Abstract
Gender differences, sex steroid effects, and sex-specific candidate therapeutics in ischemic stroke have been studied in rodents but not in nonhuman primates. In this feasibility study (n = 3 per group), we developed a model of transient focal cerebral ischemia in adult male and female rhesus macaques that consistently includes white matter injury. The animals also were used to determine whether gender-linked differences in histopathologic outcomes could be evaluated in this model in future, larger preclinical trials. Histologic brain pathology was evaluated at 4 d after 90 min of reversible occlusion of the middle cerebral artery (MCA). MCA occlusion was accomplished by using a transorbital approach and temporary placement of an aneurysm clip. Male and female rhesus macaques 7 to 11 y of age were studied. Baseline and intraischemic blood glucose, systolic blood pressure, heart rate, oxygen saturation, end-tidal CO2, and rectal temperatures were not different among groups. The variability in injury volume was comparable to that observed in human focal cerebrovascular ischemia and in other nonhuman primate models using proximal MCA occlusion. In this small sample, the volume of injury was not different between male and female subjects, but observed variability was higher in female caudate nucleus, putamen, and hemisphere. This report is the first to compare cerebral ischemic outcomes in female and male rhesus macaques. The female rhesus macaque ischemic stroke model could be used after rodent studies to provide preclinical data for clinical trials in women.
Abbreviations: BP, blood pressure; EtCO2, end-tidal CO2; MCA, middle cerebral artery; SpO2, oxygen saturation of peripheral blood
Stroke is defined as the symptomatic loss or alteration of bodily function that results from an insufficient supply of blood to part of the brain. The most common type of stroke is ischemic stroke, which accounts for 87% of stroke cases.1 Ischemic stroke occurs with greater frequency in men than in women across diverse ethnic backgrounds and nationalities.1,25,38 This sexually dimorphic epidemiology is present in childhood10 until late in life, well beyond the menopausal years.13,31 These observations suggest that the presence of female sex steroids may not completely explain stroke's sexual dimorphism.
One of the new concepts that must be considered in stroke research is that therapies and treatments for this disease operate in different genetic landscapes in women and men. Consequently, any prospective neuroprotective or injurious compound may act differently in female and male ischemic brains. For example, the Women's Health Study recently evaluated aspirin use over 10 y for primary prevention of cardiovascular events, including stroke, and in reduction of mortality from cardiovascular causes.30 Aspirin lowered ischemic stroke risk by 24% in healthy women, in contrast to results from the men-only Physicians Health study,27 in which aspirin had no effect on stroke in men. These differences in how women compared with men respond to candidate agents potentially could influence clinical trial outcomes.
Ischemic stroke models have been developed and characterized in many animal species,14 including several nonhuman primate species like baboons, macaques, marmosets, and squirrel monkeys.9,26 There are several advantages to using nonhuman primate ischemic stroke models. First, the neuroanatomy and cerebrovascular anatomy of nonhuman primates is highly similar to that in humans. For example, rodents have lissencephalic brains, whereas humans and many nonhuman primate species have gyrencephalic brains.9 Moreover, the striatal gray matter of rats and mice is interleaved with white matter, whereas the white matter tracts of nonhuman primates are organized predominantly in the internal capsule.9 This arrangement in nonhuman primates ensures homogeneity of tissue and microvascular architecture with that of humans. Second, there are well known and often paradoxical differences between hemostatic and vascular mechanisms in rodents and rabbits and in their responses to antithrombotic and fibrinolytic agents compared with those of humans.9 However, several nonhuman primate species show close analogy to human hemostatic and coagulation systems. Finally, nonhuman primate models demonstrate a close similarity to human cerebral embolism without the effect of thrombus on the ischemic territory; lower animal species frequently lack this trait.9
Most focal ischemic stroke models in nonhuman primates involve occlusion of a single artery, such as the middle cerebral artery (MCA).9,14 Vascular clipping, use of an extrinsic balloon device or snare ligature, electrocoagulation or photocoagulation, and embolization by means of an interventional approach are common methods to induce MCA occlusion in nonhuman primates.9 In an MCA occlusion model, the degree and distribution of blood flow depends on the duration of occlusion (transient occlusion for minutes to hours with or without reperfusion compared with permanent occlusion for hours to days with no reperfusion), site of occlusion along the MCA (proximal versus distal part of the artery), and the amount of collateral blood flow into the MCA territory.9,14
The study of gender differences, effects of sex steroids, and sex-specific candidate therapeutics in experimental stroke has been limited primarily to rodent models.25 These issues have not been addressed directly in nonhuman primate models, because many stroke studies involving nonhuman primate subjects reported use of male animals only,6,7,16,18,20,22,33,40 did not indicate the gender of animals used,5,15,17,21,42,44,45 or did not stratify outcomes with respect to sex.12,23 The lack of data from female animals of higher-order species severely limits the translation of experimental data to women requiring treatment for stroke or neuroprotection during stroke. Similarly, the lack of suitably controlled preclinical data in both sexes of higher-order species potentially can lead to poorly designed clinical trials or failure of clinical trials when an agent or therapy of interest may have real benefit (or lack thereof) in a single gender. Nonhuman primate models could be invaluable in stroke studies of gender differences, the response to therapeutics in each gender, and the perimenopausal state. These models might serve as a useful bridge between experimental gender-based studies in rodents and the design of clinical trials in humans, because female nonhuman primate species such as the rhesus macaque have menstrual cycles and hormonal responses analogous to those of female humans.2
We present here the results of a feasibility study in which we developed a model of transient focal cerebral ischemia in male and female rhesus macaques (Macaca mulatta) that would consistently include white matter injury. We also examined whether gender differences in histopathologic outcomes might be evaluated in this model in future, larger experimental trials examining the response to potential new stroke therapeutics initially screened and identified in rodent studies.
Materials and Methods
Animal model.
Adult male (age, 7.6 ± 0.2 y; weight, 9.0 ± 0.9 kg; n = 3) and female (age, 10.2 ± 0.4 y; weight, 5.5 ± 0.6 kg; n = 3) rhesus macaques (Macaca mulatta; Oregon National Primate Research Center, Beaverton, OR) were used for infarct volume determinations and histopathologic analysis. The female rhesus macaques had been used in previous reproductive studies involving laparoscopy and ovarian follicle aspiration, whereas male rhesus macaques had not been used in any previous research studies. Animals were single-housed for experimental studies in double cages (121.9 × 68.6 × 81.3 cm) located in a single room. Animals were fed twice daily (LabDiet 5047, PMI Nutrition International, Richmond, IN) and received produce and other treats daily. Water was available ad libitum. The lights were on 12 h daily, from 0700 to 1900, and the temperature was maintained at 24 ± 2 °C. All animals participated in behavioral management programs to ensure their psychologic health and well-being. The animal care program is compliant with federal and local regulations regarding the care and use of research animals and is AAALAC-accredited. The Oregon Health and Science University Animal Care and Use Committee approved all experiments.
Transient focal cerebral ischemia.
Animals were food-restricted 12 h before anesthesia and surgery. All surgeries were conducted under aseptic conditions by a single surgeon who was not blinded to the animal's gender. To induce stroke, a reversible MCA occlusion procedure was used. Anesthesia was induced (ketamine, 100 mg IM; atropine, 0.2 mg IM), followed by placement of a percutaneous cephalic intravenous catheter to allow for blood sampling as well as administration of fluids (lactated Ringers at 3 to 5 ml/kg hourly) and drugs intraoperatively. Anesthetic depth was increased by using hydromorphone (0.5 mg IV), and the trachea was intubated by using direct laryngoscopy (oral tube, 4.5 Fr). Mechanical ventilation with 1.2% to 1.5% isoflurane in 100% oxygen was used to create a surgical plane of anesthesia and to maintain end-tidal exhaled carbon dioxide (EtCO2) at approximately 30 mm Hg. Tidal volumes were approximately 10 to 15 ml/kg body weight. Appropriate adjustments in tidal volume were made to maintain an inspiratory pressure of less than 20 cm H2O. Anesthetic level was assessed by hemodynamic monitoring and lack of movement (no paralytic agents were used). EtCO2, rectal temperature, pulse oximetry (saturation of peripheral oxygen [SpO2]), heart rate, respiratory rate, systolic and diastolic blood pressure (BP) via a femoral arterial catheter, and arterial blood glucose were monitored throughout preischemia, ischemia, and postischemia. The animals were warmed by air convection heating during surgery and MCA occlusion to maintain comparable periischemic body temperatures among all animal subjects (36.3 ± 0.1 °C, n = 98 measurements for 6 animals).
All animals underwent 90 min of reversible MCA occlusion; this duration was established as adequate to produce marked loss of blood flow in preliminary angiographic studies and to produce histologic injury that was not severe enough to make for a greatly impaired subject. Access to the MCA was via a transorbital approach similar to that previously described.9 Enucleation of the right orbit was performed by using bipolar electrocautery with irrigation to minimize heat transfer. After complete hemostasis was achieved, a small section of the orbital bone superior and lateral to the optic nerve was removed by using a Hall-type neurosurgical drill. The dura was opened, the MCA was exposed, and 2 straight aneurysm clips were placed on the proximal MCA (Figure 1). After placement of the aneurysm clips, the orbit was filled with sterile saline for the 90 min period of occlusion. To reestablish flow, the clips were removed from the MCA, and return of distal perfusion in the artery was confirmed by visual inspection. The posterior orbit was lined with a cyanoacrylate patch, and then the orbit was filled with bone wax at room temperature. Surgery was completed through closure of the right orbit by suturing the eyelids together in a water-tight fashion and removing the femoral arterial catheter. Animals received diazepam (0.5 mg IV) and additional doses of hydromorphone (0.5 mg IV) to facilitate controlled emergence from anesthesia.
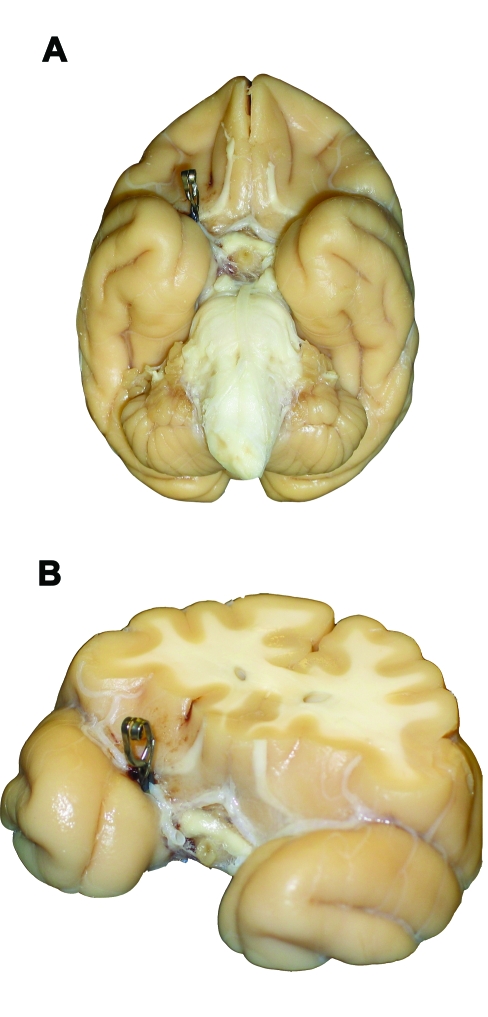
Figure 1.
Perfusion-fixed (A) ventral whole brain and (B) thick coronal section (anterior aspect up) views of a rhesus macaque brain demonstrating aneurysm clip placement on the proximal right middle cerebral artery.
On extubation and initial emergence from anesthesia, all animals demonstrated abnormal circling consistent with brain injury within the MCA-supplied brain region. When it was clear that extubation was successful, the intravenous catheter was removed. After recovery from anesthesia, animals were returned to their home cages and offered food by hand. By 6 h after surgery, all animals were eating without assistance. Postoperatively animals were housed individually and observed for 4 d. Postoperative analgesia consisted of hydromorphone HCl by either the intramuscular (1.0 mg) or oral (2.0 mg) route and buprenorphine (0.3 mg IM). Postoperative evaluations for behavior, food and water consumption, and urine and feces production were done.
Using information from the literature, we chose 4 d of reperfusion after ischemia because this timing would extend beyond the period of early edema, allowing us to make reasonable histologic conclusions. At the end of the 4 d postischemic reperfusion period, animals were placed under sodium pentobarbital (11 mg/kg IV) anesthesia after ketamine induction (15 to 25 mg/kg IM) in preparation for euthanasia and fixation and harvesting of the brain tissues. All animals were euthanized by exsanguination while under a deep surgical plane of anesthesia. After euthanasia, the thoracic cavity was opened by removal of the sternum. The heart was removed, and a 10-gauge cannula was introduced into the common trunk as it originates from the ascending aorta. The brachial arteries were occluded with hemostats at the axillae. Saline flush (1 l) followed by fixative (2 l of 4% phosphate-buffered paraformaldehyde) was delivered by peristaltic pump through the cannula. This technique permitted regional fixation of the head and its contents. The calvarium then was opened and the brain carefully removed, photographed, placed in fixative, and processed for neuropathologic study.
Histopathology.
Persons performing brain histopathologic analyses were blinded to gender. After fixation for a minimum of 1 wk, the brains were weighed and examined for evidence of external injury. Coronal slices were made 1 to 2 mm anterior to the temporal poles and at the anterior margin of the pons. The central block contained the entire extent of the basal ganglia and was prepared for histology. If a gross lesion was apparent in either the anterior or posterior blocks, these were also prepared for histology. The tissue blocks were cryoprotected in a graded series of sucrose (10%, 20%, 30%) in 4% paraformaldehyde. The tissue was frozen in crushed dry ice and then cut on a sliding microtome at a thickness of 50 μm. Sections were collected into 4% paraformaldehyde and stored at 4 °C. The first section was selected randomly, and every 25th section was mounted on a glass slide, defatted in chloroform, and stained with cresyl violet (9 to 12 sections per brain). Sections from all brains were aligned based on the section closest to the point at which the anterior commissure crosses the midline. Each slide was scanned at high resolution and saved as a jpeg file. After examination of the slides by light microscopy, the areas of infarct were outlined in Photoshop (Adobe Systems, San Jose, CA). Infarct volumes were determined for the caudate nucleus, putamen, globus pallidus, claustrum, internal capsule, external capsule, and the entire hemisphere by using SigmaScan software according to the Cavalieri principle.3,24 Any regions of basal forebrain that had traumatic injury due to surgical manipulations other than placement of the 2 aneurysm clips were excluded from analysis.
For 5 of the 6 animals (all male rhesus macaques and female rhesus macaques 1 and 2), representative blocks of pons and medulla were embedded in paraffin, cut at a thickness of 6 microns, and stained with Fluoro-Jade B.34 Sections were examined by fluorescence microscopy for evidence of descending white matter tract degeneration. The brainstem of female rhesus macaque 3 was not examined because there was no injury in the internal capsule and therefore no degeneration of the descending white matter tract.
Statistics.
Values are expressed as mean ± SEM. Differences in total regional infarction volume (mm3, percentage of volume of contralateral structure) were determined for the caudate nucleus, putamen, globus pallidus, claustrum, internal capsule, external capsule, and hemisphere by using Student t tests. Because raw infarct data (mm3) were not normally distributed, a square root transformation was performed for each experimental group, followed by Student t tests comparing between genders the infarct volumes for the caudate nucleus, putamen, globus pallidus, claustrum, internal capsule, external capsule, and hemisphere. The data were analyzed further by looking at the 9 coronal levels that were evaluated in order to determine total infarct volume for each brain region evaluated. A 2-way repeated-measures ANOVA was performed, comparing infarct area (mm2) between genders for caudate nucleus, putamen, globus pallidus, claustrum, internal capsule, external capsule, and hemisphere. The coronal level data were normalized by square root transformation. A 2-way repeated-measures ANOVA was performed that compared the normalized infarct areas (mm2) between the genders for caudate nucleus, putamen, globus pallidus, claustrum, internal capsule, external capsule, and hemisphere. Differences in age and body weight were determined with Student t tests. Physiologic parameters underwent 2-way ANOVA with post hoc Student Newman–Keuls test. Statistical significance was defined at a P value of less than 0.05. All statistical analyses were performed by using SigmaStat statistical software (version 3.0; SPSS, Chicago, IL).
Results
The female rhesus macaques in our study were older than the males (10.2 ± 0.4 y and 7.6 ± 0.2 y, respectively; P < 0.05) and weighed less than the males (5.5 ± 0.6 kg and 9.0 ± 0.9 kg, respectively; P < 0.05). Heart rate, systolic BP, EtCO2, rectal temperatures, and glucose were comparable between male and female animals during preischemia and ischemia (Table 1). Female rhesus macaques had a higher (P < 0.05) preischemic diastolic BP and a lower (P < 0.05) ischemic respiratory rate than did the male animals (Table 1). Within each experimental group, clinically small but statistically significant (P < 0.05) differences in systolic BP, diastolic BP, respiratory rate, EtCO2, rectal temperatures, and glucose between preischemia and ischemia were present (Table 1). SpO2 ranged from 90% to 100% for all experimental animals at all time intervals monitored. In addition, anesthetic requirement, perioperative drug administration, and surgical preparation times were equivalent between male and female rhesus macaques (data not shown). No mortality occurred in either experimental group. All animals demonstrated abnormal circling after experiencing ischemia, and none were excluded due to lack of neurologic deficits.
Table 1.
Summary of physiologic parameters before (preischemia) and during (ischemia) 90 min of middle cerebral artery occlusion in male and female rhesus macaques
| Heart rate (beats per minute) | Systolic BP (mm Hg) | Diastolic BP (mm Hg) | Respiratory rate (breaths per minute) | EtCO2 (mm Hg) | Rectal temperature (°C) | Glucose (mg/dl) | |
| Male macaques | |||||||
| Preischemia | 120 ± 2 (n = 26) | 74 ± 3 (n = 20) | 48 ± 2 (n = 20) | 17 ± 1 (n = 26) | 32 ± 2 (n = 26) | 35.5 ± 0.1 (n = 25) | 53 ± 6 (n = 4) |
| Ischemia | 115 ± 2 (n = 22) | 93 ± 3 (n = 22)a | 60 ± 1 (n = 22)a | 15 ± 1 (n = 19) | 27 ± 1 (n = 22)a | 36.5 ± 0.2 (n = 22)a | 69 ± 2 (n = 5)a |
| Female macaques | |||||||
| Preischemia | 122 ± 4 (n = 25) | 81 ± 5 (n = 17) | 55 ± 2 (n = 17)b | 16 ± 1 (n = 23) | 32 ± 2 (n = 25) | 35.9 ± 0.3 (n = 24) | 62 ± 5 (n = 5) |
| Ischemia | 116 ± 2 (n = 19) | 92 ± 6 (n = 19) | 59 ± 2 (n = 19) | 12 ± 0 (n = 19)ab | 25 ± 1 (n = 19)a | 37.0 ± 0.2 (n = 19)a | 64 ± 2 (n = 8) |
n, total number of measurements made from 3 animals within an experimental group during a specific time interval
P < 0.05 compared with preischemia within an experimental group
P < 0.05 compared with males within a time interval
Histologic evaluation of multiple sections from each animal showed subacute ischemic injury of variable size (Figure 2). All animals had evidence of injury to the subcortex (striatum), consistent with proximal MCA occlusion. The lesions ranged from areas of neuronal injury with relative preservation of vasculature to complete tissue necrosis. Infiltrates of neutrophils and macrophages were seen at the margins of most lesions. The animals with the smallest infarcts (females 2 and 3) showed focal injury in the external segment of the globus pallidus and putamen, with extension into the external capsule. In animals with larger infarcts (female 1, males 2 and 3), the caudate nucleus and putamen were most extensively involved and often extended into the external capsule and adjacent deep subcortical white matter. Internal capsule involvement was variable, but when present, was always adjacent to damaged areas of caudate nucleus or putamen. Isolated small lesions in white matter were not observed. Large infarcts demonstrated mass effect (tissue expansion) at 4 d of reperfusion after ischemia, with compression of the ipsilateral lateral ventricle and midline shift. Neocortical injury was not noted in any of these animals. Fluoro-Jade staining did not reveal degenerating axons in the brainstem at 4 d of reperfusion after ischemia.
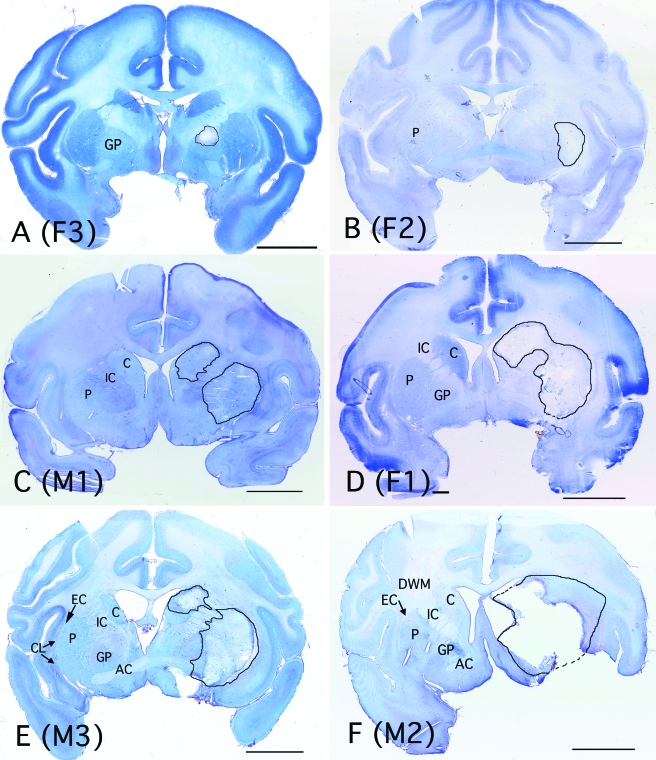
Figure 2.
Low-power images of sections from each animal at the level of the anterior commissure (except A), demonstrating the range of injury seen in this model, from (A) least to (F) most severe. The image in (A) is from a slightly more caudal level, to illustrate the maximal size of the lesion in this animal. Areas of injury are outlined in black, and the corresponding brain regions are labeled on the contralateral side. Individual animals are identified as male (M) or female (F) and by the individual animal identification numbers listed in Table 2. AC, anterior commissure; C, caudate; CL, claustrum; DWM, deep white matter; EC, external capsule; GP, globus pallidus; IC, internal capsule; and P, putamen. Bar, 1 cm.
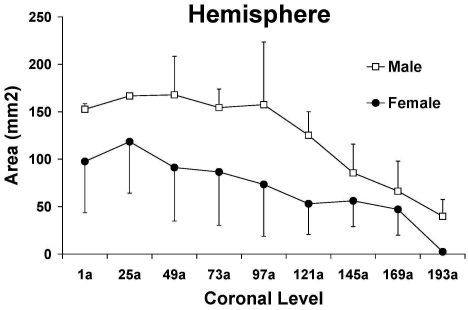
The regional injury volumes (Table 2) and representative coronal sections (Figure 2) showed no significant differences between male and female rhesus macaques (Table 2). In addition, no significant differences in infarct area were detected at each coronal level evaluated for caudate nucleus, putamen, globus pallidus, claustrum, internal capsule, external capsule, and hemisphere (Figure 3; data for hemisphere shown as representative). However, based on the larger SEMs (Table 2) and ranges (that is, minimum value subtracted from maximum value) of infarction volumes (mm3, % of volume of contralateral structure) within each gender in the two brain regions with the largest mean infarct injury, female macaques appeared to show more variability than did male macaques in ischemic damage in the caudate nucleus (female: range, 342 mm3, 120%; male: range 217 mm3, 54%) and putamen (female: range, 626 mm3, 141%; male: range, 198 mm3, 37%). In the hemisphere, female macaques had a larger SEM (Table 2) and a comparable or higher range of infarction volumes (female: range, 1321 mm3, 24%; male: range, 1323 mm3, 20%), suggesting more variability within the female group as compared with the male macaques.
Table 2.
Regional and total hemispheric infarction volumes (in mm3and % of contralateral structure; mean ± SEM) as determined in coronal sections stained with cresyl violet from male and female rhesus macaques (Macaca mulatta) subjected to 90 min of middle cerebral artery occlusion followed by 4 d of reperfusion
| Caudate nucleus mm3 (%) | Putamen mm3 (%) | Globus pallidus mm3 (%) | Claustrum mm3 (%) | Internal capsule mm3 (%) | External capsule mm3 (%) | Hemisphere mm3 (%) | |
| Male 1 | 105 (69%) | 409 (86%) | 4 (2%) | 7 (8%) | 0 (0%) | 1 (2%) | 574 (8%) |
| Male 2 | 225 (99%) | 483 (110%) | 98 (59%) | 51 (69%) | 118 (59%) | 64 (67%) | 1897 (28%) |
| Male 3 | 322 (123%) | 607 (123%) | 36 (23%) | 10 (10%) | 16 (6%) | 73 (106%) | 1314 (17%) |
| Male macaques (n = 3) | 217 ± 63 (97% ± 16%) | 499 ± 58 (106% ± 11%) | 46 ± 28 (28% ± 17%) | 23 ± 14 (29% ± 20%) | 45 ± 37 (22% ± 19%) | 46 ± 23 (58% ± 30%) | 1262 ± 383 (18% ± 6%) |
| Female 1 | 342 (120%) | 629 (141%) | 56 (36%) | 31 (38%) | 141 (69%) | 45 (109%) | 1654 (26%) |
| Female 2 | 35 (15%) | 218 (45%) | 13 (6%) | 3 (4%) | 0 (0%) | 30 (32%) | 333 (4%) |
| Female 3 | 0 (0%) | 3 (0%) | 88 (28%) | 2 (1%) | 0 (0%) | 47 (25%) | 355 (2%) |
| Female macaques (n = 3) | 125 ± 109 (45% ± 38%) | 283 ± 184 (62% ± 42%) | 53 ± 22 (23% ± 9%) | 12 ± 10 (14% ± 12%) | 47 ± 47 (23% ± 23%) | 41 ± 5 (55% ± 27%) | 781 ± 437 (10% ± 8%) |
Figure 3.
Cresyl violet-determined total hemispheric infarction area (mm2) for 9 coronal levels (anterior to posterior, 1a to 193a) approximately 1.25 mm apart in male (open symbols) and female (filled symbols) rhesus macaques subjected to 90 min of middle cerebral artery occlusion followed by 96 h reperfusion. Values are mean ± SEM.
Discussion
The present study has several important findings. First, the described rhesus macaque model of focal cerebral ischemia involving reversible MCA occlusion through a transorbital approach and temporary aneurysm clip placement is feasible and consistently produces white matter injury in both sexes. Second, the observed variability in injury volume is comparable to that in human focal cerebrovascular ischemia and in other nonhuman primate models of proximal MCA occlusion.9 Finally, the amount of injury did not differ between male and female rhesus macaques in this small sample. Given the larger observed SEMs and range of infarction volumes, female rhesus macaques appeared to show greater variability in ischemic damage within the areas of caudate nucleus, putamen, and overall hemisphere, than did their male counterparts. These data suggest that a larger study of experimental stroke in female rhesus macaques is warranted, with comparison with male rhesus macaques matched for age and disease morbidities.
One of the challenges in designing stroke studies in higher-order species such as nonhuman primates, which are expensive and of limited availability, is determining a reasonable sample size in light of statistical evaluations and logistical concerns. For example, in our study, the cost of a stroke study in a rhesus macaque (US $7000 to US $8000 per animal) is approximately 15 to 20 times the cost of doing a comparable study in mice or rats (US $200 to US $400 per animal). We used the data from this feasibility study to perform a power analysis for the 2 brain regions with the greatest damage regardless of gender (caudate nucleus and putamen) and for hemisphere. According to these power analyses, 16 to 42 animals would be needed in this model to detect significant gender differences in mm3 and 14 to 37 animals would be needed to determine significant gender differences in percentage of volume of contralateral structure in the caudate nucleus (n = 42 for mm3, n = 14 for %), putamen (n = 16 for mm3, n = 19 for %), and hemisphere (n = 36 for mm3, n = 37 for %). However, if the experimental design controls for some of the factors that potentially contribute to variability within and between these groups, reducing the group size needed to detect gender differences in our model for future studies may be possible.
Clinical relevance of model.
Human stroke is a diverse disease in terms of causes, manifestations, variability in local vascular anatomy, anatomic sites of ischemia,4 and gender-linked effects.1,25 Many animal stroke models have been described,14 but no single animal model fully mimics human stroke because of the heterogeneity of clinical disease. Because nonhuman primate stroke models have not addressed systematically the role of biologic sex on ischemic outcome, we felt it was important to validate the utility of the present model as a tool for studying differences (and similarities) in male and female brain injury. Furthermore, rodent models of proximal MCA occlusion have limitations. For example, although extensive infarction in dorsolateral cerebral cortex and underlying striatum is evident in rat and mouse,14,37 white matter injury is modest or inconsistent. Furthermore, significant temporal and topographical features differ in rodent versus primate neuronal and microvascular responses to ischemia and reperfusion.39 Therefore, the transition from rodent models to humans may be difficult without the intermediate step of evaluating therapy in gyrencephalic brains in which significant white matter injury occurs. Accordingly, current guidelines published by the Stroke Therapy Academic Industry Roundtable (STAIR) recommend that initial drug development studies be accomplished in species such as rodents, followed by testing in higher-order species, such as nonhuman primates.37
Factors contributing to ischemic variability in female rhesus macaques.
The present feasibility study used a small number of animals and so cannot support definitive statements about sex-specific responses to MCA occlusion. In addition to small sample size, multiple factors including age, parity status, and hormonal status may have contributed to variability in outcomes. Age is an important risk factor affecting stroke risk and outcome, with increasing age generally leading to greater susceptibility to and poorer recovery from stroke.1,28,32 In the present study, female rhesus macaques (age, 10.2 ± 0.4 y; range, 9.4 to 10.8 y) were significantly older than male rhesus macaques (age, 7.6 ± 0.2 y; range, 7.3 to 7.9 y), even though the female rhesus macaques (weight, 5.5 ± 0.6 kg; range, 4.9 to 6.6 kg) weighed significantly less than did the male animals (weight, 9.0 ± 0.9 kg; range, 7.4 to 10.5 kg). Future studies addressing gender differences should use animals as close in age as possible, depending on availability. The variability observed in the female rhesus macaques may also be influenced by parity status. An association between increasing parity and increased risk for cardiovascular diseases, including stroke has been suggested.11,19,29,43 The 2 female rhesus macaques with the smallest infarct volumes (nos. 2 and 3) were nulliparous, whereas the female with the largest infarct volume (female rhesus macaque 1) had had 1 stillbirth and 1 live birth approximately 3 and 3.5 y, respectively before experimental stroke surgery.
The variability of female infarct volumes may likewise be due to differences in cycling hormones, which are known to shape outcomes in experimental stroke.25,35 The number of days between each female rhesus macaque's last observed menses and surgery ranged from 34 to 58 d (45 ± 7 d). Because hormone levels and reproductive tract pathology were not evaluated concurrently with brain histopathology, we were not able to stage the menstrual phase (luteal versus follicular) of individual females at the time of ischemic injury. Therefore we cannot rule out the possibility that these females may have had different hormonal levels during cerebral ischemia and reperfusion, which in turn could have contributed to heterogeneity in response.
Future stroke studies using female rhesus macaques should control for parity status and menstrual cycle variability based on detailed reproductive records. In addition, hormone levels (estradiol, progesterone, luteinizing hormone, follicle stimulating hormone) should be measured at the time of ischemia and at reperfusion end points to correlate hormone levels with ischemic outcomes. We further suggest evaluating uterine and ovarian histopathology at the same time as brain neuropathology to stage where females are in their menstrual cycles.
Factors contributing to overall ischemic variability.
The animals used in this cerebral ischemia study were not specifically matched for age or history of comorbidities. Nevertheless, all had comparable medical histories with prior episodes of intermittent diarrhea that responded well to therapy. All of the male rhesus macaques and female rhesus macaques 1 and 2 had been treated on multiple occasions for wounds due to fighting with conspecifics or other trauma. All of the female rhesus macaques and 1 of the male rhesus macaques (male 3) had been treated for vomiting on at least 1 occasion. Another of the male rhesus macaques (male 2) had received successful treatment for an abscess, rectal prolapse, and hematuria. In addition, previous experimental use of these animals may have influenced ischemic outcomes. For example, none of the male rhesus macaques had been used in previous studies, whereas all of the female rhesus macaques had been used in reproductive studies involving ovarian follicle aspiration (females 1 and 2), gonadotropin injections (all females), and laparoscopy (female 3). We cannot rule out the possibility that previous gonadotropin exposure in all of the females may have affected outcomes as gonadotropin treatment for infertility may be linked to the development of cerebral infarction in women.8 Nevertheless, the health heterogeneity of these nonhuman primates is likely to simulate that of humans who experience ischemic stroke and cerebrovascular disease.
Variability during the perioperative period might have contributed to the observed variability observed in male and female rhesus macaques relative to ischemic damage. However, several measures were taken to reduce perisurgical variability. First, a single surgeon performed all of the MCA occlusion surgeries. Second, the time from anesthesia induction until MCA occlusion was not significantly different between male (124 ± 5 min) and female rhesus macaques (112 ± 10 min). All animals underwent 90 min of ischemia. Time from initiation of reperfusion until cessation of anesthesia was not statistically different between male (20 ± 5 min) and female rhesus macaques (25 ± 5 min). Therefore the duration of anesthesia, surgery, and ischemia was comparable among all animals regardless of gender. Third, blood loss during surgery was comparable (≤10 ml) between male and female rhesus macaques. Lastly, although all of the male rhesus macaques and 1 of the female rhesus macaques had the same anesthetist and the other 2 female rhesus macaques each had a different anesthetist, the type and amounts of medications given during the perioperative period (ketamine, atropine, hydromorphone, dextrose, valium) as well as the percentage of isoflurane used for induction and maintenance were not significantly different between male and female rhesus macaques. Therefore, it is unlikely that surgical and anesthetic variability during the perioperative period appreciably contributed to ischemic damage variability.
Vascular anatomy and thus collateral cerebral blood flow could have contributed to the infarct variability present within the female and male groups. Just as in humans, variability in local vascular anatomy can occur in macaques.9 Future stroke studies should address the issue of vascular anatomy and collateral cerebral blood flow. We recommend that periischemic cerebral blood flow imaging modalities, such as magnetic resonance imaging and computed tomography, as well as follow-up studies evaluating regional cerebral blood flow be done in future studies using this stroke model.
Although we did not measure PaCO2 in this study, we did measure EtCO2, which should correlate with PaCO2.36,41 However, in our study, the average preischemic and ischemic EtCO2 values were below 35 mm Hg (range, 22 to 55 mm Hg). Therefore, our animals likely were studied under hypocapnic conditions, although EtCO2 values were comparable between male and female rhesus macaques during preischemia and ischemia. Consequently, any effect of low EtCO2 (hypocapnic) values during preischemia and ischemia were similar and present in each experimental group, thereby still allowing us to examine the effect of gender on infarction volume outcomes.
Summary.
Our model closely mimics ischemic stroke as observed in both men and women, including considerations of comorbidities, risk factors, and heterogeneous medical histories. This report is the first to compare cerebral ischemic outcomes in female and male rhesus macaques.
Acknowledgments
The authors would like to recognize the excellent surgical support and postoperative care provided by Drs John Fanton and Theodore Hobbs and their staff in the Surgical Services Unit (Division of Animal Resources, Oregon National Primate Research Center [ONPRC], Beaverton, OR) for all of the animals used in this study. We also acknowledge the Pathology Unit at ONPRC (Beaverton, OR), which performed fixation and harvesting of all rhesus macaque brains. Lastly, the authors recognize Nahideh Nilforoushan (Department of Anesthesiology and Peri-Operative Medicine, Oregon Health and Science University, Portland, OR), who prepared the histologic sections for evaluation.
References
- 1.American Heart Association [Internet] Heart disease and stroke statistics—2008 Update [cited 24 Jan 2008]. Available at http://www.americanheart.org/downloadable/heart/1200078608862HS_Stats%202008.final.pdf.
- 2.Bellino FL, Wise PM. 2003. Nonhuman primate models of menopause workshop. Biol Reprod 68:10–18 [DOI] [PubMed] [Google Scholar]
- 3.Cavalieri B. 1635 Geometria indivibilibus continuorum. Bononi: typis Clementis Ferronij. Reprint from (1966): Geometria degli indivisibili. Torino (Italy): Unione Tipografico-Editrice Torinese; 1966 [Google Scholar]
- 4.Cramer SC. 2003. Clinical issues in animal models of stroke and rehabilitation. ILAR J 44:83–84 [DOI] [PubMed] [Google Scholar]
- 5.Crowell RM, Olsson Y, Klatzo I, Ommaya A. 1970. Temporary occlusion of the middle cerebral artery in the monkey: clinical and pathological observations. Stroke 1:439–448 [DOI] [PubMed] [Google Scholar]
- 6.D'Arceuil HE, Duggan M, He J, Pryor J, de Crespigny A. 2006. Middle cerebral artery occlusion in Macaca fascicularis: acute and chronic stroke evolution. J Med Primatol 35:78–86 [DOI] [PubMed] [Google Scholar]
- 7.Del Zoppo GJ, Copeland BR, Harker LA, Waltz TA, Zyroff J, Hanson SR, Battenberg E. 1986. Experimental acute thrombotic stroke in baboons. Stroke 17:1254–1265 [DOI] [PubMed] [Google Scholar]
- 8.Espensen MK, Baggesen KL. 2005. Ischemic cerebral infarction in a young woman undergoing gonadotropin treatment for infertility. Ugeskr Laeger 167:2901–2903 [PubMed] [Google Scholar]
- 9.Fukuda S, del Zoppo GJ. 2003. Models of focal cerebral ischemia in the nonhuman primate. ILAR J 44:96–104 [DOI] [PubMed] [Google Scholar]
- 10.Fullerton HJ, Wu YW, Zhao S, Johnston SC. 2003. Risk of stroke in children: ethnic and gender disparities. Neurology 61:189–194 [DOI] [PubMed] [Google Scholar]
- 11.Gaist D, Pedersen L, Cnattinguis S, Sorensen HT. 2004. Parity and risk of subarachnoid hemorrhage in women: a nested case-control study based on national Swedish registries. Stroke 35:28–33 [DOI] [PubMed] [Google Scholar]
- 12.Gao H, Liu Y, Lu S, Xiang B, Wang C. 2006. A reversible middle cerebral artery occlusion model using intraluminal balloon technique in monkeys. J Stroke Cerebrovasc Dis 15:202–208 [DOI] [PubMed] [Google Scholar]
- 13.Giroud M, Milan C, Beuriat P, Gras P, Essayagh E, Arveux P, Dumas R. 1991. Incidence and survival rates during a 2-year period of intracerebral and subarachnoid haemorrhages, cortical infarcts, lacunes, and transient ischaemic attacks: the stroke registry of Dijon. Int J Epidemiol 20:892–899 [DOI] [PubMed] [Google Scholar]
- 14.Graham SM, McCullough LD, Murphy SJ. 2004. Animal models of ischemic stroke: balancing experimental aims and animal care. Comp Med 54:486–496 [PubMed] [Google Scholar]
- 15.Hirouchi Y, Suzuki E, Mitsuoka C, Jin H, Kitajima S, Kohjimoto Y, Enomoto M, Kugino K. 2007. Neuroimaging and histopathological evaluation of delayed neurological damage produced by artificial occlusion of the middle cerebral artery in Cynomolgus monkeys—establishment of a monkey model for delayed cerebral ischemia. Exp Toxicol Pathol 59:9–16 [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Mocco J, Choudhri TF, Poisik A, Popilskis SJ, Emerson R, DelaPaz RL, Khandji AG, Pinsky DJ, Connolly S., Jr 2000. A modified transorbital baboon model of reperfused stroke. Stroke 31:3054–3063 [DOI] [PubMed] [Google Scholar]
- 17.Hudgins WR, Garcia JH. 1970. Transorbital approach to the middle cerebral artery of the squirrel monkey: a technique for experimental cerebral infarction applicable to ultrastructural studies. Stroke 1:107–111 [DOI] [PubMed] [Google Scholar]
- 18.Kito G, Nishimura A, Susumu T, Nagata R, Kuge Y, Yokota C, Minematsu K. 2001. Experimental thromboembolic stroke in cynomolgus monkey. J Neurosci Methods 105:45–53 [DOI] [PubMed] [Google Scholar]
- 19.Koski-Rahikkala H, Pouta A, Pietilainen K, Hartikainen AL. 2006. Does parity affect mortality among parous women? J Epidemiol Community Health 60:968–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuge Y, Yokota C, Tagaya M, Hasegawa Y, Nishimura A, Kito G, Tamaki N, Hashimoto N, Yamaguchi T, Minematsu K. 2001. Serial changes in cerebral blood flow and flow-metabolism uncoupling in primates with acute thromboembolic stroke. J Cereb Blood Flow Metab 21:202–210 [DOI] [PubMed] [Google Scholar]
- 21.Liu XG, Branston NM, Kawauchi M, Symon L. 1992. A model of acute focal ischemia in the territory of the anterior cerebral artery in baboons. Stroke 23:40–44 [DOI] [PubMed] [Google Scholar]
- 22.Maeda M, Takamatsu H, Furuichi Y, Noda A, Awaga Y, Tatsumi M, Yamammoto M, Ichise R, Nishimura S, Matsuoka N. 2005. Characterization of a novel thrombotic middle cerebral artery occlusion model in monkeys that exhibits progressive hypoperfusion and robust cortical infarction. J Neurosci Methods 146:106–115 [DOI] [PubMed] [Google Scholar]
- 23.Molinari GF, Moseley JI, Laurent JP. 1974. Segmental middle cerebral artery occlusion in primates: an experimental method requiring minimal surgery and anesthesia. Stroke 5:334–339 [DOI] [PubMed] [Google Scholar]
- 24.Mouton PR. 2002. Principles and practices of unbiased stereology: an introduction for bioscientists. Baltimore: The Johns Hopkins University Press [Google Scholar]
- 25.Murphy SJ, McCullough LD, Smith JM. 2004. Stroke in the female: role of biological sex and estrogen. ILAR J 45:147–159 [DOI] [PubMed] [Google Scholar]
- 26.Peterson JN, Evans JP. 1937. The anatomical end results of cerebral artery occlusion: an experimental and clinical correlation. Trans Am Neurol Assoc 63:83–88 [Google Scholar]
- 27.Physicians' Health Study Research Group Steering Committee 1989. Final report on the aspirin component of the ongoing Physicians' Health Study. N. Engl. J. Med. 321:129–135 [DOI] [PubMed] [Google Scholar]
- 28.Popa-Wagner A, Carmichael ST, Kokaia Z, Kessler C, Walker LC. 2007. The response of the aged brain to stroke: too much, too soon? Curr Neurovasc Res 4:216–227 [DOI] [PubMed] [Google Scholar]
- 29.Qureshi AI, Giles WH, Croft JB, Stern B. 1997. Number of pregnancies and risk for stroke and stroke subtypes. Arch Neurol 54:203–206 [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. 2005. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 352:1293–1304 [DOI] [PubMed] [Google Scholar]
- 31.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA. 1998. Stroke incidence among white, black, and Hispanic residents of an urban community: the northern Manhattan stroke study. Am J Epidemiol 147:259–268 [DOI] [PubMed] [Google Scholar]
- 32.Schaller BJ. 2007. Influence of age on stroke and preconditioning-induced ischemic tolerance in the brain. Exp Neurol 205:9–19 [DOI] [PubMed] [Google Scholar]
- 33.Scheller MS, Grafe MR, Zornow MH, Fleischer JE. 1992. Effects of ischemia duration on neurological outcome, CA1 histopathology, and nonmatching to sample learning in monkeys. Stroke 23:1471–1478 [DOI] [PubMed] [Google Scholar]
- 34.Schmued LC, Hopkins KJ. 2000. Fluoro-Jade B: a high-affinity fluorescent marker for the localization of neuronal degeneration. Brain Res 874:123–130 [DOI] [PubMed] [Google Scholar]
- 35.Singh M, Sumieh N, Kyser C, Simpkins JW. 2008. Estrogens and progesterones as neuroprotectants: what animal models teach us. Front Biosci 13:1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith JC, Danneman PJ. 2008. Monitoring of anesthesia, p 178. Fish RE, Brown MJ, Danneman PJ, Karas AZ. Anesthesia and analgesia in laboratory animals, 2nd ed. San Diego, Academic Press, Inc [Google Scholar]
- 37.Stroke Therapy Academic Industry Roundtable (STAIR) 1999. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 30:2752–2758 [DOI] [PubMed] [Google Scholar]
- 38.Sudlow CL, Warlow CP, for the International Stroke Collaboration 1997. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. Stroke 28:491–499 [DOI] [PubMed] [Google Scholar]
- 39.Tagaya M, Liu KF, Copeland B, Seiffert D, Engler R, Garcia JH, del Zoppo GJ. 1997. DNA scission after focal brain ischemia: temporal differences in two species. Stroke 28:1245–1254 [DOI] [PubMed] [Google Scholar]
- 40.Takamatsu H, Tsukada H, Kakiuchi T, Nishiyama S, Noda A, Umemura K. 2000. Detection of reperfusion injury using PET in a monkey model of cerebral ischemia. J Nucl Med 41:1409–1416 [PubMed] [Google Scholar]
- 41.Takano Y, Sakamoto O, Kiyofuji C, Ito K. 2003. A comparison of the end-tidal CO2 measured by portable capnometer and the arterial pCO2 in spontaneously breathing patients. Respir Med 97:476–481 [DOI] [PubMed] [Google Scholar]
- 42.Watanabe O, Bremer AM, West CR. 1977. Experimental regional cerebral ischemia in the middle cerebral artery territory in primates. Part 1: angio-anatomy and description of an experimental model with selective embolization of the internal carotid artery bifurcation. Stroke 8:61–70 [DOI] [PubMed] [Google Scholar]
- 43.Wolff B, Volzke H, Robinson D, Schwahn C, Ludemann J, Kessler C, John U, Felix SB. 2005. Relation of parity with common carotid intima–media thickness among women of the study of health in Pomerania. Stroke 36:938–943 [DOI] [PubMed] [Google Scholar]
- 44.Yonas H, Gur D, Claassen D, Wolfson SK, Jr, Moossy J. 1990. Stable xenon-enhanced CT measurement of cerebral blood flow in reversible focal ischemia in baboons. J Neurosurg 73:266–273 [DOI] [PubMed] [Google Scholar]
- 45.Young AR, Touzanni O, Derlon JM, Sette G, MacKenzie ET, Baron JC. 1997. Early reperfusion in the anesthetized baboon reduces brain damage following middle cerebral artery occlusion. Stroke 28:632–638 [DOI] [PubMed] [Google Scholar]