Abstract
Background
Previous studies of dose-response effects of usual sodium and potassium intake on later cardiovascular disease (CVD) have largely relied on sub-optimal measures of intake.
Methods
Two trials of sodium reduction and other interventions collected 24-hour urinary excretions intermittently over 18 months in 1987-1990 (TOHP I) and 36 months in 1990-1995 (TOHP II) among pre-hypertensive adults aged 30-54. Among those not assigned to an active sodium reduction intervention, we assessed the relation of an average of 3 to 7 measures of sodium and potassium excretion and their ratio with subsequent CVD (myocardial infarction, stroke, coronary revascularization, or CVD mortality) through 10-15 years of post-trial follow-up.
Results
Among 2,974 participants, follow-up information was obtained on 2,275 (76.5%), with 193 CVD events. After adjustment for baseline variables and lifestyle changes, there was a non-significant trend in CVD risk over gender-specific quartiles of sodium (rate ratio (RR) from lowest to highest = 1.00, 0.99, 1.16, 1.20, p-trend=0.38), and potassium (RR=1.00, 0.94, 0.91, 0.64, p-trend=0.08) excretion, but a significant trend over quartiles of the sodium-potassium ratio (RR=1.00, 0.84, 1.18, and 1.50, p-trend=0.04). In models containing both measures simultaneously, linear effects were RR=1.42 (95% confidence interval (CI) =0.99-2.04) per 100 mmol/d of sodium excretion (p=0.05) and RR=0.67 (95%CI=0.41-1.10) per 50 mmol/d of potassium excretion (p=0.12). A model containing the sodium-potassium ratio (RR=1.24 per unit, 95%CI=1.05-1.46, p=0.01) had the lowest Bayes information criterion (best fit).
Conclusion
A higher sodium/potassium ratio is associated with increased risk of later CVD, and is stronger than sodium or potassium alone.
Both observational data and randomized trials have identified a lower level of blood pressure (BP) and a reduced risk of hypertension among those with lower levels of sodium intake and higher levels of potassium intake.1-8 Recent evidence has suggested that long-term interventions aimed at sodium reduction or potassium substitution may also lead to a reduced risk of cardiovascular disease (CVD).9, 10 Dose-response relations of sodium and potassium intake, as well as the sodium-potassium ratio, with CVD, however, have not been definitively determined.11 The biologic interaction of sodium and potassium is of particular interest, since it may play a dominant role in the pathogenesis of hypertension and the development of CVD.12 Several studies have suggested that a high sodium-potassium ratio is associated with increased blood pressure as well as CVD.13-17
Available observational studies of subsequent CVD use imperfect measures of sodium and potassium intake, either a single dietary recall or a single urine excretion.15, 18 Such imprecise measures may attenuate the estimated effect on subsequent risk. During the Trials of Hypertension Prevention (TOHP)4, 19 repeated measures of 24-hour electrolyte excretion were carefully collected intermittently over an 18-month (TOHP I) or a 3-year period (TOHP II). We were thus able to assess the relationship of usual long-term average sodium and potassium excretion, as well as the sodium-potassium ratio, with subsequent CVD. A previous report compared those randomized to an active sodium intervention or a usual care control group in the same studies.9 The current analysis includes only those not assigned to an active sodium reduction intervention in order to focus on long-term usual intake.
Methods
TOHP I
TOHP I was designed to test the feasibility and efficacy of seven nonpharmacologic interventions in reducing BP among persons with high normal BP (pre-hypertension).20 These included lifestyle interventions of weight loss, sodium reduction, and stress management, and nutritional supplement interventions of calcium, magnesium, potassium, and fish oil. Eligible participants were aged 30-54 years with mean diastolic BP between 80 and 89 mmHg, not on antihypertensive medication. Randomization occurred at 10 clinic sites from September 1987 through October 1988. Of 2,182 randomized participants, 327 were assigned to a sodium reduction intervention and were excluded, leaving 1,855 eligible for these analyses.
The follow-up period for the lifestyle interventions was 18 months. The four supplement interventions, including potassium, were conducted in two stages, each of six months duration, with an intervening washout period. Follow-up visits occurred at 3 and 6 months during each supplement intervention, and for lifestyle interventions also at 12 and 18 months. Twenty-four-hour sodium excretions were collected at baseline (2 collections) for all interventions, at 6, 12, and 18 months for the lifestyle interventions, and at each of five follow-up visits for the supplement interventions. For the 178 participants randomized to the short-term active potassium supplement intervention, excretions taken during this 6 month intervention were not included in the average. Collection of final visit data ended in January, 1990.
TOHP II
TOHP II tested the effect of weight loss and sodium reduction interventions over a 3-year follow-up.21 The 2×2 factorial design included intervention groups of weight loss alone, sodium reduction alone, a combination of weight loss and sodium reduction and a usual care comparison group. Participants were aged 30-54 years, with a body mass index (BMI) representing 110-165% of desirable body weight. Eligible baseline diastolic BP was 83-89 mmHg, with systolic BP <140 mmHg, not on antihypertensive medication. A total of 2,382 participants were randomized into the trial from December, 1990, to March, 1992, at 9 clinic sites, with 1,191 assigned to an active sodium reduction intervention. These were excluded, leaving 1,191 participants eligible for this analysis.
Participants in each of the four arms were seen at clinic visits every six months for 36 to 48 months, with 24-hour excretions collected at baseline, 18, and 36 months. Additional excretions were collected from selected participants at 6, 42, and 48 months. Final visits concluded in March, 1995.
TOHP Follow-up
Observational follow-up for CVD began in 2000, approximately 10 years after the end of TOHP I and 5 years after the end of TOHP II. The cohort comprised 3,009 participants who had not been randomized to an active sodium reduction intervention An additional 20 participants had CVD events during the trial periods and 15 had no valid excretion measures. These were excluded from current analyses, leaving 2,974 participants, of whom 37 took part in both trials. For these 37, follow-up time was assigned to TOHP I until the start of TOHP II, and then to TOHP II. The follow-up was conducted by the TOHP Coordinating Center at the Brigham and Women's Hospital in Boston, MA, and approved by institutional review boards there and at participating clinic centers.
The follow-up was conducted centrally by mail and phone. Beginning in January 2000, initial questionnaires were sent out, with up to four additional requests, followed by phone calls as needed. Questionnaires sought information on cardiovascular and other health outcomes, as well as limited information on weight, BP, and several health behaviors. Additional questionnaires were sent at two-year intervals, through early 2005, with interim annual postcards to collect information on address changes and study endpoints.
The primary endpoint for the follow-up study was CVD, consisting of myocardial infarction (MI), stroke, coronary artery bypass graft (CABG), percutaneous transluminal coronary angioplasty (PTCA), or death from cardiovascular causes. Upon notification of occurrence of a primary nonfatal endpoint, consent was sought to obtain medical records. The medical records were reviewed by a study physician blinded to treatment assignment to confirm reported events using standardized endpoint criteria. Of 301 nonfatal endpoints reported, including multiples per person, consent for medical records was obtained for 239 (79.4%), and medical records were obtained for 213 (89.1%) of these. Of the reported endpoints with records, 193 (90.6%) were confirmed. Due to the high confirmation rate, to enhance statistical power all reported endpoints were included in these analyses (n=193 individuals), except for those that were disconfirmed following record review. A search of the National Death Index identified deaths up through December 2003 among non-respondents to the questionnaires.
Statistical Methods
We conducted an observational analysis of the association of usual sodium and potassium intake ascertained during the trial periods with subsequent CVD or mortality endpoints. Exposure was defined as the average excretion during the trial period, with outcomes ascertained following the trial. The primary outcome for this analysis was CVD following the end of the trial. We also examined the composite outcome of CVD or death from any cause, and total mortality, as well as other combinations of the components of the primary endpoint, including coronary heart disease, MI, and stroke.
Usual intake of sodium or potassium or their ratio was the average of available excretion measures at 5 (lifestyle interventions) or 7 (supplement interventions) scheduled collections over 18 months in TOHP I, and at 3 or up to 5 scheduled collections over a 3-year period in TOHP II. Mean excretion levels were examined across categories of baseline characteristics and tested using linear regression. Because of large differences by gender, gender-specific quartile categories of sodium and potassium excretion, as well as their ratio, were formed.
Cox regression analysis was used to assess the rate (hazard) ratio (RR) for quartile categories of excretion from the end of the trial period to the end of follow-up. Models were stratified by trial, and first adjusted for clinic, age, race, sex, and treatment assignment (Model 1). Additional models adjusted for education, family history, baseline weight, alcohol, smoking, and exercise (Model 2) and also for changes in weight, smoking and exercise over the trial periods (Model 3). Other analyses additionally controlled for BP, change in BP, and antihypertensive medication use during the trials.
To examine the shape of the relationship to CVD, we first fit linear terms for each excretion measure, and used penalized splines,22 or flexible nonlinear functions, to assess linearity. Models were fit for sodium and potassium separately, together, and as a ratio. No improvement was found when a log transformation for the excretion variables or when an interaction term for sodium and potassium was included. The additional predictive information of the sodium-potassium ratio was examined by comparing standardized coefficients for sodium, potassium, and the ratio,23, 24 and assessing model fit through the Bayes information criteria.25 Possible modification of the linear effect by age, race, gender, baseline BMI, smoking, trial, and participation in an active weight loss intervention, was examined and tested using interaction terms.
Finally, despite averaging several repeated measures of sodium and potassium, remaining within-person variability, as well as the correlation between measures, could impact the estimated effects.15, 26, 27 We estimated the joint between and within-individual variance components using a mixed model for measures over time,28, 29 and corrected for within-person variability using multivariate regression calibration.15, 30 Splines were fit in SPlus, and all other analyses were conducted in SAS 9.1.
Results
Follow-up response
Of the 2,974 eligible participants, 2,207 provided follow-up information on study questionnaires, and 68 deaths occurred prior to the start of follow-up. Thus, endpoint information was obtained for 2,275 participants (2306 including duplicates in both phases), for an overall response rate of 76.5%. Response was higher in TOHP II (78.4%), than in TOHP I (75.3%) participants. Name or other contact information was unavailable for 65 participants (2.2%), and 226 participants had unforwardable addresses (7.6%). A total of 102 participants (3.4%) were not willing to provide study information, and 306 (10.3%) did not respond. Of those with a known address, 84.8% responded. Response varied considerably by study clinic site, but there was no difference by quartile of average sodium excretion after adjustment for phase and clinic.
A total of 193 participants experienced study endpoints (8.5%), with a higher proportion among those participating in TOHP I vs. TOHP II (9.3% vs. 7.3%) due to the longer follow-up period. First events included 68 MI's, 22 strokes (one reported both MI and stroke), 77 revascularizations, 27 CVD deaths, and 51 non-CVD deaths. Eighty deaths occurred during the follow-up period, two following a nonfatal event.
Analysis of usual intake
Participants had an average of 4.8 excretion measures (median = 5, range=1 to 7) in TOHP I and 3.6 measures (median = 4, range=1 to 5) in TOHP II. The overall median sodium excretion was 158 mmol/24h (inter-quartile range (IQR) = 127-194 mmol/24h). The median was higher among men (171 mmol/24h) than women (134 mmol/24h), and among those in TOHP II (172 mmol/24h) than in TOHP I (149 mmol/24h), likely associated with the higher weight criterion. The median potassium excretion was 60 mmol/24h (IQR=48-73 mmol/24h), and the median sodium-potassium ratio was 2.8 (IQR=2.2-3.4 mmol/24h), again with higher levels in TOHP II.
We compared mean urinary excretion levels by categories of baseline characteristics among men and women separately due to the large gender differences (Table 1). Means varied significantly with several of these, including age, race, education, baseline systolic BP, smoking, alcohol use, exercise, and baseline BMI. Differences were modest except for the positive relation of BMI with sodium excretion.
Table 1.
Mean levels of urinary excretion variables by gender and baseline characteristics, adjusted for trial.
| N | Sodium | Potassium | Sodium/Potassium | |||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | Men | Women | |
| Overall | 1601 | 705 | 176.1 | 138.3 | 66.5 | 50.7 | 2.88 | 2.97 |
| Age | ||||||||
| 30-44 | 915 | 366 | 176.5 | 142.9 | 65.2 | 49.2 | 2.94 | 3.15 |
| 45-54 | 686 | 339 | 175.0 | 134.6 | 68.2 | 52.5 | 2.78 | 2.79 |
| p | 0.57 | 0.014 | 0.0028 | 0.0039 | 0.0012 | <0.0001 | ||
| Race | ||||||||
| White | 1418 | 504 | 177.1 | 137.6 | 67.8 | 53.0 | 2.83 | 2.80 |
| Black | 139 | 183 | 170.0 | 139.5 | 53.6 | 43.4 | 3.43 | 3.49 |
| Other | 44 | 18 | 153.1 | 169.3 | 66.0 | 63.3 | 2.54 | 2.78 |
| p (Black vs. white) | 0.14 | 0.63 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| p (Other vs white) | 0.004 | 0.0032 | 0.54 | 0.0027 | 0.051 | 0.93 | ||
| Education | ||||||||
| <=High school | 486 | 374 | 184.4 | 141.4 | 64.3 | 50.0 | 3.11 | 3.07 |
| College degree | 710 | 220 | 175.4 | 138.1 | 66.5 | 51.8 | 2.87 | 2.92 |
| Grad degree | 404 | 111 | 166.3 | 132.3 | 69.1 | 51.5 | 2.59 | 2.80 |
| p-trend | <0.0001 | 0.059 | 0.0003 | 0.19 | <0.0001 | 0.0074 | ||
| Systolic BP | ||||||||
| <125 mmHg | 762 | 298 | 174.9 | 138.9 | 65.5 | 48.5 | 2.90 | 3.10 |
| 125+ mmHg | 839 | 407 | 176.7 | 139.0 | 67.5 | 52.4 | 2.85 | 2.89 |
| p | 0.49 | 0.98 | 0.049 | 0.0009 | 0.38 | 0.0072 | ||
| Diastolic BP | ||||||||
| 80-84 mmHg | 894 | 387 | 177.5 | 139.4 | 67.2 | 50.6 | 2.85 | 2.97 |
| 85-89 mmHg | 707 | 318 | 173.8 | 138.4 | 65.6 | 51.0 | 2.90 | 2.98 |
| p | 0.19 | 0.77 | 0.13 | 0.72 | 0.33 | 0.88 | ||
| Smoking | ||||||||
| Current | 157 | 81 | 171.5 | 140.5 | 60.9 | 50.0 | 3.07 | 3.02 |
| Past | 593 | 187 | 180.2 | 133.8 | 69.8 | 51.5 | 2.79 | 2.80 |
| Never | 850 | 437 | 173.6 | 140.8 | 65.2 | 50.7 | 2.90 | 3.04 |
| p (Current vs. never) | 0.65 | 0.95 | 0.012 | 0.71 | 0.039 | 0.84 | ||
| p (Past vs. never) | 0.023 | 0.074 | <0.0001 | 0.53 | 0.036 | 0.0058 | ||
| Alcohol use | ||||||||
| >=1 drink/wk | 783 | 183 | 171.3 | 134.6 | 67.3 | 52.9 | 2.78 | 2.74 |
| None | 818 | 522 | 180.2 | 140.4 | 65.7 | 50.0 | 2.96 | 3.06 |
| p | 0.0011 | 0.14 | 0.12 | 0.026 | 0.0003 | 0.0004 | ||
| Exercise | ||||||||
| None | 446 | 281 | 183.0 | 141.0 | 64.6 | 50.2 | 3.05 | 3.07 |
| 1-2 times/wk | 520 | 190 | 177.5 | 140.2 | 67.0 | 51.0 | 2.86 | 2.97 |
| 3+ times/wk | 622 | 228 | 169.9 | 136.2 | 67.6 | 51.5 | 2.76 | 2.88 |
| p-trend | <0.0001 | 0.24 | 0.020 | 0.34 | <0.0001 | 0.034 | ||
| Family history of CVD* | ||||||||
| Yes | 142 | 61 | 177.6 | 140.4 | 66.0 | 51.6 | 2.90 | 2.81 |
| No | 1452 | 641 | 175.8 | 138.8 | 66.6 | 50.7 | 2.87 | 2.99 |
| p | 0.71 | 0.79 | 0.71 | 0.65 | 0.70 | 0.18 | ||
| Baseline BMI | ||||||||
| <25 kg/m2 | 238 | 138 | 145.1 | 123.1 | 64.6 | 49.9 | 2.46 | 2.65 |
| 25-<30 kg/m2 | 777 | 279 | 169.8 | 134.6 | 65.7 | 50.1 | 2.80 | 2.95 |
| 30+ kg/m2 | 586 | 288 | 196.3 | 150.7 | 68.4 | 51.9 | 3.14 | 3.16 |
| p-trend | <0.0001 | <0.0001 | 0.0084 | 0.16 | <0.0001 | <0.0001 | ||
| Change in weight | ||||||||
| Lost 5+ lbs | 402 | 145 | 181.9 | 139.1 | 69.0 | 53.8 | 2.83 | 2.81 |
| No change | 641 | 276 | 172.4 | 137.1 | 66.6 | 49.2 | 2.80 | 3.03 |
| Gained 5+ lbs | 432 | 213 | 177.4 | 142.4 | 65.9 | 51.6 | 2.94 | 3.02 |
| p-trend | 0.24 | 0.41 | 0.024 | 0.32 | 0.095 | 0.085 | ||
Father or mother died due to cardiovascular disease.
In Cox regression models there was some evidence of a trend in CVD risk over quartiles of sodium excretion, but this was not statistically significant and was attenuated after adjustment for baseline characteristics (Table 2). Extreme quartiles represent a difference in medians of 122 mmol/24h in men and 95 mmol/24h in women. The RR for those in the highest vs. the lowest sodium quartile was 1.20 (95%CI = 0.73-1.97, with p for trend=0.38) after full adjustment. There was a significant inverse trend over quartiles of potassium excretion, with a 45% reduction in risk among those in the highest vs. lowest quartile (RR=0.55, 95%CI=0.35-0.87, p for trend = 0.01) after adjustment for baseline characteristics, and a 36% reduction after additional adjustment for lifestyle changes during the trials (p for trend = 0.08). The trend over quartiles of the sodium-potassium ratio was the strongest and statistically significant with RR=1.50 (95%CI =0.94-2.39, p for trend= 0.039) over extreme quartiles in fully adjusted analyses.
Table 2.
Cardiovascular events following the trials by overall quartiles of average excretion levels across time among responders not in an active sodium intervention.
| Total CVD by Quartile | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p-trend | |
| Sodium Excretion | |||||
| N (events/total) | 47/563 | 39/573 | 56/589 | 51/581 | 0.25* |
| % | 8.3 | 6.8 | 9.5 | 8.8 | |
| Model 1 RR† | 1.00 | 0.84 | 1.18 | 1.25 | 0.14 |
| 95% CI | (ref) | 0.55-1.30 | 0.79-1.76 | 0.83-1.90 | |
| Model 2 RR | 1.00 | 0.84 | 1.06 | 1.10 | 0.48 |
| 95% CI | (ref) | 0.54-1.30 | 0.70-1.62 | 0.70-1.72 | |
| Model 3 RR | 1.00 | 0.99 | 1.16 | 1.20 | 0.38 |
| 95% CI | (ref) | 0.62-1.58 | 0.73-1.84 | 0.73-1.97 | |
| Potassium Excretion | |||||
| N (events/total) | 50/543 | 53/579 | 51/589 | 39/595 | 0.12* |
| % | 9.2 | 9.2 | 8.7 | 6.6 | |
| Model 1 RR | 1.00 | 0.90 | 0.89 | 0.57 | 0.018 |
| 95% CI | (ref) | 0.60-1.33 | 0.60-1.34 | 0.37-0.89 | |
| Model 2 RR | 1.00 | 0.85 | 0.82 | 0.55 | 0.014 |
| 95% CI | (ref) | 0.56-1.27 | 0.54-1.25 | 0.35-0.87 | |
| Model 3 RR | 1.00 | 0.94 | 0.91 | 0.64 | 0.080 |
| 95% CI | (ref) | 0.61-1.47 | 0.58-1.44 | 0.39-1.05 | |
| Sodium/Potassium Excretion Ratio | |||||
| N (events/total) | 45/587 | 43/585 | 49/580 | 56/554 | 0.066* |
| % | 7.7 | 7.4 | 8.4 | 10.1 | |
| Model 1 RR† | 1.00 | 1.06 | 1.35 | 1.77 | 0.004 |
| 95% CI | (ref) | 0.70-1.62 | 0.89-2.05 | 1.17-2.69 | |
| Model 2 RR | 1.00 | 0.95 | 1.16 | 1.61 | 0.019 |
| 95% CI | (ref) | 0.61-1.46 | 0.76-1.80 | 1.04-2.49 | |
| Model 3 RR | 1.00 | 0.84 | 1.18 | 1.50 | 0.039 |
| 95% CI | (ref) | 0.53-1.34 | 0.75-1.87 | 0.94-2.39 | |
Cochran-Mantel-Haenszel test stratified by trial.
- Model 1: clinic, treatment assignment, age, sex, and race.
- Model 2: Model 1 variables plus education, family history, baseline weight, alcohol, smoking, and exercise.
- Model 3: Model 2 variables plus changes in weight, smoking, and exercise.
Linear effects on CVD
In analyses treating average sodium excretion as a linear term, there was a trend toward increasing risk of CVD with increasing sodium excretion that was attenuated in fully adjusted analyses (Table 3). A 100 mmol/24h higher level of sodium excretion was associated with a 25% increase in risk (RR=1.25, 95%CI = 0.91-1.72, p=0.18). The RR was similar after additional adjustment for baseline BP, change in BP, and use of anti-hypertensive medications during the trials (RR=1.25, 95%CI = 0.90-1.72, p=0.18). Spline plots suggested an increasing risk with higher sodium with p-linear=0.15, and tests for non-linearity were non-significant (p=0.82).
Table 3.
Linear effect of average excretion on total cardiovascular disease following the trial in the non-sodium interventions. The rate ratio (RR) is that associated with an increase of 100 mmol/24h in sodium excretion, of 50 mmol/24h in potassium excretion, and of 1 unit of the sodium-potassium excretion ratio.
| Regression Coefficient | ||||||
|---|---|---|---|---|---|---|
| Events | Beta | SE | p | RR | 95% CI | |
| Sodium Excretion (mmol/d) Only | ||||||
| Model 1* | 193 | 0.00275 | 0.00137 | 0.044 | 1.32 | 1.01-1.72 |
| Model 2 | 190 | 0.00200 | 0.00152 | 0.19 | 1.22 | 0.91-1.65 |
| Model 3 | 166 | 0.00220 | 0.00163 | 0.18 | 1.25 | 0.91-1.72 |
| Potassium Excretion (mmol/d) Only | ||||||
| Model 1 | 193 | -0.00674 | 0.00407 | 0.097 | 0.71 | 0.48-1.06 |
| Model 2 | 190 | -0.00668 | 0.00425 | 0.12 | 0.71 | 0.47-1.08 |
| Model 3 | 166 | -0.00378 | 0.00453 | 0.40 | 0.83 | 0.53-1.29 |
| Both Sodium and Potassium Excretion (mmol/d) | ||||||
| Model 1 | 193 | |||||
| Sodium | 0.00475 | 0.00155 | 0.0021 | 1.61 | 1.19-2.18 | |
| Potassium | -0.01268 | 0.00458 | 0.0056 | 0.53 | 0.34-0.83 | |
| Model 2 | 190 | |||||
| Sodium | 0.00377 | 0.00168 | 0.025 | 1.46 | 1.05-2.03 | |
| Potassium | -0.01118 | 0.00476 | 0.019 | 0.57 | 0.36-0.91 | |
| Model 3 | 166 | |||||
| Sodium | 0.00352 | 0.00183 | 0.054 | 1.42 | 0.99-2.04 | |
| Potassium | -0.00802 | 0.00509 | 0.12 | 0.67 | 0.41-1.10 | |
| Sodium/Potassium Excretion Ratio | ||||||
| Model 1 | 193 | 0.23104 | 0.06175 | 0.0002 | 1.26 | 1.12-1.42 |
| Model 2 | 190 | 0.19360 | 0.06529 | 0.0030 | 1.21 | 1.07-1.38 |
| Model 3 | 166 | 0.21203 | 0.08450 | 0.012 | 1.24 | 1.05-1.46 |
- Model 1: clinic, treatment assignment, age, sex, and race.
- Model 2: Model 1 variables plus education, family history, baseline weight, alcohol, smoking, and exercise.
- Model 3: Model 2 variables plus changes in weight, smoking, and exercise.
A linear inverse relation of potassium excretion with CVD risk was suggested but not statistically significant in fully adjusted models (RR=0.83 per 50 mmol/24h, 95%CI = 0.53-1.29, p=0.40) (Table 3). Spline plots did not show a significant linear trend (p=.62). Some curvature in the relationship was suggested, but confidence intervals were wide, and the test for nonlinearity was not significant (p=0.22).
The correlation between average sodium and potassium excretions was 0.49, with slight variation by phase and gender. When sodium and potassium excretion were included in the same model, effects strengthened for both measures. There was a 42% estimated increase in CVD per 100 mmol/d increase in sodium excretion and a 33% risk reduction in CVD per 50 mmol/d increase in potassium excretion (Table 3). Expressed per SD unit, the RRs were 1.22 per 55.5 mmol/d increase (95%CI = 1.00-1.48, p=0.054) for sodium and 0.82 per 25.0 mmol/d increase (95%CI = 0.64-1.05, p=0.12) for potassium, with regression coefficients that were equal but opposite in direction. An interaction term for sodium and potassium was not statistically significant (p=0.24).
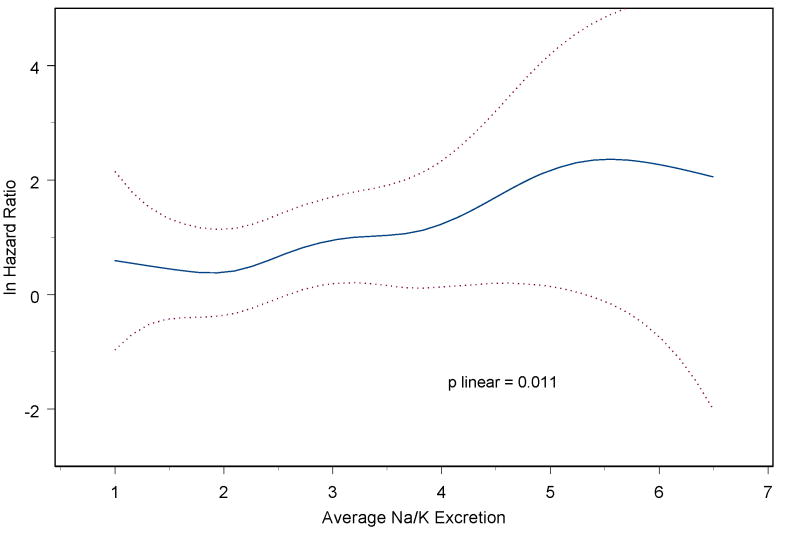
The sodium-potassium ratio, however, exhibited a statistically significant linear association with risk of CVD, with an RR of 1.24 per unit (95%CI = 1.05-1.46, p=0.012) (Table 3). Adjustment for BP, change in BP, and BP medication use during the trials produced little change in the estimated effect (RR=1.25, 95%CI = 1.06-1.48, p=0.009). The relationship appeared to be linear (Figure 1) (p-linear=0.01), with no evidence of non-linearity in the relation (p for nonlinearity = 0.47). Expressed per SD unit, the RR for CVD was 1.24 per 1.01 units (95%CI = 1.05-1.46, p=0.01). The model using the ratio demonstrated better fit according to the Bayes information criteria25 than the model including both excretion measures.
Figure 1.
Spline plot of log hazard ratio for total cardiovascular disease by average sodium/potassium excretion ratio, among those not in an active sodium reduction intervention, stratified by trial and adjusted for clinic, treatment assignment, age, sex, race, education, family history, baseline weight, alcohol, smoking, exercise, and changes in weight, smoking, and exercise. The p-value is from a test of linearity using penalized splines.
The repeated measures obtained during the trial allowed us to examine variation over time and correct for measurement error in both sodium and potassium during the trials. The correlation over time (reliability) of the excretions was 0.35 for sodium, 0.40 for potassium, and 0.32 for the ratio. The within-person correlation of sodium and potassium was 0.47 and the between-person correlation was 0.52. When correcting for remaining measurement error in both excretion measures, the RR for sodium increased to 1.84 per 100 mmol/d, and that for potassium decreased to 0.59 per 50 mmol/d. The corrected RR for the sodium/potassium ratio increased to 1.35 per unit.
In fully adjusted analyses, estimated effects of the sodium-potassium ratio were similar for coronary heart disease (CHD) (MI, coronary revascularization, or CHD death, n=154; RR=1.26, 95%CI=1.06-1.50) and for stroke (including cerebrovascular death, n=21; RR=1.25, 95%CI=0.86-1.82), but not total mortality (n=66; RR=1.01, 95%CI=0.76-1.32). There was little evidence of modification of the rate ratio in subgroup analyses (Table 4). All interactions were non-significant, and effects were relatively consistent across subgroups.
Table 4.
Linear effect of average sodium/potassium ratio on cardiovascular disease following the trial in the non-sodium interventions by subgroups.*
| Effect per unit | ||||||
|---|---|---|---|---|---|---|
| N | Events | RR | 95% CI | p | p for interaction | |
| Sodium/Potassium Excretion Ratio | ||||||
| Sex | ||||||
| Male | 1459 | 141 | 1.26 | 1.04-1.53 | 0.020 | 0.77 |
| Female | 625 | 25 | 1.21 | 0.79-1.85 | 0.39 | |
| Race | ||||||
| White | 1743 | 141 | 1.24 | 1.04-1.49 | 0.020 | 0.58 |
| Black | 284 | 19 | 1.85 | 0.94-3.63 | 0.073 | |
| Age | ||||||
| 30-44 | 1153 | 67 | 1.39 | 1.05-1.84 | 0.022 | 0.13 |
| 45-54 | 931 | 99 | 1.20 | 0.96-1.49 | 0.11 | |
| Body mass index | ||||||
| < 30 kg/m2 | 1286 | 100 | 1.16 | 0.90-1.49 | 0.24 | 0.26 |
| ≥ 30 kg/m2 | 798 | 66 | 1.33 | 1.05-1.68 | 0.018 | |
| Smoking | ||||||
| Current | 201 | 25 | 1.39 | 0.76-2.51 | 0.28 | 0.54 |
| Past | 709 | 67 | 1.08 | 0.78-1.51 | 0.64 | 0.75 |
| Never | 1174 | 74 | 1.21 | 0.96-1.54 | 0.11 | |
| Trial | ||||||
| TOHP I | 1219 | 104 | 1.13 | 0.91-1.41 | 0.29 | 0.37 |
| TOHP II | 865 | 62 | 1.34 | 1.06-1.70 | 0.015 | |
| Active weight loss intervention | ||||||
| Yes | 643 | 58 | 1.31 | 1.01-1.70 | 0.045 | 0.12 |
| No | 1441 | 108 | 1.15 | 0.92-1.44 | 0.21 | |
| Active weight loss intervention | ||||||
| TOHP I | ||||||
| Yes | 217 | 22 | 1.34 | 0.79-2.26 | 0.27 | 0.90 (Trial) |
| No | 1002 | 82 | 1.06 | 0.82-1.36 | 0.66 | 0.22 (wt loss) |
| TOHP II | ||||||
| Yes | 426 | 36 | 1.22 | 0.87-1.71 | 0.25 | |
| No | 439 | 26 | 1.48 | 0.95-2.31 | 0.084 | |
From Cox regression analysis stratified by trial and adjusted for clinic, treatment assignment, age, sex, race, education, family history, baseline weight, alcohol, smoking, exercise, and changes in weight, smoking, and exercise.
Discussion
In observational analyses of average excretion over a 1½ to 3 year period, we found a suggested positive relation of sodium excretion and an inverse relation of potassium excretion with risk of CVD, but neither were statistically significant when considered separately. Both measures strengthened when modeled jointly, with opposite but similar effects on risk. However, the sodium-potassium ratio displayed the strongest and most statistically significant association, with a 24% increase in risk per sodium/potassium unit that was similar for CHD and stroke and consistent within subgroups.
Few epidemiologic studies have jointly examined the relation of sodium and potassium or their ratio to CVD. Most large longitudinal studies of potassium intake have used food frequency questionnaires,31, 32 which have difficulty fully capturing sodium intake. The sodium-potassium ratio has, however, been found to be a somewhat stronger predictor of BP than sodium alone in data from TOHP I,15 INTERSALT,13, 14 and Southern California.16 Also, in a meta-analysis of trials of potassium supplementation, the effect on blood pressure was modified by average sodium intake.5 We found that the ratio was the strongest of the three measures in predicting CVD, and that the effect of both sodium and potassium were enhanced when the other was included in the model, supporting the notion that the joint activity of these two electrolytes may play an important biologic role.12
Liew et al24 demonstrate that using a ratio makes the implicit assumption that the regression coefficients for the two variables are equal in magnitude but opposite in direction. This was true in our data when sodium and potassium were expressed per SD unit. Further, the ratio provided a better fit to the data, and the addition of a multiplicative interaction term was not statistically significant.23 It is possible that the ratio offers a correction for characteristics of the urine collection, such as completeness over the 24-hour period and correlated measurement errors.27 Whether the improved fit of the ratio is due to a inherent biologic synergism12 or to an artifact of the excretion measurements is unclear.
Previous studies have largely relied on dietary records or recalls for estimating sodium intake, which tend to be less precise than urinary measures, and results have been inconsistent.33-37 Four studies have examined a single measure of urinary sodium excretion and CHD endpoints. One found an inverse relation with MI in hypertensive men but not women,38-40 one found a positive association with CHD in women only,41 and one found a positive relation with CHD and total mortality that appeared stronger in overweight men.42 Most recently, the Rotterdam Study found no effect of sodium, potassium or the ratio on CVD or mortality, except for an effect of the ratio on mortality among those with BMI≥25 kg/m2.43 That study used a single overnight urine, however, which may have included more measurement error and attenuated any effects. Exposure in TOHP was an average of 3 to 7 24-hour excretions taken over a period of 1 to 3 years, reducing measurement error and within-person variability.15, 18
Fewer studies have examined potassium and cardiovascular disease, with most attention paid to stroke. Higher dietary potassium has generally been associated with decreased stroke incidence or mortality,23, 31, 44-46 although results are sometimes marginal.32, 47 Few studies have examined urinary potassium and risk of CHD.41 We did not find an inverse association with stroke in adjusted analyses, but this was limited by the small number of strokes in our data. A stronger, though non-significant, association was found with total CVD.
Some observational studies have found effects of sodium and potassium on stroke or CVD that may be independent of effects on BP36, 42, 44 or hypertension,31, 32 as in the present study. Urinary sodium excretion has been found to be positively related to urinary albumin excretion,48-50 suggesting that the effect of sodium on CVD may be partially mediated by endothelial damage. Other proposed mechanisms for a cardiovascular effect of sodium independent of BP include a direct effect on left-ventricular mass51 or increased blood flow and vascular reactivity with higher sodium exposure.52, 53 The present study found an effect on CVD outcomes even after controlling for blood pressure. We did not have measured follow-up BP, however, so cannot determine if the sodium observation is fully independent of blood pressure.
Other limitations of the present study include a lack of complete follow-up of trial participants for nonfatal events. Response, however, did not appear to be related to excretion measures. We also did not have a full complement of CVD risk factors, such as lipids, for adjustment. In addition, we used urinary excretion as our exposure, which is assumed to adequately reflect dietary intake. We also had no sodium excretions during follow-up and thus could not account for possible changes during the follow-up period.
The current analysis of the TOHP follow-up data included only those not in an active sodium intervention to better represent long-term usual intake. Previous independent analyses of the TOHP trials found a significant reduction in total CVD as well as a suggested reduction in total mortality among those assigned to the sodium reduction intervention.9 A cluster-randomized trial conducted among elderly residents of a veterans retirement home in Taiwan compared potassium-enriched salt, containing less sodium, to regular salt, and found a significant 41% reduction in CVD mortality in the experimental group.10 Thus, recent randomized trial data support our findings of a reduced risk of CVD among those with lower sodium and/or higher potassium. The 2005 US Dietary Guidelines54 recommend consuming potassium-rich foods, such as fruits and vegetables, as well as consuming little salt. The current totality of evidence suggests that such lowering of dietary sodium intake while increasing potassium consumption at the population level might reduce the incidence of cardiovascular disease.
Acknowledgments
TOHP I and II were supported by cooperative agreements HL37849, HL37852, HL37853, HL37854, HL37872, HL37884, HL37899, HL37904, HL37906, HL37907, and HL37924, and the TOHP Follow-up Study was supported by grant HL057915, all from the National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD. Dr. Cook had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors have no conflicts of interest to report.
References
- 1.Intersalt Cooperative Research Group. Intersalt: an international study of electrolyte excretion and blood pressure: results for 24-hour urinary sodium and potassium excretion. BMJ. 1988;297:319–328. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Law MR, Frost CD, Wald NJ. By how much does dietary salt reduction lower blood pressure? I-Analysis of observation data among populations. Br Med J. 1991;302:811–815. doi: 10.1136/bmj.302.6780.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frost CD, Law MR, Wald NJ. By how much does dietary salt reduction lower blood pressure? II-Analysis of observation data within populations. Br Med J. 1991;302:815–818. doi: 10.1136/bmj.302.6780.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high- normal blood pressure. The Trials of Hypertension Prevention, Phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997;157:657–667. [PubMed] [Google Scholar]
- 5.Whelton PK, He J, Cutler JA, et al. The effects of oral potassium on blood pressure: a quantitative overview of randomized, controlled clinical trials. J Amer Med Assoc. 1997;277:1624–1632. doi: 10.1001/jama.1997.03540440058033. [DOI] [PubMed] [Google Scholar]
- 6.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 7.He FJ, MacGregor GA. Effect of modest salt reduction on blood pressure: a meta-analysis of randomized trials. Implications for public health. J Human Hypertens. 2002;16:761–770. doi: 10.1038/sj.jhh.1001459. [DOI] [PubMed] [Google Scholar]
- 8.Geleijnse JM, Kok FJ, Grobbee DE. Blood pressure response to changes in sodium and potassium intake: a metaregression analysis of randomised trials. J Human Hypertens. 2003;17:471–480. doi: 10.1038/sj.jhh.1001575. [DOI] [PubMed] [Google Scholar]
- 9.Cook NR, Cutler JA, Obarzanek E, et al. The long-term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the Trials of Hypertension Prevention. Br Med J. 2007;334:885–888. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang HY, Hu YW, Yue CSJ, et al. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am J Clin Nutr. 2006;83:1289–1296. doi: 10.1093/ajcn/83.6.1289. [DOI] [PubMed] [Google Scholar]
- 11.Hooper L, Bartlett C, Davey Smith G, et al. Systematic review of long term effects of advice to reduce dietary salt in adults. BMJ. 2002;325:628–637. doi: 10.1136/bmj.325.7365.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adrogue HJ, Madias NE. Sodium and potassium in the pathogensis of hypertension. N Engl J Med. 2007;356:1966–1978. doi: 10.1056/NEJMra064486. [DOI] [PubMed] [Google Scholar]
- 13.Dyer AR, Elliott P, Shipley M. INTERSALT Cooperative Research Group. Urinary electrolyte excretion in 24 hours and blood pressure int eh INTERSALT Study. II. Estimates of electrolyte-blood pressure associations corrected for regression dilution bias. Am J Epidemiol. 1994;139:940–951. doi: 10.1093/oxfordjournals.aje.a117100. for the. [DOI] [PubMed] [Google Scholar]
- 14.Elliott P, Stamler J, Nichols R, et al. Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. Br Med J. 1996;312:1249–1253. doi: 10.1136/bmj.312.7041.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook NR, Kumanyika SK, Cutler JA. Effect of change in sodium excretion on change in blood pressure corrected for measurement errror: the Trials of Hypertension Prevention, Phase I. Am J Epidemiol. 1998;148:431–444. doi: 10.1093/oxfordjournals.aje.a009668. [DOI] [PubMed] [Google Scholar]
- 16.Khaw KT, Barrett-Connor E. The association between blood pressure, age, and dietary sodium and potassium: a population study. Circ. 1988;77:53–56. doi: 10.1161/01.cir.77.1.53. [DOI] [PubMed] [Google Scholar]
- 17.Xie JX, Sasaki S, Joosens JV, Kesteloot H. The relationship between urinary cations obtained from the INTERSALT study and cerebrovascular mortality. J Human Hypertens. 1992;6:17–21. [PubMed] [Google Scholar]
- 18.Liu K. Measurement error and its impact on partial correlation and multiple linear regression analysis. Am J Epidemiol. 1988;127:864–874. doi: 10.1093/oxfordjournals.aje.a114870. [DOI] [PubMed] [Google Scholar]
- 19.The Trials of Hypertension Prevention Collaborative Research Group. The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. J Amer Med Assoc. 1992;267:1213–1220. doi: 10.1001/jama.1992.03480090061028. [DOI] [PubMed] [Google Scholar]
- 20.Satterfield S, Cutler JA, Langford HG, et al. Trials of hypertension prevention. Phase I design. Ann Epidemiol. 1991;1:455–471. doi: 10.1016/1047-2797(91)90014-4. [DOI] [PubMed] [Google Scholar]
- 21.Hebert PR, Bolt RJ, Borhani NO, et al. Design of a multicenter trial to evaluate long-term life-style intervention in adults with high-normal blood pressure levels. Trials of Hypertension Prevention (phase II). Trials of Hypertension Prevention (TOHP) Collaborative Research Group. Ann Epidemiol. 1995;5:130–139. doi: 10.1016/1047-2797(94)00057-z. [DOI] [PubMed] [Google Scholar]
- 22.Eilers PHC, Marx BD. Flexible smoothing with B-splines and penalties. Stat Sci. 1996;11:89–121. [Google Scholar]
- 23.Green DM, Ropper AH, Kronmal RA, Psaty BM, Burke GL. Cardiovascular Health Study. Serum potassium level and dietary potassium intake as risk factors for stroke. Neurol. 2002;59:314–320. doi: 10.1212/wnl.59.3.314. for the. [DOI] [PubMed] [Google Scholar]
- 24.Liew G, Sharrett AR, Kronmal R, et al. Measurement of retinal vascular caliber: issues and alternatives to using the arteriole to venule ratio. Invest Ophthalmol Vis Sci. 2007;48:52–57. doi: 10.1167/iovs.06-0672. [DOI] [PubMed] [Google Scholar]
- 25.Harrell FE., Jr . Regression Modeling Strategies. New York: Springer; 2001. [Google Scholar]
- 26.Espeland MA, Kumanyika S, Yunis C, et al. Electrolyte intake and nonpharmacologic blood pressure control. Ann Epidemiol. 2002;12:587–595. doi: 10.1016/s1047-2797(01)00318-0. [DOI] [PubMed] [Google Scholar]
- 27.Espeland MA, Kumanyika S, Wilson AC, et al. Statistical issues in analyzing 24-hour dietary recall and 24-hour urine collection data for sodium and potassium intakes. Am J Epidemiol. 2001;153:996–1006. doi: 10.1093/aje/153.10.996. [DOI] [PubMed] [Google Scholar]
- 28.SAS Institute Inc. SAS OnlineDoc® 9.1.3. Cary, NC: SAS Institute Inc.; 2007. [Google Scholar]
- 29.Thiébaut R, Jacqmin-Gadda H, Chêne G, Leport C, Commenges D. Bivariate linear mixed models using SAS proc MIXED. Comp Meth Prog Biomed. 2002;69:249–256. doi: 10.1016/s0169-2607(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 30.Fraser GE, Stram DO. Regression calibration in studies with correlated variables measured with error. Am J Epidemiol. 2001;154:836–844. doi: 10.1093/aje/154.9.836. [DOI] [PubMed] [Google Scholar]
- 31.Ascherio A, Rimm EB, Hernan MA, et al. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circ. 1998;98:1198–1204. doi: 10.1161/01.cir.98.12.1198. [DOI] [PubMed] [Google Scholar]
- 32.Iso H, Stampfer MJ, Manson JE, et al. Prospective study of calcium, potassium, and magnesium intake and risk of stroke in women. Stroke. 1999;30:1772–1779. doi: 10.1161/01.str.30.9.1772. [DOI] [PubMed] [Google Scholar]
- 33.Khaw KT. Dietary potassium and stroke (letter) N Engl J Med. 1987;317:509–510. doi: 10.1056/nejm198708203170815. [DOI] [PubMed] [Google Scholar]
- 34.Kagan A, Popper JS, Rhoads GG, Yano K. Dietary and other risk factors for stroke in Hawaiian Japanese men. Stroke. 1985;16:390–396. doi: 10.1161/01.str.16.3.390. [DOI] [PubMed] [Google Scholar]
- 35.Alderman MH, Cohen JD, Madhaven S. Dietary sodium intake and mortality: the National Health and Nutrition Examination Survey (NHANES I) Lancet. 1998;351:781–785. doi: 10.1016/S0140-6736(97)09092-2. [DOI] [PubMed] [Google Scholar]
- 36.He J, Ogden LG, Vupputuri S, Bazzano LA, Loria C, Whelton PK. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. J Amer Med Assoc. 1999;282:2027–2034. doi: 10.1001/jama.282.21.2027. [DOI] [PubMed] [Google Scholar]
- 37.Nagata C, Takatsuka N, Shimizu N, Shimizu H. Sodium intake and risk of death from stroke in Japanese men and women. Stroke. 2004;35:1543–1547. doi: 10.1161/01.STR.0000130425.50441.b0. [DOI] [PubMed] [Google Scholar]
- 38.Alderman MH, Madhaven S, Cohen H, Sealey JH, Laragh JH. Low urinary sodium is associated with greater risk of myocardial infarction among treated hypertensive men. Hypertens. 1995;25:1144–1152. doi: 10.1161/01.hyp.25.6.1144. [DOI] [PubMed] [Google Scholar]
- 39.MacGregor G. Low urinary sodium and myocardial infarction. Hypertens. 1996;27:156. [PubMed] [Google Scholar]
- 40.Cook NR, Cutler JA, Hennekens CH. An unexpected result for sodium - causal or casual. Hypertens. 1995;25 doi: 10.1161/01.hyp.25.6.1153. [DOI] [PubMed] [Google Scholar]
- 41.Tunstall-Pedoe H, Woodward M, Tavendale R, Brook RA, McCluskey MK. Comparison of the prediction by 27 different factors of coronary heart disease and death in men and women of the Scottish Heart Health Study. Br Med J. 1997;315:722–729. doi: 10.1136/bmj.315.7110.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuomilehto J, Jousilahti P, Rastenyte D, et al. Urinary sodium excretion and cardiovascular mortality in Finland: a prospective study. Lancet. 2001;357:848–851. doi: 10.1016/S0140-6736(00)04199-4. [DOI] [PubMed] [Google Scholar]
- 43.Geleijnse JM, Witteman JCM, Stijnen T, Kloos MW, Hofman A, Grobbee DE. Sodium and potassium intake and risk of cardiovascular events and all-cause mortality: the Rotterdam Study. Eur J Epidemiol. 2007;22:763–770. doi: 10.1007/s10654-007-9186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khaw KT. Dietary potassium and stroke-associated mortality: a 12-year prospective population study. N Engl J Med. 1987;316:235–240. doi: 10.1056/NEJM198701293160502. [DOI] [PubMed] [Google Scholar]
- 45.Lee CN, Reed DM, MacLean CJ, Yano K, Chiu D. Dietary potassium and stroke (letter) N Engl J Med. 1988;318:995–996. doi: 10.1056/NEJM198804143181516. [DOI] [PubMed] [Google Scholar]
- 46.Bazzano LA, He J, Ogden LG, et al. Dietary potassium intake and risk of stroke in US men and women: National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Stroke. 2001;32:1473–1480. doi: 10.1161/01.str.32.7.1473. [DOI] [PubMed] [Google Scholar]
- 47.Fang J, Madhaven S, Alderman MH. Dietary potassium intake and stroke mortality. Stroke. 2000;31:1532–1537. doi: 10.1161/01.str.31.7.1532. [DOI] [PubMed] [Google Scholar]
- 48.Verhave JC, Hillege HL, Burgerhof JGM, et al. Sodium intake affects urinary albumin excretion especially in overweight subjects. J Int Med. 2004;256:324–330. doi: 10.1111/j.1365-2796.2004.01390.x. [DOI] [PubMed] [Google Scholar]
- 49.du Cailar G, Ribstein J, Mimran A. Dietary sodium and target organ damage in essential hypertension. Am J Hypertens. 2002;15:222–229. doi: 10.1016/s0895-7061(01)02287-7. [DOI] [PubMed] [Google Scholar]
- 50.Fox CS, Larson MG, Hwang SJ, et al. Cross-sectional relations of serum aldosterone and urine sodium excretion to urinary albumin excretion in a community-based sample. Kidney Int. 2006;69:2064–2069. doi: 10.1038/sj.ki.5000378. [DOI] [PubMed] [Google Scholar]
- 51.Frohlich ED, Varagic J. The role of sodium in hypertension is more complex than simply elevating arterial pressure. Nat Clin Pract Cardiovasc Med. 2004;1:24–30. doi: 10.1038/ncpcardio0025. 1(1), 24-30. [DOI] [PubMed] [Google Scholar]
- 52.Simon G. Experimental evidence for blood pressure-independent vascular effects of high sodium diet. Am J Hypertens. 2003;16:1074–1078. doi: 10.1016/j.amjhyper.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 53.Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular disease. Physiol Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- 54.U.S. Department of Health and Human Services and U.S. Department of Agriculture. Dietary Guidelines for Americans, 2005. 6th. Washington, DC: U.S. Government Printing Office; Jan, 2005. [Google Scholar]