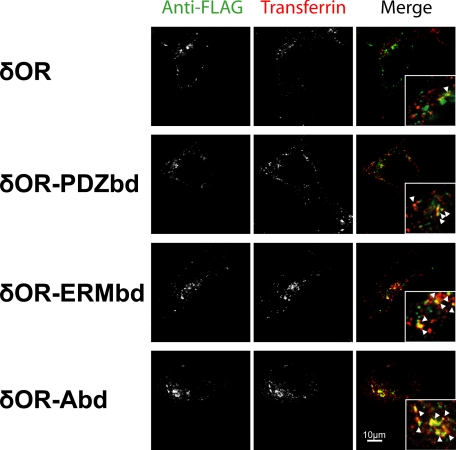
Recycling Promoted by Either Direct or Indirect Actin Connectivity Utilizes a Similar Membrane Pathway—To further investigate the degree to which downstream actin connectivity mimics the recycling effect of the PDZbd, we compared the subcellular localization of engineered receptor constructs after agonist-induced endocytosis to that of internalized transferrin, which marks the shared recycling pathway traversed by the wild-type β2AR (38). HEK293 cells were transfected with expression constructs encoding the FLAG-tagged δOR, δOR-PDZbd, δOR-ERMbd, or δOR-Abd, and surface-accessible receptors were labeled with anti-FLAG monoclonal antibody. Cells were then incubated at 37 °C in the presence of 10 μm DAMGO to drive endocytosis of the GPCR, and with 10 μg/ml Texas Red-labeled transferrin (Tf-TR) to label endocytosed transferrin receptors in the same cells. After incubation at 37 °C for 40 min, a time period sufficient to achieve steady state labeling of transferrin receptors in the conserved recycling pathway (39), cells were fixed and the localization of the engineered receptors was compared by dual channel confocal microscopy (Fig. 4). Internalized δOR (top row of images, left panel) appeared in a vesicular pattern largely distinct from that of labeled transferrin (middle panel). This was confirmed in the merged image displaying δOR and transferrin in green and red, respectively (right panel). Examination of dual receptor localization at higher magnification (inset) emphasized that internalized δOR was present in endocytic compartments largely distinct from those mediating the conserved recycling pathway marked by internalized Tf-TR, while structures containing detectable amounts of both receptors were relatively rare (an example is indicated by arrowhead in inset). Internalized δOR-PDZbd, in contrast, was visualized in a vesicular pattern more similar to that of Tf-TR (second row), with considerable overlap indicated by yellow structures observed in the merged color image (right, several examples are indicated by arrowheads in inset). Internalized δOR-ERMbd (third row) and δOR-Abd (fourth row) also exhibited substantial overlap with the shared recycling pathway. These observations indicate that fusion of either the ERMbd or Abd indeed promote trafficking of internalized receptors via a pathway similar to that promoted by the PDZbd, which overlaps significantly with the shared recycling pathway marked by transferrin receptors.
FIGURE 4.
Engineered recycling sequences promote receptor trafficking via transferrin-containing endosomes. The indicated δOR-derived constructs expressed in HEK293 cells were surface-labeled with M1 anti-FLAG monoclonal antibody, then cells were then incubated in the combined presence of 10 μm DADLE (to promote endocytosis of the labeled receptor constructs) and Texas Red-conjugated transferrin (to label endocytosed transferrin receptors marking the conserved recycling pathway). Cells were fixed and stained under permeabilized conditions with Alexa488-conjugated anti-mouse IgG and imaged by confocal fluorescence microscopy to selectively visualize internalized δOR-derived receptor constructs (green) and internalized transferrin (red) in the same cells. The corresponding merged images are shown to the right, and insets show a region of the cytoplasm at 2.5× higher magnification to help distinguish endosomes labeled selectively with one receptor (appearing red or green) or co-labeled for both receptors (appearing yellow, examples indicated by arrows). Images shown are representative of at three independent experiments for each receptor construct.
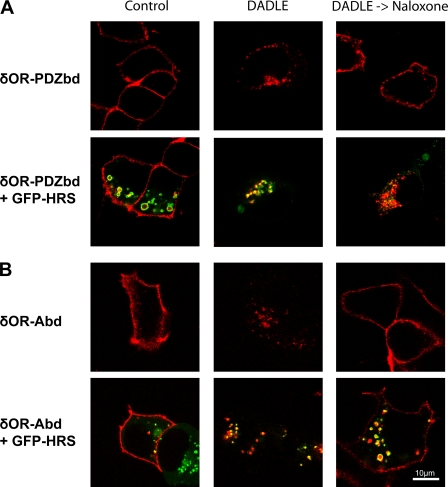
Downstream Protein Connectivity to Actin Fails to Recapitulate Hrs-dependent Regulation of Recycling—Given that both the ERMbd and Abd promoted actin-dependent recycling with similarly high efficiency as the PDZbd, and did so via a similar vesicular pathway, we continued to investigate the degree to which downstream protein interaction modules mimic PDZ-dependent recycling. Another characteristic feature of PDZ-dependent recycling of the β2AR is its regulation by Hrs (hepatocyte growth factor receptor substrate) (11), a conserved endosome-associating sorting protein (7, 40). Overexpression of an epitope-tagged Hrs construct has been shown to inhibit PDZ-dependent recycling of the wild-type β2AR, without detectably affecting default recycling of either the transferrin receptor (40) or a mutant GPCR apparently devoid of all endocytic sorting determinants (11). Thus we examined the sensitivity of the engineered receptors to overexpression of Hrs. We first approached this question using dual channel, confocal microscopy to visualize trafficking of the δOR-PDZbd fusion receptor in stably transfected cells in which an EGFP-tagged Hrs construct (GFP-Hrs) was subsequently expressed by transient transfection. This allowed direct visualization of receptor trafficking in cells overexpressing tagged Hrs at various levels, as estimated by EGFP fluorescence intensity. We also noted that GFP-Hrs overexpression produced a visible increase in endosome diameter, characteristic of the dominant-negative inhibition of endosome function described previously (11, 40). The δOR-PDZbd fusion receptor was targeted to the plasma membrane and exhibited rapid endocytosis upon addition of DADLE, irrespective of GFP-Hrs expression level (Fig. 5A, left and middle panels). Following agonist washout, however, redistribution of the δOR-PDZbd fusion protein from endosomes to the plasma membrane was visibly reduced in cells overexpressing GFP-Hrs (Fig. 5A, right column, compare top and bottom panels). These observations suggested that Hrs overexpression inhibits recycling of the δOR-PDZbd fusion receptor, as it does the wild-type β2AR. A markedly different result was obtained in parallel experiments conducted on the δOR-ERMbd and δOR-Abd fusion receptors. While overexpression of GFP-Hrs again had no detectable effect on surface targeting or agonist-induced endocytosis (representative images of δOR-Abd-expressing cells are shown in Fig. 5B, bottom row, left and middle columns), overexpression of GFP-Hrs, even at apparently high levels (as indicated by GFP fluorescence intensity and endosome enlargement), did not visibly interfere with recycling of either the δOR-ERMbd or δOR-Abd fusion receptors (δOR-Abd image Fig. 5B, bottom right panel).
FIGURE 5.
Receptors fused to distinct recycling sequences localize to Hrs-associated endosomes but differ in Hrs-dependent regulation of recycling. A, confocal optical sections of cells showing the localization of labeled δOR-PDZbd (red) and co-expressed GFP-Hrs (green) in HEK293 cells incubated in the absence of ligand (control), in the presence of 10 μm DADLE for 25 min (DADLE), or with 10 μm DADLE followed by washout and subsequent incubation for 45 min in the presence of 10 μm naloxone (DADLE->Naloxone). Top panels show a typical example of cells not expressing GFP-Hrs, and lower panels show a typical example of cells expressing GFP-Hrs at levels that produce visible endosome enlargement. B, similar experiments conducted in cells expressing the δOR-Abd. Images shown are representative of at least four independent experiments.
A pronounced difference in Hrs-sensitivity of the receptor constructs was confirmed quantitatively using a modification of the flow cytometric assay. Stably transfected cell clones expressing the indicated receptor were transiently transfected with the GFP-Hrs construct, producing a range of Hrs overexpression in cells expressing receptors uniformly (see “Experimental Procedures”). Receptor recycling was then analyzed simultaneously in two cell populations differing substantially in average fluorescence intensity of GFP-Hrs (region R2 versus R3, respectively, Fig. 6A), allowing the inhibitory effect of increased Hrs expression to be assessed in an internally controlled manner. Inhibition of δOR-PDZbd recycling was substantially greater in cells expressing GFP-Hrs at higher levels (region R3) compared with lower levels (region R2) in the same transfected samples (Fig. 6B, compare left and right bars), and recycling in both cell populations displayed a trend of inhibition relative to that observed in the parental cell clone not expressing GFP-Hrs (compare with Fig. 2B). By calculating the difference in receptor recycling observed in these internally controlled populations, dose-dependent inhibition of δOR-PDZbd recycling by Hrs overexpression was clearly confirmed. In contrast, increased expression of GFP-Hrs did not detectably inhibit recycling of the other engineered receptor constructs (Fig. 6C). Together these results indicate that, while both the ERMbd and Abd were sufficient to mimic the actin-dependent recycling activity of the PDZbd, neither of these proposed downstream interaction modules recapitulated Hrs-dependent regulation that is characteristic of PDZ-dependent recycling of both wild-type β2AR (11) and engineered (δOR-PDZbd) receptors.
DISCUSSION
The present results indicate that a modular ERMbd conserved in NHERF/EBP50-family proteins, as well as an Abd conserved in ERM proteins, is sufficient to promote recycling of an engineered GPCR with similarly high efficiency as the PDZbd derived from the β2AR. Because the recycling activity of the PDZbd can be effectively bypassed by either of these protein-interaction modules mediating the proposed downstream connectivity, and in the absence of any other known functional domains present in NHERF/EBP50 or ERM proteins, the present results suggest that a relatively simple architecture of actin connectivity is truly sufficient to promote sequence-directed recycling. Of particular interest, fusion of the Abd by itself was sufficient to promote efficient recycling of receptors, and we confirmed biochemically that the engineered receptor was indeed capable of direct binding to actin filaments. We further verified that recycling promoted by all of the interaction modules exhibited an actin-dependence that is characteristic of PDZ-dependent recycling of the wild-type β2AR (17). Actin connectivity is known to affect the mobility of various signaling receptors in the plasma membrane (41) and can influence the clathrin-dependent endocytic mechanism (42) but, to our knowledge, the present results are the first to show that direct actin connectivity is sufficient to promote recycling of GPCRs after endocytosis.
An important question for future study, therefore, is precisely how protein connectivity to actin filaments mediates this endocytic sorting function. In principle, receptor linkage to actin structures could control the movement of individual GPCR-containing endocytic vesicles directed back to the plasma membrane. Alternatively, actin linkage could influence the lateral partitioning of receptors in the endosome membrane, or bring endocytosed receptors in proximity to other proteins that subsequently dictate sorting fate (16).
While fusion of either the ERMbd or Abd promoted receptor recycling with similarly high efficiency as the PDZbd, bypassing the proximal PDZ interaction using the engineered receptor approach did not fully recapitulate the features of PDZ-dependent recycling. In particular, manipulating the cellular concentration of the endosomal sorting protein Hrs, which is known to regulate recycling of the wild-type β2AR (11), affected recycling of engineered receptors promoted by the PDZbd but not that promoted by the ERMbd or Abd. Thus, while the present results argue strongly that ERM-actin linkage is a sufficient basis for a core mechanism of sequence-dependent recycling, it is likely that additional regulation of recycling occurs physiologically at the level of the PDZ protein itself. It will be interesting in future studies to investigate the potential role of various PDZ proteins and non-ERM protein interactions with NHERF/EBP50 family members (22, 23, 25) in regulating, and perhaps conferring additional specificity on, PDZ-dependent recycling that occurs in a native cellular context (20, 43).
Considering that many PDZ proteins link directly or indirectly (via additional protein interactions) to actin (44), it is tempting to speculate that actin connectivity might play a rather general role in promoting GPCR recycling by various PDZ-linked protein complexes. We note, for example, that hScrib/ßPIX/GIT1-dependent recycling of thyrotropin receptors is regulated by the GTPase Arf6 and AKAP79/SAP97-dependent recycling of β1-adrenergic receptors involves PKA-mediated phosphorylation of the receptor (30, 31). Thus, if actin connectivity contributes to recycling via these alternative PDZ-linked protein complexes, it is likely that there exists additional cellular regulation dependent on the proximal PDZ domain-mediated interaction with the receptor that occursnaturally. Also, given that PDZ-dependent recycling of GPCRs occurs in diverse cell types, including neurons (43) and cardiac muscle cells (20), the possibility that distinct PDZ-linked complexes also confer cell type-specific regulation merits future study. It is also interesting to note that efficient recycling of the α1b-adrenergic receptor, although not directed by a PDZ-mediated protein interaction, is actin-dependent and requires an ERM protein binding domain present in the receptor (33). Thus, it seems likely that actin connectivity represents a biochemical principle of receptor recycling that is deeply conserved.
In summary, the present results define a relatively simple network of protein connectivity that is sufficient to promote the core function of sequence-dependent recycling in the absence of a natural GPCR-derived recycling sequence. While indirect protein connectivity to actin likely represents a fundamental and conserved biochemical principle of sequence-dependent recycling, the present results also establish that such connectivity is not sufficient to fully recapitulate the cellular regulation observed in the endocytic trafficking of naturally occurring GPCRs.
Acknowledgments
We thank Anthony Bretscher, Harald Stenmark, R. Dyche Mullins, Margot Quinlan, Orkun Akin, and J. Bradley Zuchero for valuable discussion and critical reagents. We thank James Hislop, Aylin Hanyaloglu, Manoj Puthenveedu, Robert Gage, Eric Lontok, and Cindy Yang for helpful comments, instruction, and technical assistance with some of the preliminary experiments.
This work was supported, in whole or in part, by research grants from the National Institutes of Health. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: GPCR, G protein-coupled receptor; PDZbd, PSD-95/Discs Large/ZO-1 protein-binding determinant; Hrs, hepatocyte growth factor-regulated substrate; GFP, green fluorescent protein; Abd, actin binding domain; AR, adrenergic receptor; OR, opioid receptor; ERMbd, ERM protein binding domain.
References
- 1.Palczewski, K. (2006) Annu. Review Biochem. 75 743-767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cherezov, V., Rosenbaum, D. M., Hanson, M. A., Rasmussen, S. G. F., Thian, F. S., Kobilka, T. S., Choi, H.-J., Kuhn, P., Weis, W. I., Kobilka, B. K., and Stevens, R. C. (2007) Science 318 1258-1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen, S. G. F., Choi, H.-J., Rosenbaum, D. M., Kobilka, T. S., Thian, F. S., Edwards, P. C., Burghammer, M., Ratnala, V. R. P., Sanishvili, R., Fischetti, R. F., Schertler, G. F. X., Weis, W. I., and Kobilka, B. K. (2007) Nature 450 383-387 [DOI] [PubMed] [Google Scholar]
- 4.Lefkowitz, R. J. (2007) Acta Physiologica 190 9-19 [DOI] [PubMed] [Google Scholar]
- 5.Sorkin, A., and Von Zastrow, M. (2002) Nat. Rev. Mol. Cell Biol. 3 600-614 [DOI] [PubMed] [Google Scholar]
- 6.Lefkowitz, R. J., Pitcher, J., Krueger, K., and Daaka, Y. (1998) Adv. Pharmacol. 42 416-420 [DOI] [PubMed] [Google Scholar]
- 7.Marchese, A., Chen, C., Kim, Y.-M., and Benovic, J. L. (2003) Trends Biochem. Sci. 28 369-376 [DOI] [PubMed] [Google Scholar]
- 8.Law, P. Y., and Loh, H. H. (1999) J. Pharmacol. Exp. Ther. 289 607-624 [PubMed] [Google Scholar]
- 9.Tsao, P., and von Zastrow, M. (2000) Curr. Opin. Neurobiol. 10 365-369 [DOI] [PubMed] [Google Scholar]
- 10.Pippig, S., Andexinger, S., and Lohse, M. J. (1995) Mol. Pharmacol. 47 666-676 [PubMed] [Google Scholar]
- 11.Hanyaloglu, A. C., McCullagh, E., and von Zastrow, M. (2005) EMBO J. 24 2265-2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsao, P. I., and von Zastrow, M. (2000) J. Biol. Chem. 275 11130-11140 [DOI] [PubMed] [Google Scholar]
- 13.Gruenberg, J., and Maxfield, F. R. (1995) Curr. Opin. Cell Biol. 7 552-563 [DOI] [PubMed] [Google Scholar]
- 14.Hanyaloglu, A. C., and von Zastrow, M. (2008) Annu. Rev. Pharmacol. Toxicol. 48 537-568 [DOI] [PubMed] [Google Scholar]
- 15.Saksena, S., Sun, J., Chu, T., and Emr, S. D. (2007) Trends Biochem. Sci. 32 561-573 [DOI] [PubMed] [Google Scholar]
- 16.Marchese, A., Paing, M. M., Temple, B. R. S., and Trejo, J. (2008) Annu. Rev. Pharmacol. Toxicol. 48 601-629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao, T. T., Deacon, H. W., Reczek, D., Bretscher, A., and von Zastrow, M. (1999) Nature 401 286-290 [DOI] [PubMed] [Google Scholar]
- 18.Gage, R. M., Kim, K. A., Cao, T. T., and von Zastrow, M. (2001) J. Biol. Chem. 276 44712-44720 [DOI] [PubMed] [Google Scholar]
- 19.Gage, R. M., Matveeva, E. A., Whiteheart, S. W., and von Zastrow, M. (2004) J. Biol. Chem. 280 3305-3313 [DOI] [PubMed] [Google Scholar]
- 20.Wang, Y., Lauffer, B., Von Zastrow, M., Kobilka, B. K., and Xiang, Y. (2007) Mol. Pharmacol. 72 429-439 [DOI] [PubMed] [Google Scholar]
- 21.Hall, R. A., Premont, R. T., Chow, C. W., Blitzer, J. T., Pitcher, J. A., Claing, A., Stoffel, R. H., Barak, L. S., Shenolikar, S., Weinman, E. J., Grinstein, S., and Lefkowitz, R. J. (1998) Nature 392 626-630 [DOI] [PubMed] [Google Scholar]
- 22.He, J., Bellini, M., Inuzuka, H., Xu, J., Xiong, Y., Yang, X., Castleberry, A. M., and Hall, R. A. (2005) J. Biol. Chem. 281 2820-2827 [DOI] [PubMed] [Google Scholar]
- 23.Bretscher, A., Chambers, D., Nguyen, R., and Reczek, D. (2000) Annu. Rev. Cell Dev. Biol. 16 113-143 [DOI] [PubMed] [Google Scholar]
- 24.Reczek, D., and Bretscher, A. (1998) J. Biol. Chem. 273 18452-18458 [DOI] [PubMed] [Google Scholar]
- 25.Reczek, D., and Bretscher, A. (2001) J. Cell Biol. 153 191-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turunen, O., Wahlstrom, T., and Vaheri, A. (1994) J. Cell Biol. 126 1445-1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galet, C., Hirakawa, T., and Ascoli, M. (2004) Mol. Endocrinol. 18 434-446 [DOI] [PubMed] [Google Scholar]
- 28.Tanowitz, M., and von Zastrow, M. (2003) J. Biol. Chem. 278 45978-45986 [DOI] [PubMed] [Google Scholar]
- 29.Vargas, G. A., and Von Zastrow, M. (2004) J. Biol. Chem. 279 37461-37469 [DOI] [PubMed] [Google Scholar]
- 30.Lahuna, O., Quellari, M., Achard, C., Nola, S., Meduri, G., Navarro, C., Vitale, N., Borg, J. P., and Misrahi, M. (2005) EMBO J. 24 1364-1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gardner, L. A., Naren, A. P., and Bahouth, S. W. (2007) J. Biol. Chem. 282 5085-5099 [DOI] [PubMed] [Google Scholar]
- 32.Paasche, J. D., Attramadal, T., Kristiansen, K., Oksvold, M. P., Johansen, H. K., Huitfeldt, H. S., Dahl, S. G., and Attramadal, H. (2005) Mol. Pharmacol. 67 1581-1590 [DOI] [PubMed] [Google Scholar]
- 33.Stanasila, L., Abuin, L., Diviani, D., and Cotecchia, S. (2006) J. Biol. Chem. 281 4354-4363 [DOI] [PubMed] [Google Scholar]
- 34.Bache, K. G., Raiborg, C., Mehlum, A., and Stenmark, H. (2003) J. Biol. Chem. 278 12513-12521 [DOI] [PubMed] [Google Scholar]
- 35.Gordon, D. J., Eisenberg, E., and Korn, E. D. (1976) J. Biol. Chem. 251 4778-4786 [PubMed] [Google Scholar]
- 36.Koenig, J. A., and Edwardson, J. M. (1997) Trends Pharm. Sci. 18 276-287 [DOI] [PubMed] [Google Scholar]
- 37.Petaja-Repo, U. E., Hogue, M., Laperriere, A., Walker, P., and Bouvier, M. (2000) J. Biol. Chem. 275 13727-13736 [DOI] [PubMed] [Google Scholar]
- 38.von Zastrow, M., and Kobilka, B. K. (1992) J. Biol. Chem. 267 3530-3538 [PubMed] [Google Scholar]
- 39.Dunn, K. W., McGraw, T. E., and Maxfield, F. R. (1989) J. Cell Biol. 109 3303-3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raiborg, C., and Stenmark, H. (2002) Cell Struct. Funct. 27 403-408 [DOI] [PubMed] [Google Scholar]
- 41.Haggie, P. M., Kim, J. K., Lukacs, G. L., and Verkman, A. S. (2006) Mol. Biol. Cell 17 4937-4945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puthenveedu, M. A., and von Zastrow, M. (2006) Cell 127 113-124 [DOI] [PubMed] [Google Scholar]
- 43.Yudowski, G. A., Puthenveedu, M. A., and von Zastrow, M. (2006) Nat. Neurosci. 9 622-627 [DOI] [PubMed] [Google Scholar]
- 44.Kim, E., and Sheng, M. (2004) Nat. Rev. Neurosci. 5 771-781 [DOI] [PubMed] [Google Scholar]