Abstract
Rationale
Most patients who develop persistent airflow limitation do so either as a manifestation of chronic obstructive pulmonary disease that is largely related to smoking or as a consequence of persistent asthma. We sought to compare the natural course of lung function associated with persistent airflow limitation in subjects with and without asthma from early to late adult life.
Methods
We studied 2552 participants aged 25 or more who had multiple questionnaire and lung function data from the long-term prospective population-based Tucson Epidemiological Study of Airway Obstructive Disease. Persistent airflow limitation was defined as FEV1/FVC ratio consistently < 70% in all completed surveys subsequent to the first survey with airflow limitation. Participants were divided into nine groups based on the combination of their physician-confirmed asthma status (never, onset ≤ 25 years, or onset > 25 years) and the presence of airflow limitation during the study follow-up (never, inconsistent, or persistent).
Results
Among subjects with an asthma onset ≤ 25 years, blood eosinophilia increased significantly the odds of developing persistent airflow limitation (adjOR: 3.7, 1.4–9.5), whereas cigarette smoking was the strongest risk factor for persistent airflow limitation among non-asthmatics and among subjects with asthma onset after age 25 years. Among subjects with persistent airflow limitation, the natural course of lung function differed between subjects with asthma onset ≤ 25 years and non-asthmatics, with the former having lower FEV1 levels at age 25 (predicted value for a 175-cm tall male of 3,400 versus 4,090 ml, respectively; p<0.001) and the latter having greater FEV1 loss between age 25 and 75 (1,590 versus 2,140 ml; p=0.003).
Conclusion
In subjects who have asthma onset before 25 years of age and persistent airflow limitation in adult life, the bulk of the FEV1 deficit is already established before age 25 years.
Keywords: asthma, COPD, eosinophilia, airflow limitation
Introduction
Airflow limitation that is variable and reversible either spontaneously or with treatment is a defining feature of asthma1, 2. Yet, subgroups of patients with long-term persistent asthma may develop irreversible airflow limitation3–5, which has been associated with markers of disease severity5 and overall mortality risk6. The possible progression of persistent asthma into chronic airflow limitation is consistent with observations showing that, at the population level, co-existing diagnoses of asthma, chronic bronchitis and emphysema are frequently reported by the same patient7, 8 and that active asthma increases substantially the risk of acquiring a subsequent diagnosis of chronic obstructive pulmonary disease (COPD)9. Indeed, in the presence of irreversible airflow limitation, a differential diagnosis between asthma and COPD can be quite difficult using the physiologic tests that are routinely utilized in the clinical setting10–12.
Understanding the natural course of lung function and the patterns of risk factors for development of persistent airflow limitation among asthmatics, as contrasted with those of subjects with classical smoking-related COPD, is essential to determine whether, how, and when prevention and treatment strategies can be implemented to reduce the long-term sequelae of this disease. However, population-based long-term prospective studies on persistent airflow limitation in subjects with asthma are scant. Seminal work by Burrows and colleagues1 assessed the course and prognosis of chronic airway obstruction in 27 asthmatics who already had moderate to severe airflow limitation at their enrollment in the Tucson Epidemiological Study of Airway Obstructive Disease (TESAOD). They found that subjects with asthma had a slower FEV1 decline than did subjects with smoking-related emphysema, but they did not study how the natural course of lung function from young to late adult life differed in these two groups. The large cohort of the TESAOD study has now covered a sufficiently long period with prospective follow-up of well-characterized respiratory phenotypes and repeated lung function tests over a substantial proportion of adult life for most of the participants, to address this issue. The goal of the present study was to determine risk factors and natural course of lung function associated with persistent airflow limitation in subjects with and without asthma in the TESAOD study.
Methods
Study Population
TESAOD is a population-based prospective cohort study initiated in Tucson, AZ in 1972. Details of the enrollment process have been previously reported13. During the follow-up, new enrollees were added by marriages and births for a final total of 5,377 white participants.
TESAOD is composed of surveys that took place approximately every two years between 1972 and 1996. All participants were eligible to take part in the first 12 surveys, whereas survey 13 was conducted on selected subgroups based on previous reports of respiratory symptoms/disease or residence in Arizona. During each survey, participants completed a standardized questionnaire and, with the exception of survey 4, performed spirometric lung function tests with a pneumotachygraph device, which was calibrated daily, according to ATS guidelines14. In survey 13, spirometric tests were repeated 15 minutes after inhalation of 180 µg of albuterol via a pressured metered-dose inhaler and a valved holding chamber.
For the present study, we used data from 2,552 participants who completed questionnaire and lung function tests in at least two TESAOD surveys in which they were 25 years or older. Only lung function data from surveys completed at or after age 25 were used to minimize potential confounding by individual variation in onset and length of the plateau phase of lung function growth. Of the 2,552 participants, 515 (20%) were 25 years or younger at the time of enrollment into TESAOD.
Definitions and Measurements
Participants were considered to have physician-confirmed asthma if they reported to have ever had asthma and to have been seen, diagnosed, or treated for asthma by a doctor at any completed survey. Participants with asthma were classified into two mutually exclusive groups based on whether they had disease onset at / before age 25 years or after age 25 years. We selected this age cut-off to be consistent with the above mentioned age cut-off that was chosen as initial point for analyses on lung function. Asthma onset ≤ 25 years was defined as having asthma (as described above) at any survey before age 25 or, for subjects enrolled after age 25, as having asthma at enrollment into the study and reporting being ≤ 25 years at first asthma attack or having had ‘respiratory trouble’ before age 1615. Asthma onset > 25 years was defined as either having no asthma at enrollment and having new asthma at any subsequent survey after age 25 or as having asthma at enrollment into the study after age 25 and reporting age at first asthma attack > 25 years and no ‘respiratory trouble’ before age 16.
Airflow limitation was defined as FEV1/FVC ratio < 70%. Airflow limitation was defined as “persistent” if the subject had FEV1/FVC values < 70% in all completed lung function tests subsequent to the first survey with airflow limitation. Otherwise, it was coded as “inconsistent”. The categories of no, inconsistent, and persistent airflow limitation were created based on available observations and missing observations were assumed to be at random in the statistical analyses. Both subjects with prevalent airflow limitation and subjects with incident airflow limitation were included in this study to avoid selective removal of subjects with early onset of airflow limitation. Because the FEV1/FVC ratio declines significantly with aging, analyses on lung function were also repeated after defining airflow limitation based on lower limit of normal equations for FEV1/FVC16.
We categorized subjects as ever smokers if they had smoked at least 1 pack-year by the time they completed their last survey.
A detailed description of assessment methods for skin prick tests, IgE, eosinophil measurements, and other clinical variables is included in Table E1 in the online appendix.
Study Design and Statistical Analyses
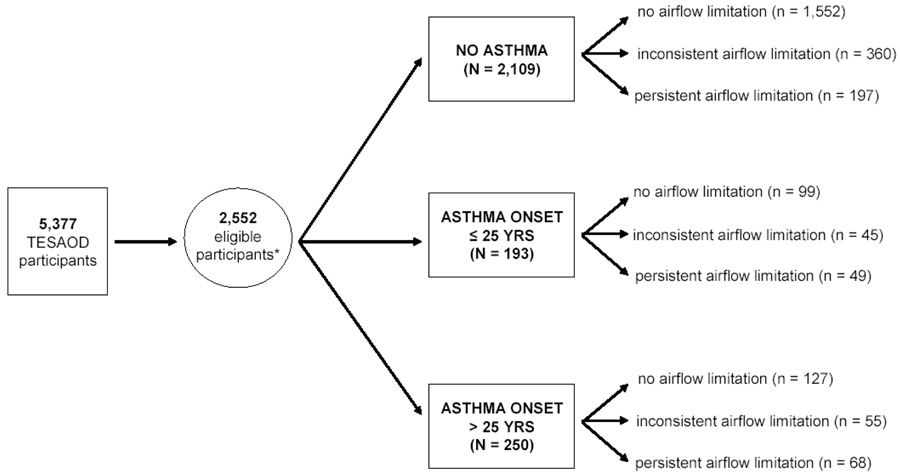
The 2,552 study subjects were divided into nine mutually exclusive groups based on the combination of their physician-confirmed asthma status (no asthma, asthma onset ≤ 25 years, or asthma onset > 25 years) and the presence of airflow limitation during the study follow-up (never, inconsistent, or persistent). The study design is summarized in Figure 1.
Figure 1.
Summary of study design. The nine study groups were created based on the combination of physician-confirmed asthma status (no asthma, asthma onset ≤ 25 years, or asthma onset > 25 years) and the presence of airflow limitation during the study follow-up (never, inconsistent, or persistent).
* Only participants who completed questionnaire and lung function tests in at least two surveys in which they were ≥ 25 years or older were eligible for the current study.
Multinomial logistic regression was used to determine factors associated with persistent and inconsistent airflow limitation, as compared with no airflow limitation, in the total population and separately for subjects with no asthma, asthma onset ≤ 25 years, and asthma onset > 25 years. Robust variance estimates were used to minimize the effects of correlation within households. In order to compare FEV1 trends over time across the groups with persistent airflow limitation while adjusting for the intra-subject serial correlation of repeated observations and reducing the impact of missing observations17, we used random coefficients models that included as fix covariates a categorical indicator of study groups, sex, height, length of follow-up, age, age squared, an interaction term between study groups and age, and an interaction term between study groups and age squared. Because FEV1 is part of the defining criteria of persistent airflow limitation, FEV1 trends were compared using linear contrasts only between pairs of groups with persistent airflow limitation. In addition, the slope of FEV1 was computed for each subject with at least three lung function tests ≥ 25 years of age by regressing FEV1 against age. Two multivariate logistic regression models were then used to determine factors associated with being in the fastest tertile and being in the fastest quintile of FEV1 decline (i.e., having an FEV1 slope of at least 33 ml per year or at least 45 ml per year, respectively). Statistical analyses were completed using the statistical packages SPSS version 15.0 and Stata version 9.0.
The study was approved by the Human Subjects Committee at the University of Arizona and all participants provided informed consent.
Results
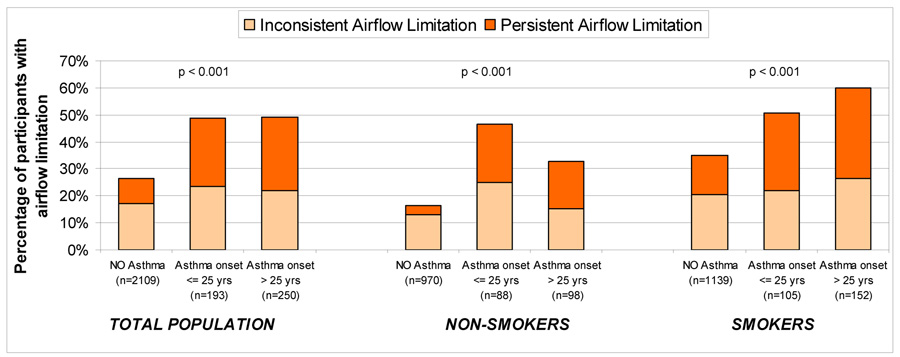
Of the 2,552 subjects included in this study, 1,778 (70%) had no airflow limitation, 460 (18%) inconsistent airflow limitation, and 314 (12%) persistent airflow limitation. Figure 2 shows the frequency of persistent and inconsistent airflow limitation among subjects with no asthma, asthma onset ≤ 25 years, and asthma onset > 25 years. Subjects in either asthma group were at increased risk of inconsistent and persistent airflow limitation as compared with subjects with no asthma. When analyses were stratified by smoking, the associations between asthma and airflow limitation were significant both among non-smokers and smokers.
Figure 2.
Proportion of subjects with inconsistent and persistent airflow limitation in the groups with no asthma, asthma onset ≤ 25 years, and asthma onset > 25 years. Data are presented for the total population as well as stratified by the groups of non-smokers and smokers.
P values refer to the comparison of proportion of any airflow limitation among subjects with no asthma, asthma onset ≤ 25 years, and asthma onset > 25 years.
Factors Associated with Airflow Limitation
Table I shows the characteristics of the subjects in the nine study groups (see methods). Factors potentially associated with inconsistent and persistent airflow limitation were tested in multinomial logistic regression models for the total population and separately for subjects with no asthma, asthma onset ≤ 25 years, and asthma onset > 25 years (Table II). Male sex, age, pack-years, elevated IgE, eosinophilia, and asthma status were significantly associated with persistent airflow limitation in the total population. The risk for persistent airflow limitation was 8 times higher for subjects with asthma onset ≤ 25 years than subjects with no asthma. The corresponding RR was lower (4.9) but still highly significant for subjects with asthma onset > 25 years. Age and smoking were significantly associated with persistent airflow limitation in all models stratified by asthma status. However, the RRs for the association between pack-years and persistent airflow limitation were substantially higher among subjects with no asthma or asthma onset > 25 years than among subjects with asthma onset ≤ 25 years. In contrast, eosinophilia was associated with an almost 4-fold increased risk of developing persistent airflow limitation only among subjects with asthma onset ≤ 25 years. Of note, in this group the RR for persistent airflow limitation associated with eosinophilia was particularly high among never smokers (6.8; 1.6–28.9). Table E2 in the online data supplement shows multinomial logistic regression models for inconsistent and persistent airflow limitation separately for males and females. The increased risk for persistent airflow limitation associated with eosinophilia appeared stronger among females than males, with the corresponding RRs being 2.1 (1.3–3.5) and 1.5 (0.9–2.5), respectively. This trend was particularly evident among subjects with asthma onset ≤ 25 years. In this group, eosinophilia was associated with a RR of 6.8 (1.6–28.4) among females and a RR of 1.9 (0.5–7.3) among males, although the interaction term between eosinophilia and sex was not significant (data not shown).
Table I.
Characteristics of the subjects in the nine study groups. N is 2552 unless otherwise specified.
| NO Asthma (n = 2109) | Asthma onset ≤ 25 years (n = 193) | Asthma onset > 25 years (n = 250) | Overall p value* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No Airflow Limitation | Inconsistent Airflow Limitation | Persistent Airflow Limitation | No Airflow Limitation | Inconsistent Airflow Limitation | Persistent Airflow Limitation | No Airflow Limitation | Inconsistent Airflow Limitation | Persistent Airflow Limitation | ||
| N | 1552 | 360 | 197 | 99 | 45 | 49 | 127 | 55 | 68 | N/A |
| Sex: % female |
57.9 | 55.3 | 34.5 | 53.5 | 62.2 | 46.9 | 73.2 | 58.2 | 47.1 | < 0.001 |
| Age at first survey in years: mean ± SD |
45 ± 18 | 58 ± 15 | 59 ± 15 | 35 ± 14 | 43 ± 14 | 48 ± 16 | 43 ± 17 | 56 ± 14 | 60 ± 13 | < 0.001 |
| Number of completed lung function tests over age 25: mean ± SD |
5.4 ± 3 | 7.2 ± 3 | 5.9 ± 3 | 5.4 ± 3 | 7.4 ± 3 | 6.4 ± 3 | 5.3 ± 3 | 6.9 ± 3 | 5.7 ± 3 | < 0.001 |
| Years of follow-up on lung function over age 25: mean ± SD |
10.7 ± 7 | 13.2 ± 7 | 10.9 ± 7 | 10.5 ± 6 | 14.8 ± 6 | 11.6 ± 7 | 11.5 ± 6 | 13.2 ± 6 | 9.8 ± 7 | < 0.001 |
| Years of formal education (n = 2512): % with more than 12 years |
55.0 | 39.9 | 33.8 | 69.1 | 54.5 | 58.3 | 59.7 | 41.8 | 23.5 | < 0.001 |
| Chronic Bronchitis (n = 2550): | < 0.001 | |||||||||
| % Never | 84.6 | 67.8 | 49.7 | 76.8 | 53.3 | 28.6 | 67.7 | 56.4 | 29.4 | |
| % Sporadic | 10.8 | 22.8 | 32.5 | 12.1 | 33.3 | 26.5 | 21.3 | 25.5 | 27.9 | |
| % Frequent | 4.5 | 9.4 | 17.8 | 11.1 | 13.3 | 44.9 | 11.0 | 18.2 | 42.6 | |
| Dyspnea (MMRC^ score ≥ 2) (n = 2551): | < 0.001 | |||||||||
| % Never | 78.1 | 64.4 | 50.8 | 63.6 | 46.7 | 34.7 | 52.8 | 45.5 | 16.2 | |
| % Sporadic | 13.8 | 22.2 | 21.8 | 22.2 | 35.6 | 22.4 | 22.8 | 27.3 | 22.1 | |
| % Frequent | 8.1 | 13.3 | 27.4 | 14.1 | 17.8 | 42.9 | 24.4 | 27.3 | 61.8 | |
| Physician-confirmed emphysema (n = 2,551) % ever |
2.3 | 9.7 | 31.0 | 4.0 | 13.3 | 42.9 | 8.7 | 25.5 | 51.5 | < 0.001 |
| Pack-Years at last completed survey: | < 0.001 | |||||||||
| % < 1 pack-year | 52.3 | 34.7 | 17.3 | 47.5 | 48.9 | 38.8 | 52.0 | 27.3 | 25.0 | |
| % 1–19.9 pack-years | 18.7 | 15.3 | 8.1 | 27.3 | 8.9 | 12.2 | 25.2 | 20.0 | 11.8 | |
| % 20–49.9 pack-years | 21.6 | 28.6 | 26.9 | 22.2 | 24.4 | 26.5 | 17.3 | 38.2 | 22.1 | |
| % ≥ 50 pack-years | 7.5 | 21.4 | 47.7 | 3.0 | 17.8 | 22.4 | 5.5 | 14.5 | 41.2 | |
| Allergy Skin Tests (n = 2,390): % ever positive ^^ |
49.6 | 42.8 | 45.4 | 80.2 | 87.8 | 68.1 | 70.1 | 65.5 | 42.4 | < 0.001 |
| Elevated IgE (n = 2,238): % ever ¥¥ |
12.4 | 15.0 | 21.2 | 27.2 | 45.0 | 46.5 | 24.5 | 51.0 | 27.9 | < 0.001 |
| Eosinophilia (n = 2,116): % ever ## |
12.8 | 18.2 | 19.0 | 23.4 | 30.0 | 41.9 | 24.8 | 25.5 | 31.6 | < 0.001 |
P value for the comparison across the nine groups.
Modified Medical Research Council dyspnea scale33
Wheal at least two mm larger than the control wheal for at least one tested allergen in at least one of the three test surveys
IgE z score at least one standard deviation higher than the mean of the subject’s sex- and age-specific category in any of the measurements performed during the stdy34. For example, this would correspond to an IgE level > 174 IU / ml for a woman between age 25 and 35 years.
Eosinophils > 4% or eosinophil z scores at least one standard deviation higher than the sex-specific mean in any of the measurements performed during the study. For example, this would correspond to eosinophils > 274 per mm3 for a woman.
Table II.
Multinomial logistic regressions to predict inconsistent and persistent airflow limitation in the total population and separately for subjects with no asthma, asthma onset ≤ 25 years, and asthma onset > 25 years. Sex, age, pack-years, skin tests, IgE, eosinophilia, and asthma status (in model 1) were tested as independent variables. All models were also adjusted for duration of follow-up. Factors that were significant in Model 1 were retained in all models.
| MODEL 1: TOTAL STUDY POPULATION (n = 2552) | MODEL 2: NO ASTHMA (n = 2109) | MODEL 3: ASTHMA onset ≤ 25 yrs (n = 193) | MODEL 4: ASTHMA onset > 25 yrs (n = 250) | |||||
|---|---|---|---|---|---|---|---|---|
| (reference group = subjects with no airflow limitation) | (reference group = subjects with no asthma and no airflow limitation) | (reference group = subjects with asthma onset ≤ 25 yrs and no airflow limitation) | (reference group = subjects with asthma onset > 25 yrs and no airflow limitation) | |||||
| Inconsistent Airflow Limitation | Persistent Airflow Limitation | Inconsistent Airflow Limitation | Persistent Airflow Limitation | Inconsistent Airflow Limitation | Persistent Airflow Limitation | Inconsistent Airflow Limitation | Persistent Airflow Limitation | |
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Sex (male) | 1.14 (0.90–1.45) | 1.79 (1.34–2.38) | 1.10 (0.84–1.43) | 1.82 (1.29–2.58) | 0.79 (0.31–2.02) | 1.17 (0.50–2.72) | 2.21 (1.00–4.88) | 2.49 (1.17–5.26) |
| Age at first survey (RR associated with 10-yrs increase) | 1.67 (1.55–1.81) | 1.67 (1.52–1.84) | 1.67 (1.53–1.82) | 1.59 (1.42–1.78) | 1.56 (1.17–2.09) | 1.91 (1.43–2.55) | 1.85 (1.45–2.35) | 1.98 (1.54–2.54) |
| Pack-years (RR associated with 10-packyears increase) | 1.20 (1.15–1.26) | 1.34 (1.27–1.42) | 1.20 (1.14–1.26) | 1.35 (1.27–1.44) | 1.23 (1.03–1.46) | 1.22 (1.05–1.43) | 1.28 (1.06–1.53) | 1.43 (1.19–1.73) |
| Elevated IgE ^ | 1.35 (0.99–1.82) | 1.47 (1.02–2.12) | 1.05 (0.72–1.53) | 1.44 (0.90–2.32) | 1.87 (0.77–4.56) | 2.23 (0.92–5.41) | 3.36 (1.52–7.46) | 1.45 (0.61–3.45) |
| Eosinophilia # | 1.25 (0.92–1.70) | 1.74 (1.21–2.51) | 1.29 (0.92–1.83) | 1.36 (0.84–2.20) | 1.72 (0.62–4.72) | 3.66 (1.41–9.54) | 1.01 (0.40–2.53) | 2.16 (0.84–5.51) |
| Asthma: | ||||||||
| Onset ≤ 25 yrs vs Never | 3.64 (2.40–5.55) | 8.10 (5.11–12.8) | N/A - | N/A - | N/A - | N/A - | N/A - | N/A - |
| Onset > 25 yrs vs Never | 2.01 (1.40–2.88) | 4.86 (3.36–7.03) | ||||||
IgE z score at least one standard deviation higher than the mean of the subject’s sex- and age-specific category in any of the measurements performed during the study34
Eosinophils > 4% or eosinophil z scores at least one standard deviation higher than the sex-specific mean in any of the measurements performed during the study
Lung Function
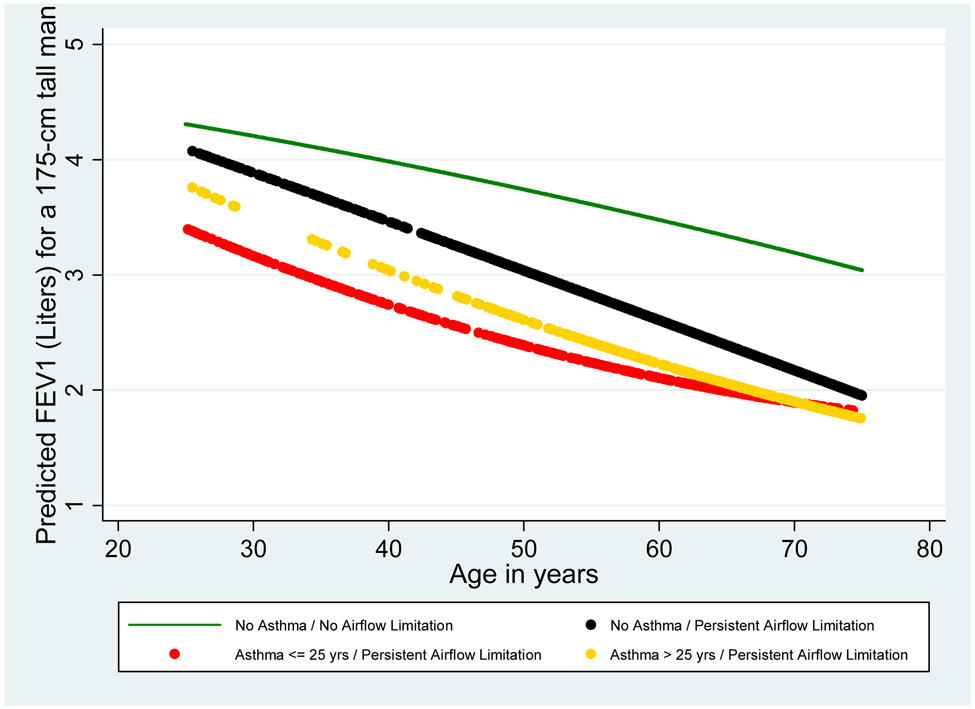
FEV1 levels during adult age were assessed in the study groups based on lung function tests completed over age 25 (see Figure E1 for distribution of available observations by age). Figure 3 shows that, inherent in the classification, the groups with persistent airflow limitation had lower FEV1 values as compared with controls, but the natural history of their lung function differed based on the presence and time of onset of asthma. As compared with controls with no asthma and no airflow limitation, subjects with no asthma and persistent airflow limitation had only moderate deficits of FEV1 at age 25 (mean deficit, 95% CI: 213, 16–410 ml), but they had 874 (665–1,084) ml excess loss of FEV1 between age 25 and 75. In contrast, subjects with asthma onset ≤ 25 years and persistent airflow limitation had larger deficits of FEV1 at age 25 (904, 637–1,171 ml) and a moderate excess loss of FEV1 between age 25 and 75 (322, 15–630 ml). Subjects with asthma onset > 25 years and persistent airflow limitation had a mean 524 (145–904) ml deficit of FEV1 at age 25 and a 763 (372–1,154) ml excess loss of FEV1 between age 25 and 75. Linear contrasts indicated that, as compared with subjects with no asthma and persistent airflow limitation, subjects with asthma onset ≤ 25 years and persistent airflow limitation had significantly lower FEV1 levels at age 25 (predicted values for a 175-cm tall male: 4,090 versus 3,400 ml, respectively; p<0.001) and a significantly smaller FEV1 loss between age 25 and 75 (2,140 versus 1,590 ml, respectively; p=0.003). Predicted FEV1 values and FEV1 loss are shown for each of the study groups in Figure 3. In the online data supplement, Figure E2a–d shows predicted FEV1 values from the best-fitting random coefficients models stratified by sex and smoking.
Figure 3.
Levels and decline of FEV1 during adult age for the study groups of subjects with no asthma and persistent airflow limitation (black line; number of subjects = 197; number of observations = 1,159), subjects with asthma onset ≤ 25 years and persistent airflow limitation (red line; number of subjects = 49; number of observations = 313), and subjects with asthma onset > 25 years and persistent airflow limitation (gold line; number of subjects = 68; number of observations = 385). Predicted values for subjects with no asthma and no airflow limitation (green line; number of subjects = 1,552; number of observations = 8,433) are also reported for comparison. Depicted values represent predicted values for a 175-cms tall male from the best-fitting random coefficients model.
| Subjects with no asthma / no airflow limitation | Subjects with no asthma / persistent airflow limitation | Subjects with asthma onset ≤ 25 yrs / persistent airflow limitation | Subjects with asthma onset > 25 yrs / persistent airflow limitation | |
|---|---|---|---|---|
| Predicted FEV1 values (95% CI) in Liters at age 25 | 4.31 (4.26–4.35) | 4.09 (3.90–4.29) | 3.40 (3.14–3.67)* | 3.78 (3.40–4.16) |
| Predicted FEV1 loss (95% CI) in Liters between age 25 and 75 | 1.26 (1.21–1.32) | 2.14 (1.94–2.34) | 1.59 (1.29–1.89)** | 2.03 (1.64–2.42) |
In order to estimate slopes of FEV1 decline for each of the groups with persistent airflow limitation, random coefficients models were re-run after excluding the age squared term. The expected FEV1 decline in controls with no asthma and no airflow limitation was 26 ml per year. Subjects with no asthma and persistent airflow limitation had a significantly faster FEV1 decline than did subjects with asthma onset ≤ 25 years and persistent airflow limitation or subjects with asthma onset > 25 years and persistent airflow limitation (43 [95% CI: 40–46] ml per year, 30 [24–36] ml per year, and 35 [29–40] ml per year; respectively).
We used multivariate logistic regression models to identify factors associated with being in the fastest tertile of FEV1 decline (i.e., having an FEV1 slope of at least 33 ml per year). Only male sex and packyears, but not IgE levels or eosinophilia, were associated with increased odds of being in the fastest tertile of FEV1 decline (adjusted ORs: 1.98, 1.63–2.41, for male sex; and 1.09, 1.05–1.13, for a 10-packyears increase). Similarly, male sex and packyears were the only significant risk factors for being in the fastest quintile of FEV1 decline (i.e., having an FEV1 slope of at least 45 ml per year), with the corresponding adjusted ORs being 2.24, 1.77–2.84; and 1.10, 1.05–1.15; respectively.
Sensitivity Analyses
To evaluate potential loss-to-follow-up bias, analyses were repeated and results confirmed after selecting for the 1,149 subjects who had ≥ 6 lung function tests (Figure E3) and after removing observations from survey 13, in which only subgroups of participants were eligible to participate (Figure E4). Results were also unchanged when persistent airflow limitation was defined as FEV1/FVC ratio consistently < 70% plus FEV1 % predicted < 80% during or after the first survey with FEV1/FVC < 70% (Figure E5). Random coefficients models including only observations over age 40 years returned very similar trends across the study groups (Figure E6).
Because the ratio FEV1/FVC declines significantly with aging in the general population, random coefficients models for lung function were also repeated after defining the study groups based on airflow limitation as assessed by an FEV1/FVC ratio below the lower limit of normal16 (Figure E7). The main differences in the natural course of lung function associated with persistent airflow limitation between subjects with asthma onset ≤ 25 years and non-asthmatics were confirmed, with the former having lower FEV1 levels at age 25 and the latter having greater FEV1 loss between age 25 and 75. In these models, the natural course of lung function of subjects with persistent airflow limitation was similar between the groups with asthma onset ≤ 25 years and asthma onset > 25 years.
Finally, using data from a subgroup of 418 adult subjects who completed pulmonary function tests before and after albuterol in survey 13, we found that 70% (23/33) of subjects with persistent airflow limitation had GOLD-defined COPD (FEV1/FVC < 70% after bronchodilator) in survey 13. This proportion was 60% (9/15) and 78% (14/18) among subjects with and without asthma, respectively.
Discussion
Using a population-based long-term prospective cohort, we identified subjects who had persistent airflow limitation during the study and compared risk factors and natural course of their disease based on the presence and age at onset of asthma. Findings of this study provide novel evidence that: 1) subjects with asthma account for a significant proportion of persistent airflow limitation in the general population; 2) the profile of risk factors for developing persistent airflow limitation is dependent upon the presence and the age at onset of asthma, with blood eosinophilia being the strongest risk factor among subjects with asthma onset before age 25 years; 3) among patients who have asthma before age 25, development of persistent airflow limitation in adult age is mainly associated with deficits that are already established by the time these subjects reach the growth plateau of their lung function.
Factors Associated with Persistent Airflow Limitation
Consistent with the well-known inverse relationship between the ratio FEV1/FVC and age18, 19, aging was strongly associated with airflow limitation both among subjects with and without asthma. However, lifetime exposure to cigarette smoking was associated with an increased risk for persistent airflow limitation that was almost twice as high among subjects with no asthma or asthma onset > 25 years than among subjects with asthma onset ≤ 25 years. In contrast, among subjects with asthma onset ≤ 25 years, eosinophilia was the strongest risk factor for persistent airflow limitation. Eosinophilia is a known risk factor for the development of respiratory symptoms20, 21 and, among asthmatics, is associated with lung function impairment22, 23 and mortality risk24, 25. In a cross-sectional study of 132 nonsmoking outpatients with severe asthma, sputum eosinophils ≥ 2% were associated with an almost 8-fold increase in the odds of having postbronchodilator FEV1 or FEV1/FVC < 75% predicted22. Similarly, among children with asthma in the Childhood Asthma Management Program cohort levels of sputum and circulating eosinophils were higher in subjects with at least 1% per year loss in FEV1 % predicted than in subjects with non-significant FEV1 loss23. However, the present study is the first population-based study showing that eosinophilia predicts development of persistent airflow limitation among adults who had asthma onset in their first 25 years of life. Our findings support a major role for eosinophilia in the smoking-independent component of the phenotypic overlap between asthma and COPD, as suggested by the particularly strong association between eosinophilia and persistent airflow limitation among non-smoker asthmatics.
Natural History of Lung Function
Because FEV1 represents the numerator of the ratio ‘FEV1/FVC’, levels of FEV1 are expected to be reduced in most patients with airflow limitation. However, in adults these FEV1 deficits can be the result of any combination between lower FEV1 levels at the beginning of adult age or an accelerated FEV1 decline during adulthood26. One of the major findings of our study is that, among subjects who had asthma onset ≤ 25 years, persistent airflow limitation in adult age was strongly associated with FEV1 deficits that were already established by young adulthood. Whether these deficits are related to early airway remodeling or impaired lung development, or both, remains to be determined. These findings are consistent with those of several other prospective studies – including the Dunedin Multidisciplinary Health and Development Study18, the Melbourne Asthma Study27, and the British 1958 Birth Cohort28 – in which persistent childhood asthma was associated with lung function deficits that are present before adult life begins and track over time from childhood to mid-adult life. In the European Community Respiratory Health Survey, percent predicted FEV1 values at age 20 – 44 years were strong predictors of asthma severity and persistence during the follow-up period29. In addition, in the birth cohort of the Children’s Respiratory Study our group has previously found that children who start life with low levels of lung function have lower expiratory flows throughout childhood30 as well as lower FEV1/FVC levels at age 2231 as compared with their peers, suggesting that early exposures and/or genetic factors also play a role in determining levels of lung function that will be attained before entering adult life. In our study, the impact of asthma on early deficits of lung function appeared stronger among males (Figure E2a), who are known to be at increased risk for severe and/or persistent childhood asthma as compared with females, and among non-smokers (Figure E2c).
Conversely, subjects with no asthma developed persistent airflow limitation mainly in response to cigarette smoking and, thus, they showed only mild FEV1 deficits at age 25 but steeper FEV1 decline over adult life. The natural course of lung function of subjects who had asthma onset after age 25 years included both moderate FEV1 deficits in young adulthood and accelerated FEV1 decline thereafter, although these results should be interpreted with caution because of the possibility of reverse causality (i.e., acquiring a diagnosis of adult-onset asthma might be the consequence rather than the cause of the steep decline of lung function) and the small number of observations before age 40 in this group.
The different natural history of lung function between subjects who developed persistent airflow limitation with or without asthma is consistent with the observation that eosinophilia, the strongest risk factor for persistent airflow limitation among subjects with asthma onset ≤ 25 years, was not associated with FEV1 decline. This observation suggests that eosinophilia influences lung function among subjects with childhood asthma mainly by affecting FEV1 levels that are attained before young adult age rather than by accelerating FEV1 decline in adult life.
Within our study design, we were unable to study potential effects of anti-inflammatory treatments32 on asthma progression. In addition, the small sample size of some of our study groups suggests the importance of other studies to replicate these findings. As with most large epidemiological studies, post-bronchodilator lung function tests were not available for the vast majority of TESAOD participants. Thus, to what extent persistent airflow limitation can be used as a surrogate of GOLD-defined COPD is not known, although sensitivity analyses from a sub-group of 418 TESAOD participants suggested acceptable correlations between these two phenotypes. We also acknowledge that, although a maximum 24-year follow-up was possible in TESAOD, subjects enrolled in this study were followed on average for 11 years. Thus, we studied the natural course of the disease over adult life by combining information from multiple sub-cohorts of subjects who differed in age at enrollment into the study and length of follow-up. However, this is an unavoidable limitation of most large prospective studies in adults. The relatively homogeneous distribution of available observations over the entire span of adult life (Figure E1) and the use of statistical techniques that are specifically designed for analysis of unbalanced longitudinal data17 should have minimized the impact of this limitation in our study.
Conclusions
In summary, development of persistent airflow limitation in adult patients with asthma is a common event and accounts for a significant proportion of the public health burden of obstructive lung disease. We showed that blood eosinophilia is the strongest risk factor for the development of persistent airflow limitation among patients with asthma onset ≤ 25 years and that, among these patients, the bulk of lung function impairment is already established by young adulthood. Therefore, future prevention programs will need to identify and target these patients before they enter adult life.
Supplementary Material
Acknowledgements
The TESAOD was initiated in 1972 as a result of the long-term vision and leadership of Benjamin Burrows, MD. We gratefully acknowledge Dr Burrows’ enduring contributions to our understanding of chronic obstructive lung diseases. We are also thankful to Dr Michael D Lebowitz who had an essential role in the design and implementation of the TESAOD study and to Dr Ronald Knudson who directed pulmonary function testing. Finally, we thank Bobbe Boyer, RN who has caringly followed the cohort subjects since 1972 and the several thousands of TESAOD participants for their extraordinary dedication to the study.
This study was funded in part by a grant award by the American Thoracic Society / Alpha1 Foundation, grants HL14136 and HL085195 by the National Heart, Lung, and Blood Institute, grant 0660059Z by the American Heart Association, and an unrestricted grant from the Barry and Janet Lang Donor Advised Fund.
This study was conducted with the support of the General Clinical Research Center of the University of Arizona.
Dr Guerra is the recipient of a Parker B. Francis Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
SG, DLS, MK-S, CV, MH, and SFQ have no conflicts of interest to disclose in relation to this manuscript. FDM has served on the Merck Advisory Board and as a consultant for GlaxoSmithKline, Pfizer and Genentech. In the last three years he has also received lecture fees from speaking at the invitation of Merck and Genentech. In each of the last three years he has been selected as the Pfizer Visiting Scholar, a program meant to increase opportunities for scientific exchange and education at medical schools, teaching hospitals and other organizations. Values of each service are determined by quantity of time and effort required.
References
- 1.Burrows B, Bloom JW, Traver GA, Cline MG. The course and prognosis of different forms of chronic airways obstruction in a sample from the general population. N Engl J Med. 1987;317:1309–1314. doi: 10.1056/NEJM198711193172103. [DOI] [PubMed] [Google Scholar]
- 2.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2006 [Google Scholar]
- 3.Vonk JM, Jongepier H, Panhuysen CI, Schouten JP, Bleecker ER, Postma DS. Risk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow up. Thorax. 2003;58:322–327. doi: 10.1136/thorax.58.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kesten S, Rebuck AS. Is the short-term response to inhaled beta-adrenergic agonist sensitive or specific for distinguishing between asthma and COPD? Chest. 1994;105:1042–1045. doi: 10.1378/chest.105.4.1042. [DOI] [PubMed] [Google Scholar]
- 5.Ulrik CS, Backer V. Nonreversible airflow obstruction in life-long nonsmokers with moderate to severe asthma. Eur Respir J. 1999;14:892–896. doi: 10.1034/j.1399-3003.1999.14d27.x. [DOI] [PubMed] [Google Scholar]
- 6.Hansen EF, Phanareth K, Laursen LC, Kok-Jensen A, Dirksen A. Reversible and irreversible airflow obstruction as predictor of overall mortality in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:1267–1271. doi: 10.1164/ajrccm.159.4.9807121. [DOI] [PubMed] [Google Scholar]
- 7.Soriano JB, Davis KJ, Coleman B, Visick G, Mannino D, Pride NB. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest. 2003;124:474–481. doi: 10.1378/chest.124.2.474. [DOI] [PubMed] [Google Scholar]
- 8.Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest. 2002;121:121S–126S. doi: 10.1378/chest.121.5_suppl.121s. [DOI] [PubMed] [Google Scholar]
- 9.Silva GE, Sherrill DL, Guerra S, Barbee RA. Asthma as a Risk Factor for COPD in a Longitudinal Study. Chest. 2004;126:59–65. doi: 10.1378/chest.126.1.59. [DOI] [PubMed] [Google Scholar]
- 10.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global Strategy for the Diagnosis, Management, and Prevention of COPD - 2006 Update. Am J Respir Crit Care Med. 2007 doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 11.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 12.Kraft M. Asthma and chronic obstructive pulmonary disease exhibit common origins in any country! Am J Respir Crit Care Med. 2006;174:238–240. doi: 10.1164/rccm.2604007. discussion 44. [DOI] [PubMed] [Google Scholar]
- 13.Lebowitz MD, Knudson RJ, Burrows B. Tucson epidemiologic study of obstructive lung diseases. I: Methodology and prevalence of disease. Am J Epidemiol. 1975;102:137–152. doi: 10.1093/oxfordjournals.aje.a112141. [DOI] [PubMed] [Google Scholar]
- 14.ATS statement--Snowbird workshop on standardization of spirometry. Am Rev Respir Dis. 1979;119:831–838. doi: 10.1164/arrd.1979.119.5.831. [DOI] [PubMed] [Google Scholar]
- 15.Burrows B, Knudson RJ, Lebowitz MD. The relationship of childhood respiratory illness to adult obstructive airway disease. Am Rev Respir Dis. 1977;115:751–760. doi: 10.1164/arrd.1977.115.5.751. [DOI] [PubMed] [Google Scholar]
- 16.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 17.Brown H, Prescott R. Applied mixed models in medicine. Chichester, UK: John Wiley & Sons, LTD; 2001. [Google Scholar]
- 18.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 19.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 20.Mensinga TT, Schouten JP, Rijcken B, Weiss ST, Speizer FE, van der Lende R. The relationship of eosinophilia and positive skin test reactivity to respiratory symptom prevalence in a community-based population study. J Allergy Clin Immunol. 1990;86:99–107. doi: 10.1016/s0091-6749(05)80129-0. [DOI] [PubMed] [Google Scholar]
- 21.Jansen DF, Schouten JP, Vonk JM, Rijcken B, Timens W, Kraan J, et al. Smoking and airway hyperresponsiveness especially in the presence of blood eosinophilia increase the risk to develop respiratory symptoms: a 25-year follow-up study in the general adult population. Am J Respir Crit Care Med. 1999;160:259–264. doi: 10.1164/ajrccm.160.1.9811015. [DOI] [PubMed] [Google Scholar]
- 22.ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. Factors associated with persistent airflow limitation in severe asthma. Am J Respir Crit Care Med. 2001;164:744–748. doi: 10.1164/ajrccm.164.5.2011026. [DOI] [PubMed] [Google Scholar]
- 23.Covar RA, Spahn JD, Murphy JR, Szefler SJ. Progression of asthma measured by lung function in the childhood asthma management program. Am J Respir Crit Care Med. 2004;170:234–241. doi: 10.1164/rccm.200308-1174OC. [DOI] [PubMed] [Google Scholar]
- 24.Ulrik CS, Frederiksen J. Mortality and markers of risk of asthma death among 1,075 outpatients with asthma. Chest. 1995;108:10–15. doi: 10.1378/chest.108.1.10. [DOI] [PubMed] [Google Scholar]
- 25.Hospers JJ, Schouten JP, Weiss ST, Rijcken B, Postma DS. Asthma attacks with eosinophilia predict mortality from chronic obstructive pulmonary disease in a general population sample. Am J Respir Crit Care Med. 1999;160:1869–1874. doi: 10.1164/ajrccm.160.6.9811041. [DOI] [PubMed] [Google Scholar]
- 26.Weiss ST, Ware JH. Overview of issues in the longitudinal analysis of respiratory data. Am J Respir Crit Care Med. 1996;154:S208–S211. doi: 10.1164/ajrccm/154.6_Pt_2.S208. [DOI] [PubMed] [Google Scholar]
- 27.Phelan PD, Robertson CF, Olinsky A. The Melbourne Asthma Study: 1964–1999. J Allergy Clin Immunol. 2002;109:189–194. doi: 10.1067/mai.2002.120951. [DOI] [PubMed] [Google Scholar]
- 28.Marossy AE, Strachan DP, Rudnicka AR, Anderson HR. Childhood Chest Illness and the Rate of Decline of Adult Lung Function Between Ages 35 and 45 Years. Am J Respir Crit Care Med. 2006 doi: 10.1164/rccm.200607-1023OC. [DOI] [PubMed] [Google Scholar]
- 29.de Marco R, Marcon A, Jarvis D, Accordini S, Almar E, Bugiani M, et al. Prognostic factors of asthma severity: a 9-year international prospective cohort study. J Allergy Clin Immunol. 2006;117:1249–1256. doi: 10.1016/j.jaci.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, et al. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172:1253–1258. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–764. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lange P, Scharling H, Ulrik CS, Vestbo J. Inhaled corticosteroids and decline of lung function in community residents with asthma. Thorax. 2006;61:100–104. doi: 10.1136/thx.2004.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 34.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.