Abstract
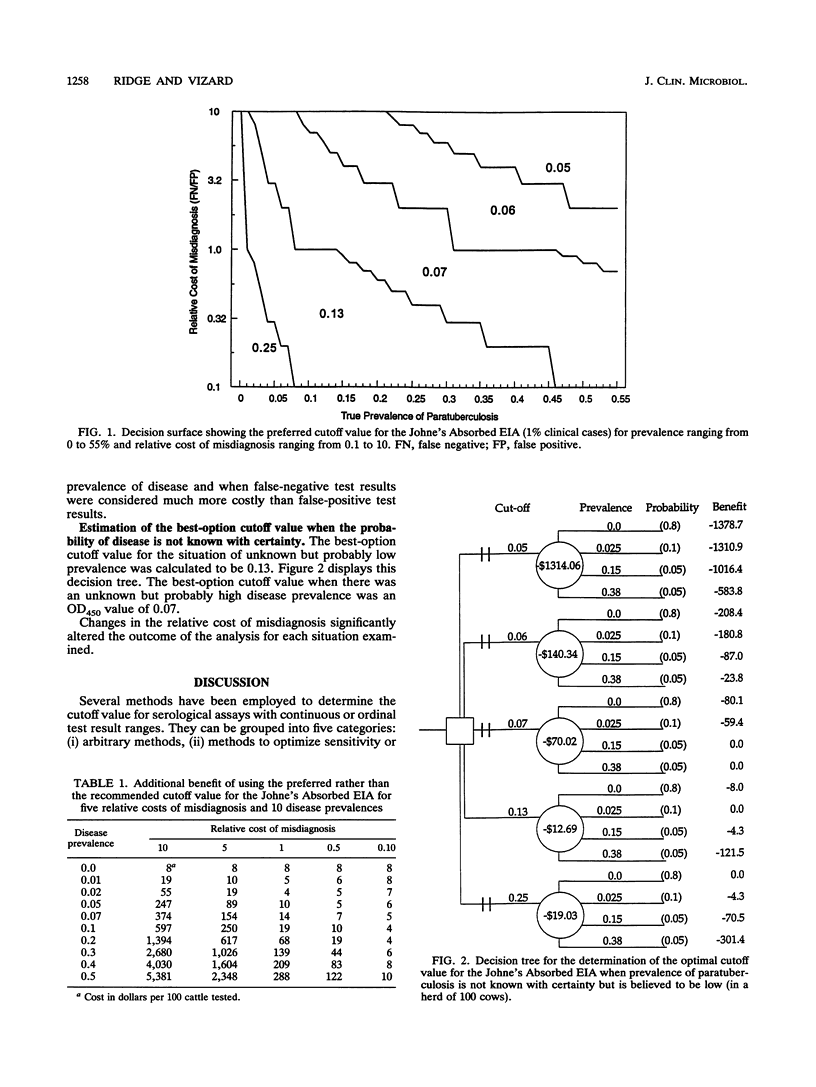
Traditionally, in order to improve diagnostic accuracy, existing tests have been replaced with newly developed diagnostic tests with superior sensitivity and specificity. However, it is possible to improve existing tests by altering the cutoff value chosen to distinguish infected individuals from uninfected individuals. This paper uses data obtained from an investigation of the operating characteristics of the Johne's Absorbed EIA to demonstrate a method of determining a preferred cutoff value from several potentially useful cutoff settings. A method of determining the financial gain from using the preferred rather than the current cutoff value and a decision analysis method to assist in determining the optimal cutoff value when critical population parameters are not known with certainty are demonstrated. The results of this study indicate that the currently recommended cutoff value for the Johne's Absorbed EIA is only close to optimal when the disease prevalence is very low and false-positive test results are deemed to be very costly. In other situations, there were considerable financial advantages to using cutoff values calculated to maximize the benefit of testing. It is probable that the current cutoff values for other diagnostic tests may not be the most appropriate for every testing situation. This paper offers methods for identifying the cutoff value that maximizes the benefit of medical and veterinary diagnostic tests.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castillo R. M., Grados P., Carcamo C., Miranda E., Montenegro T., Guevara A., Gilman R. H. Effect of treatment on serum antibody to Hymenolepis nana detected by enzyme-linked immunosorbent assay. J Clin Microbiol. 1991 Feb;29(2):413–414. doi: 10.1128/jcm.29.2.413-414.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell F. A., Koepsell T. D. Measures of gain in certainty from a diagnostic test. Am J Epidemiol. 1985 May;121(5):744–753. doi: 10.1093/aje/121.5.744. [DOI] [PubMed] [Google Scholar]
- Cox J. C., Drane D. P., Jones S. L., Ridge S., Milner A. R. Development and evaluation of a rapid absorbed enzyme immunoassay test for the diagnosis of Johne's disease in cattle. Aust Vet J. 1991 May;68(5):157–160. doi: 10.1111/j.1751-0813.1991.tb03167.x. [DOI] [PubMed] [Google Scholar]
- Decoster A., Slizewicz B., Simon J., Bazin C., Darcy F., Vittu G., Boulanger C., Champeau Y., Demory J. L., Duhamel M. Platelia-Toxo IgA, a new kit for early diagnosis of congenital toxoplasmosis by detection of anti-P30 immunoglobulin A antibodies. J Clin Microbiol. 1991 Oct;29(10):2291–2295. doi: 10.1128/jcm.29.10.2291-2295.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio J. R., Varga F. J., Conwell D. L., Kraft J. A., Kozak K. J., Willis D. H. Development of a rapid enzyme immunoassay for Clostridium difficile toxin A and its use in the diagnosis of C. difficile-associated disease. J Clin Microbiol. 1991 Dec;29(12):2724–2730. doi: 10.1128/jcm.29.12.2724-2730.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdman D. D., Anderson L. J., Adams D. R., Stewart J. A., Markowitz L. E., Bellini W. J. Evaluation of monoclonal antibody-based capture enzyme immunoassays for detection of specific antibodies to measles virus. J Clin Microbiol. 1991 Jul;29(7):1466–1471. doi: 10.1128/jcm.29.7.1466-1471.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M., Kirihara J., Mills J. Enzyme-linked immunoassay for human immunodeficiency virus type 1 envelope glycoprotein 120. J Clin Microbiol. 1991 Jan;29(1):142–147. doi: 10.1128/jcm.29.1.142-147.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonik B., Seibel M., Berkowitz A., Woodin M. B., Mills K. Comparison of two enzyme-linked immunosorbent assays for detection of herpes simplex virus antigen. J Clin Microbiol. 1991 Mar;29(3):436–438. doi: 10.1128/jcm.29.3.436-438.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K., Pii K., Lebech A. M. Improved immunoglobulin M serodiagnosis in Lyme borreliosis by using a mu-capture enzyme-linked immunosorbent assay with biotinylated Borrelia burgdorferi flagella. J Clin Microbiol. 1991 Jan;29(1):166–173. doi: 10.1128/jcm.29.1.166-173.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T. M., Malone G. E., Khabbaz R. F., Lal R. B., Kaplan J. E. Evaluation of a recombinant human T-cell lymphotropic virus type I (HTLV-I) p21E antibody detection enzyme immunoassay as a supplementary test in HTLV-I/II antibody testing algorithms. J Clin Microbiol. 1991 Jun;29(6):1125–1127. doi: 10.1128/jcm.29.6.1125-1127.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles D. P., Jr, Perryman L. E., Kappmeyer L. S., Hennager S. G. Detection of equine antibody to Babesia equi merozoite proteins by a monoclonal antibody-based competitive inhibition enzyme-linked immunosorbent assay. J Clin Microbiol. 1991 Sep;29(9):2056–2058. doi: 10.1128/jcm.29.9.2056-2058.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal R. B., Heneine W., Rudolph D. L., Present W. B., Hofhienz D., Hartley T. M., Khabbaz R. F., Kaplan J. E. Synthetic peptide-based immunoassays for distinguishing between human T-cell lymphotropic virus type I and type II infections in seropositive individuals. J Clin Microbiol. 1991 Oct;29(10):2253–2258. doi: 10.1128/jcm.29.10.2253-2258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. W. The evaluation of tests. Can J Comp Med. 1977 Jan;41(1):19–25. [PMC free article] [PubMed] [Google Scholar]
- Metz C. E. Basic principles of ROC analysis. Semin Nucl Med. 1978 Oct;8(4):283–298. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- Montagna R. A., Papsidero L., Poiesz B. J. Evaluation of a solid-phase immunoassay for the simultaneous detection of antibodies to human immunodeficiency virus type 1 and human T-cell lymphotropic virus type I. J Clin Microbiol. 1991 May;29(5):897–900. doi: 10.1128/jcm.29.5.897-900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R., Plambeck T., Foged N. T. Blocking enzyme-linked immunosorbent assay for detection of antibodies to Actinobacillus pleuropneumoniae serotype 2. J Clin Microbiol. 1991 Apr;29(4):794–797. doi: 10.1128/jcm.29.4.794-797.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J. G., Scott T. W., Lorenz L. H., Hubbard J. L. Enzyme immunoassay for detection of antibodies against eastern equine encephalomyelitis virus in sentinel chickens. J Clin Microbiol. 1991 Jul;29(7):1457–1461. doi: 10.1128/jcm.29.7.1457-1461.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerregaard A., Shand G. H., Gaarslev K., Espersen F. Comparison of crossed immunoelectrophoresis, enzyme-linked immunosorbent assays, and tube agglutination for serodiagnosis of Yersinia enterocolitica serotype O:3 infection. J Clin Microbiol. 1991 Feb;29(2):302–309. doi: 10.1128/jcm.29.2.302-309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge S. E., Morgan I. R., Sockett D. C., Collins M. T., Condron R. J., Skilbeck N. W., Webber J. J. Comparison of the Johne's absorbed EIA and the complement-fixation test for the diagnosis of Johne's disease in cattle. Aust Vet J. 1991 Aug;68(8):253–257. doi: 10.1111/j.1751-0813.1991.tb03230.x. [DOI] [PubMed] [Google Scholar]
- Sandin R. L., Knapp C. C., Hall G. S., Washington J. A., Rutherford I. Comparison of the Vitek Immunodiagnostic Assay System with an indirect immunoassay (Toxostat Test Kit) for detection of immunoglobulin G antibodies to Toxoplasma gondii in clinical specimens. J Clin Microbiol. 1991 Dec;29(12):2763–2767. doi: 10.1128/jcm.29.12.2763-2767.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz T. F., Modrow S., Hottenträger B., Höflacher B., Jäger G., Scharti W., Sumazakl R., Wolf H., Middeldorp J., Roggendorf M. New oligopeptide immunoglobulin G test for human parvovirus B19 antibodies. J Clin Microbiol. 1991 Mar;29(3):431–435. doi: 10.1128/jcm.29.3.431-435.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockett D. C., Carr D. J., Richards W. D., Collins M. T. A repository of specimens for comparison of diagnostic testing procedures for bovine paratuberculosis. J Vet Diagn Invest. 1992 Apr;4(2):188–191. doi: 10.1177/104063879200400213. [DOI] [PubMed] [Google Scholar]
- Toth I., Barrett T. J., Cohen M. L., Rumschlag H. S., Green J. H., Wachsmuth I. K. Enzyme-linked immunosorbent assay for products of the 60-megadalton plasmid of Escherichia coli serotype O157:H7. J Clin Microbiol. 1991 May;29(5):1016–1019. doi: 10.1128/jcm.29.5.1016-1019.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajstman A. C. Diagnostic tests, sensitivity, specificity, efficiency and prevalence. Aust Vet J. 1979 Oct;55(10):501–501. doi: 10.1111/j.1751-0813.1979.tb00382.x. [DOI] [PubMed] [Google Scholar]
- Verstijnen C. P., Ly H. M., Polman K., Richter C., Smits S. P., Maselle S. Y., Peerbooms P., Rienthong D., Montreewasuwat N., Koanjanart S. Enzyme-linked immunosorbent assay using monoclonal antibodies for identification of mycobacteria from early cultures. J Clin Microbiol. 1991 Jul;29(7):1372–1375. doi: 10.1128/jcm.29.7.1372-1375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöller L., Krämer I., Kappe R., Sonntag H. G. Enzyme immunoassays for invasive Candida infections: reactivity of somatic antigens of Candida albicans. J Clin Microbiol. 1991 Sep;29(9):1860–1867. doi: 10.1128/jcm.29.9.1860-1867.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oirschot J. T. Comparative evaluation of an enzyme-linked immunosorbent assay (ELISA) to detect antibodies directed against glycoprotein I of pseudorabies virus and a conventional ELISA and neutralization tests. J Clin Microbiol. 1991 Jan;29(1):5–9. doi: 10.1128/jcm.29.1.5-9.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Oever H. L., Loffeld R. J., Stobberingh E. E. Usefulness of a new serological test (Bio-Rad) to diagnose Helicobacter pylori-associated gastritis. J Clin Microbiol. 1991 Feb;29(2):283–286. doi: 10.1128/jcm.29.2.283-286.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


