Abstract
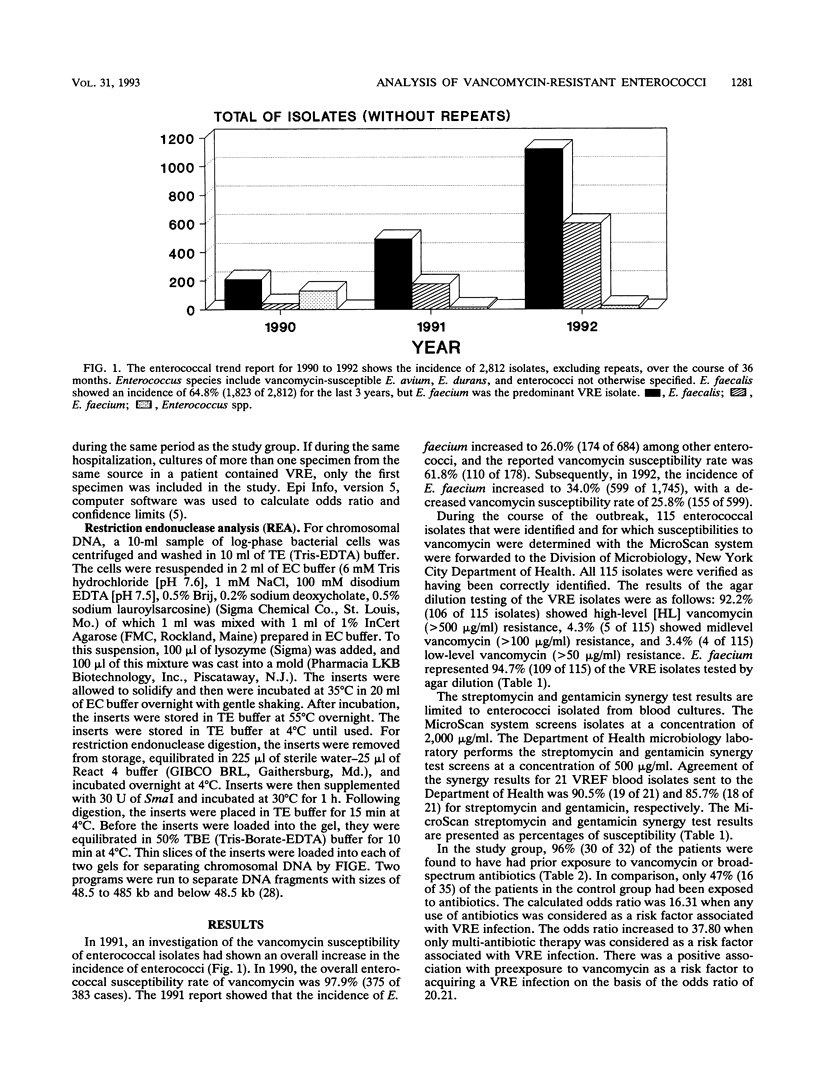
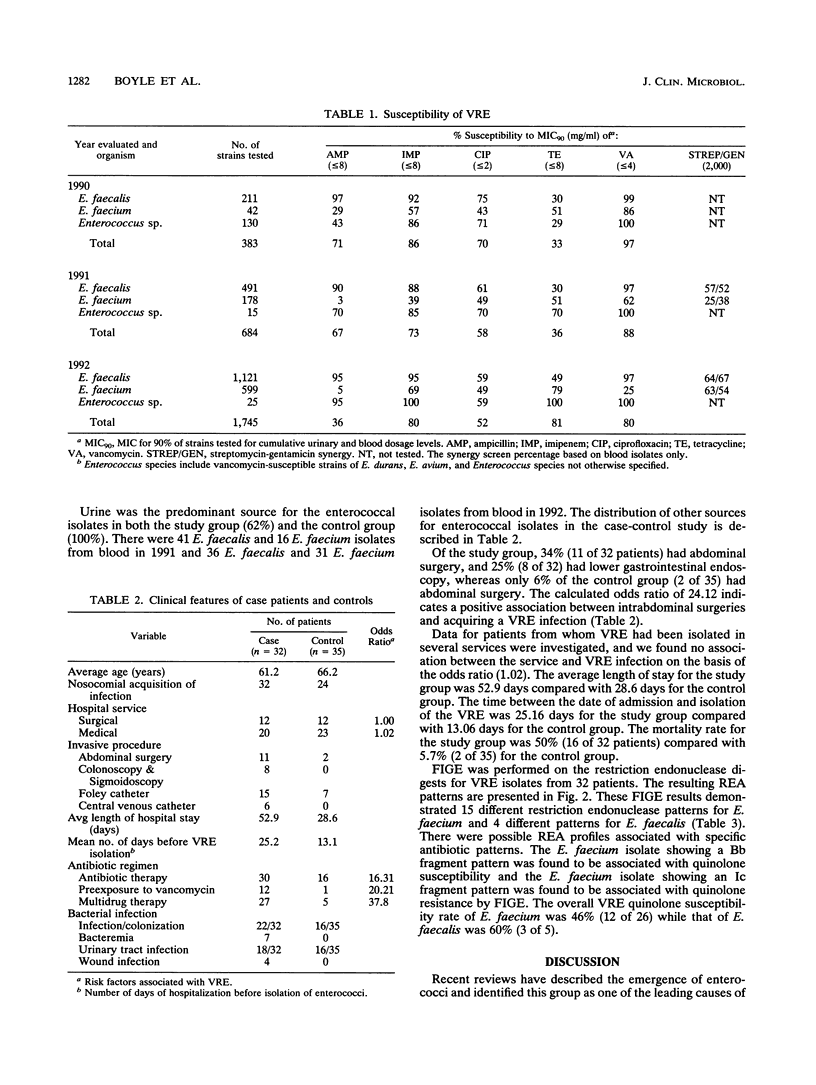
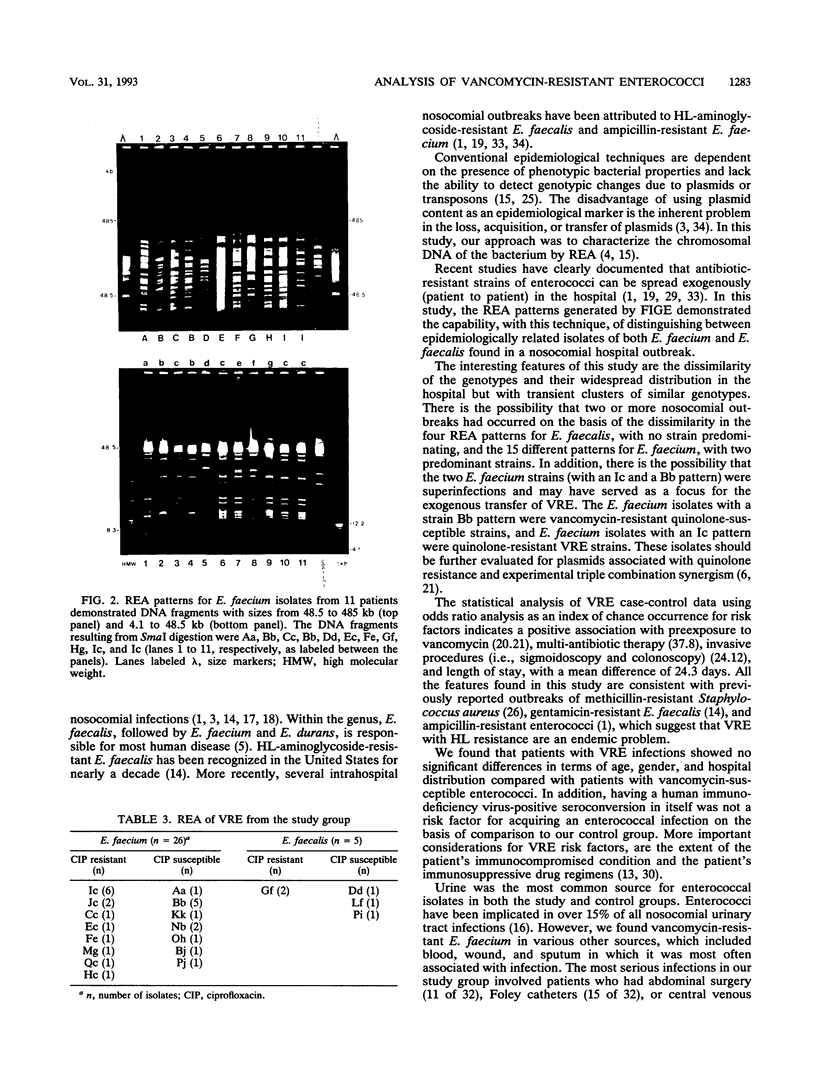
We are reporting on a nosocomial outbreak of 213 cases of vancomycin-resistant enterococcus infection involving 2,812 enterococcal isolates from patients over a period of 36 months. In 1990, the Enterococcus faecium vancomycin susceptibility rate was found to be 85.7% (36 of 42 cases), and an incidence of 10.9% (42 of 383) was noted. The 1991 data showed E. faecium with a vancomycin susceptibility rate of 61.8% (110 of 178) and an incidence of 26.0% (178 of 684). Subsequently, in 1992, the incidence of E. faecium increased to 34.0% (599 of 1,745), with a decreased vancomycin susceptibility rate of 25.8% (155 of 599). The E. faecalis vancomycin susceptibility rate remained near 97% (1,768 of 1,823) over the 36-month period. Of 115 vancomycin-resistant enterococcus (VRE) clinical isolates identified by the MicroScan MIC Combo-6 panels (Baxter Healthcare, Sacramento, Calif.), the agar dilution method indicated the resistance rate to be 92.3% (106 of 115) (high level), 3.5% (4 of 115) midlevel, and 3.5% (4 of 115) (low level). Genotypic characterization of 32 different VRE isolates by field-inversion gel electrophoresis demonstrated 19 dissimilar restriction endonuclease patterns, with 9 patterns associated with VRE quinolone resistance. Statistical analysis of case-control data for 32 patients with VRE infections indicated a positive association with intrabdominal surgical procedures (odds ratio, 24.12), multidrug therapy (odds ratio, 37.80), preexposure to vancomycin (odds ratio, 20.21), and death (odds ratio, 17.50).
Full text
PDF


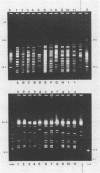
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyce J. M., Opal S. M., Potter-Bynoe G., LaForge R. G., Zervos M. J., Furtado G., Victor G., Medeiros A. A. Emergence and nosocomial transmission of ampicillin-resistant enterococci. Antimicrob Agents Chemother. 1992 May;36(5):1032–1039. doi: 10.1128/aac.36.5.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudron P. E., Markowitz S. M., Wong E. S. Isolation of a beta-lactamase-producing, aminoglycoside-resistant strain of Enterococcus faecium. Antimicrob Agents Chemother. 1992 May;36(5):1125–1126. doi: 10.1128/aac.36.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. Genotypic approach to the study of bacterial resistance to antibiotics. Antimicrob Agents Chemother. 1991 Jun;35(6):1019–1023. doi: 10.1128/aac.35.6.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. Resistance of enterococci to glycopeptides. Antimicrob Agents Chemother. 1990 Dec;34(12):2291–2296. doi: 10.1128/aac.34.12.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin B., Carbon C. In vivo antibiotic synergism: contribution of animal models. Antimicrob Agents Chemother. 1992 May;36(5):907–912. doi: 10.1128/aac.36.5.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flejzor B., Bokkenheuser V. D. Performance of the prompt system in identification and antimicrobial susceptibility testing of clinical isolates. J Clin Microbiol. 1985 Feb;21(2):267–268. doi: 10.1128/jcm.21.2.267-268.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. A., Low D. E., Simor A. E. Evaluation of a commercial microtiter system (MicroScan) using both frozen and freeze-dried panels for detection of high-level aminoglycoside resistance in Enterococcus spp. J Clin Microbiol. 1990 May;28(5):1051–1053. doi: 10.1128/jcm.28.5.1051-1053.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann L., Billot-Klein D., al-Obeid S., Klare I., Francoual S., Collatz E., van Heijenoort J. Inducible carboxypeptidase activity in vancomycin-resistant enterococci. Antimicrob Agents Chemother. 1992 Jan;36(1):77–80. doi: 10.1128/aac.36.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. P., Uttley A. H., Woodford N., George R. C. Resistance to vancomycin and teicoplanin: an emerging clinical problem. Clin Microbiol Rev. 1990 Jul;3(3):280–291. doi: 10.1128/cmr.3.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Duval J., Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988 Jul 21;319(3):157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- Matzke G. R., McGory R. W., Halstenson C. E., Keane W. F. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984 Apr;25(4):433–437. doi: 10.1128/aac.25.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederski-Samoraj B. D., Murray B. E. High-level resistance to gentamicin in clinical isolates of enterococci. J Infect Dis. 1983 Apr;147(4):751–757. doi: 10.1093/infdis/147.4.751. [DOI] [PubMed] [Google Scholar]
- Miranda A. G., Singh K. V., Murray B. E. DNA fingerprinting of Enterococcus faecium by pulsed-field gel electrophoresis may be a useful epidemiologic tool. J Clin Microbiol. 1991 Dec;29(12):2752–2757. doi: 10.1128/jcm.29.12.2752-2757.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering R. C., Jr Emergence of Enterococcus as a significant pathogen. Clin Infect Dis. 1992 Jun;14(6):1173–1176. doi: 10.1093/clinids/14.6.1173. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Krogstad D. J., Greenblatt D. J. Vancomycin therapy in patients with impaired renal function: a nomogram for dosage. Ann Intern Med. 1981 Mar;94(3):343–346. doi: 10.7326/0003-4819-94-3-343. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Lopardo H. A., Rubeglio E. A., Frosolono M., Singh K. V. Intrahospital spread of a single gentamicin-resistant, beta-lactamase-producing strain of Enterococcus faecalis in Argentina. Antimicrob Agents Chemother. 1992 Jan;36(1):230–232. doi: 10.1128/aac.36.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan R. Antibacterial activities and modes of action of vancomycin and related glycopeptides. Antimicrob Agents Chemother. 1991 Apr;35(4):605–609. doi: 10.1128/aac.35.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi N., Yoshida S., Wakebe H., Inoue M., Mitsuhashi S. Mechanisms of clinical resistance to fluoroquinolones in Enterococcus faecalis. Antimicrob Agents Chemother. 1991 Jun;35(6):1053–1059. doi: 10.1128/aac.35.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Wu C. Y., Hobbs J. N., Jr, Preston D. A., Allen N. E. Characterization of vancomycin resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob Agents Chemother. 1989 Jul;33(7):1121–1124. doi: 10.1128/aac.33.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M. A., Wakefield D. S., Hollis R., Fredrickson M., Evans E., Massanari R. M. The clinical microbiology laboratory as an aid in infection control. The application of molecular techniques in epidemiologic studies of methicillin-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis. 1991 May-Jun;14(3):209–217. doi: 10.1016/0732-8893(91)90034-d. [DOI] [PubMed] [Google Scholar]
- Raviglione M. C., Boyle J. F., Mariuz P., Pablos-Mendez A., Cortes H., Merlo A. Ciprofloxacin-resistant methicillin-resistant Staphylococcus aureus in an acute-care hospital. Antimicrob Agents Chemother. 1990 Nov;34(11):2050–2054. doi: 10.1128/aac.34.11.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm D. F., Kissinger J., Gilmore M. S., Murray P. R., Mulder R., Solliday J., Clarke B. In vitro susceptibility studies of vancomycin-resistant Enterococcus faecalis. Antimicrob Agents Chemother. 1989 Sep;33(9):1588–1591. doi: 10.1128/aac.33.9.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttley A. H., Collins C. H., Naidoo J., George R. C. Vancomycin-resistant enterococci. Lancet. 1988 Jan 2;1(8575-6):57–58. doi: 10.1016/s0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]
- Wells V. D., Wong E. S., Murray B. E., Coudron P. E., Williams D. S., Markowitz S. M. Infections due to beta-lactamase-producing, high-level gentamicin-resistant Enterococcus faecalis. Ann Intern Med. 1992 Feb 15;116(4):285–292. doi: 10.7326/0003-4819-116-4-285. [DOI] [PubMed] [Google Scholar]
- Willey B. M., Kreiswirth B. N., Simor A. E., Willaims G., Scriver S. R., Phillips A., Low D. E. Detection of vancomycin resistance in Enterococcus species. J Clin Microbiol. 1992 Jul;30(7):1621–1624. doi: 10.1128/jcm.30.7.1621-1624.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R., Al-Obeid S., Shlaes J. H., Goldstein F. W., Shlaes D. M. Inducible resistance to vancomycin in Enterococcus faecium D366. J Infect Dis. 1989 Jun;159(6):1095–1104. doi: 10.1093/infdis/159.6.1095. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Dembinski S., Mikesell T., Schaberg D. R. High-level resistance to gentamicin in Streptococcus faecalis: risk factors and evidence for exogenous acquisition of infection. J Infect Dis. 1986 Jun;153(6):1075–1083. doi: 10.1093/infdis/153.6.1075. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Kauffman C. A., Therasse P. M., Bergman A. G., Mikesell T. S., Schaberg D. R. Nosocomial infection by gentamicin-resistant Streptococcus faecalis. An epidemiologic study. Ann Intern Med. 1987 May;106(5):687–691. doi: 10.7326/0003-4819-106-5-687. [DOI] [PubMed] [Google Scholar]