Abstract
Selectins are adhesion molecules that resist large tensile forces applied by hydrodynamic forces to leukocytes binding to vessel walls. In crystals, the liganded (high-affinity) and unliganded (low-affinity) conformations differ in orientation between their tandem lectin and EGF domains. I examine how tensile force exerted on a selectin–ligand complex in vivo could favor the more extended, high-affinity conformation. Allostery is transmitted from the EGF–lectin domain interface to the ligand-binding interface on the lectin domain, 30 Å away. Trp-1 of the lectin domain and the long axis of the EGF domain form an L-shaped prybar that is welded together by hydrogen bonds to the Trp-1 α-amino group. Pivoting of the prybar induced by force demolishes an interface between the Trp-1 side chain and the lectin domain at a switch1 region. These changes are transmitted by rigid body movement of the switch2 region to rearrangements in the switch3 region at the ligand binding site. Another switch region corresponds to a single residue in the EGF domain with large effects on ligand binding and rolling adhesion. Allostery in selectins, and the alignment of tensile force on a selectin–ligand complex with the transition pathway for conformational change, explain much of the structural basis for selectin mechanochemistry.
Selectins are a unique family of 3 adhesion molecules that are expressed and function only on cells in the vasculature (1). In contrast to other classes of adhesion molecules, selectins are found only in chordata, correlating with the evolution of an enclosed circulatory system.
Selectins mediate the first step required for emigration of leukocytes from the bloodstream, tethering of a leukocyte in flow. Subsequently a highly transient adhesive interaction through selectins permits leukocytes to remain in contact with the vascular surface as they roll downstream in response to hydrodynamic drag. Only a small number of selectin–ligand bonds are formed at any one time between a rolling leukocyte and the vessel wall (2).
Selectins have receptor–ligand bonds that are unusually strong; i.e., the koff increases only moderately as force is applied (3). Furthermore, selectins exhibit catch-bond behavior at low forces and slip-bond behavior at higher forces (4, 5). In other words, as force on the selectin–ligand bond increases, the off-rate decreases, reaches a minimum, and then increases at higher force.
Selectins have an N-terminal C-type (Ca2+-binding) lectin domain, followed by a single EGF domain, 2–9 sushi domains, a single transmembrane domain, and a short cytoplasmic domain (1). Elegant crystal structures of selectin fragments containing the lectin and EGF domains reveal 2 conformational states, termed here bent and extended (6, 7). These states differ in the orientation between the tandem lectin and EGF domains and in the conformation of the ligand binding site. Crystallization in the absence of ligand yields the bent selectin conformation (6). When sialyl-Lewisx, a tetrasaccharide that represents the minimal ligand substructure, was soaked into preformed bent-conformation crystals, it bound to the bent conformation. However, cocrystals formed with P-selectin and a P-selectin ligand glycoprotein-1 (PSGL-1) fragment yielded the extended conformation (7). These structures revealed how selectins bind sialyl-Lewisx. Furthermore, the cocrystal with PSGL-1 revealed how P-selectin recognizes the PSGL-1 peptide moiety, including binding to 2 sulfated tyrosines that are more N-terminal than the Thr that is O-linked to the hexasaccharide bearing sialyl-Lewisx (7).
Because of the importance of describing how selectins bind ligands, conformational change received less attention in what remains the sole structural report on the extended conformation of selectins (7). Rearrangement of two regions in the lectin domain and the change in angle at the lectin–EGF interface were described. However, the mechanism for conveying conformational change between the ligand binding site and the lectin–EGF domain interface in selectins has not previously been described. Based on casual inspection, it is difficult to understand how shape shifting could be conveyed all the way across the selectin lectin domain from the EGF interface to the ligand binding face 30 Å away (Fig. 1). Indeed, most movement in the lectin domain is opposite in direction to the displacement of the EGF domain (see Fig. 2), and as described in an accompanying article (8), an interface between 2 lobes in the lectin domain is opened. How then could conformational change be conveyed across what appears at first glimpse to be a widening gulf?
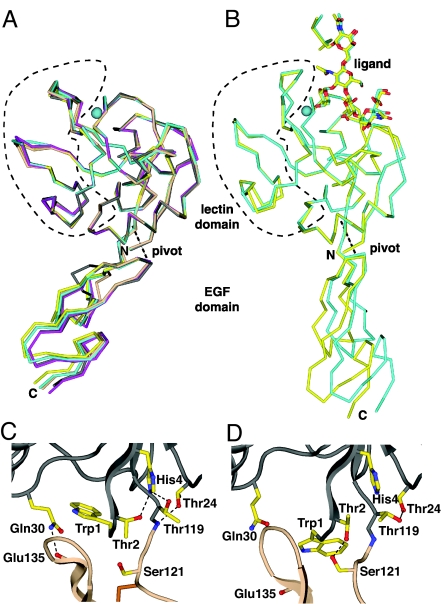
Fig. 1.
The discrete bent and extended selectin conformations. (A and B). Cα traces of molecules crystallized in different lattice environments, all superimposed on the lectin domain. (A) In absence of ligand: E-selectin (PDB ID code 1G1T), gray; P-selectin (PDB ID code 1G1Q) chains A–D, yellow, magenta, cyan, and wheat, respectively. (B) In presence of ligand: P-selectin (PDB ID code 1G1S) chains A and B, cyan and yellow, respectively. Ligand carbohydrate residues are shown in stick with red oxygen and blue nitrogen atoms. The divalent cation in the ligand binding site is a cyan sphere. (C and D). The lectin (gray backbone) and EGF (wheat backbone) domain interface for bent (C) and extended (D) P-selectin. Side chains are shown as yellow sticks with blue nitrogens and red oxygens.
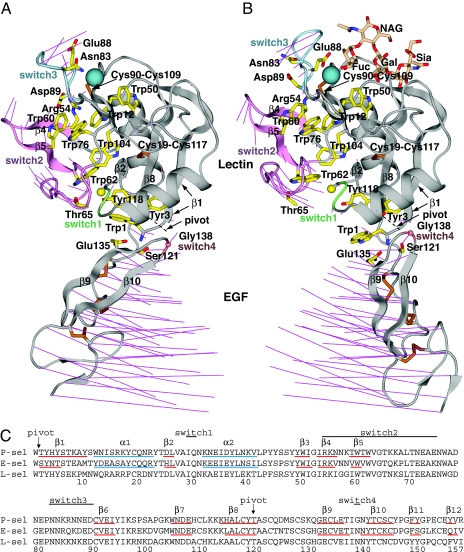
Fig. 2.
Overall conformational movements in selectin allostery. (A and B). Ribbon cartoons of bent conformation (PDB ID code 1G1Q, chain A) (A) and extended conformation (PDB ID code 1G1S, chain A) (B) (7) in identical orientations after superposition on the lectin domain. The sialyl LewisX moiety of PSGL-1 is shown as sticks in wheat. Thin magenta lines in A and B connect the same Cα atoms in the bent and extended structures for all residues. Switch region ribbons are color-coded. Side chains are shown as yellow sticks with blue nitrogens and red oxygens, except cysteine side chains are gold. The Cβ of Ala-28 and Cα of Gly-138 are shown as spheres. The Ca2+ (or equivalent Sr2+) ion is shown as a large teal sphere. (C) Sequence alignment. Secondary structure elements determined with DSSP (24) are marked.
Allostery with force as an effector could increase the affinity of selectins when they mediate tethering and rolling. Tensile force, by definition, favors extension and, when exerted on a selectin molecule between its N-terminal ligand-binding lectin domain and C-terminal points of attachment to the cell at the transmembrane and cytoplasmic domains, will favor the extended conformation (9–12). It was originally suggested that the extended PSGL-1-bound conformation represented the high-affinity state (7), as confirmed by the higher affinity of a glycan wedge mutation designed to stabilize the more obtuse angle between the lectin and EGF domains in the extended conformation (10) and a mutation within the lectin domain, at a point intermediate between the EGF interface and ligand binding site (8).
An alternative sliding-rebinding theory was stimulated by findings that substitution of Gly for Asn-138 in the EGF domain at its interface with the lectin domain markedly increases adhesiveness through L-selectin and the stability of rolling interactions to applied force (10, 11, 13–15). This theory postulates that flexibility of the hinge affects ligand binding at a distance by (i) “promoting rotational diffusion of the lectin domain, thereby contributing to the on-rate for ligand binding” and (ii) tilting the ligand-binding interface to align with the force direction (11, 16). This model does not propose that extension is linked to the conformation of the ligand binding site.
Here, we elucidate the pathway for transmission of allostery in selectins and find a number of previously unnoted features. The results provide a structural basis for selectin mechanochemistry.
Results and Discussion
Bent and Extended Conformations Are Discrete.
Does the lectin–EGF interface exhibit a wide range of orientations distributed randomly between liganded and unliganded structures, suggesting flexibility? Or does the interface assume discrete orientations that correlate with ligand binding, suggesting allostery? There are five independent examples of unliganded selectin molecules crystallized in unique lattice environments, 1 for E-selectin and 4 for P-selectin (6, 7). All show similar orientations between the lectin and EGF domains (Fig. 1A), measured between the metal ion, Trp-1 Cα, and Cys-144 Cα, of 116° to 121°. In cocrystals formed with P-selectin and ligand, there are 2 independent molecules. The angles between their lectin and EGF domains are similar, at 149° and 154° (Fig. 1B) and in a markedly different range from the 116° to 121° seen for unliganded molecules. Other loops in the lectin domain, outlined in Fig. 1, are very similar among the 5 bent molecules, and among the 2 extended molecules, but markedly different between the 2 groups. These findings suggest that the orientation between lectin and EGF domains is allosterically linked to reshaping of the loops between the lectin-EGF interface and the ligand binding interface. A caveat has been raised that a molecule of 2-methyl-2,4-pentanediol used in crystallization binds near the Trp-1 side chain in the lectin–EGF interface of the liganded, extended conformation; however, this precipitant was also used in the crystallization of the unliganded, bent conformation, and did not induce its extension (7).
Hydrogen bonds around the lectin–EGF interface stabilize its two preferred orientations (Fig. 1 C and D). A hydrogen bond between the Gln-30 side chain and Glu-135 backbone (Fig. 1C) is present only in the bent conformation. Hydrogen-bond networks involving the side chains of Thr-2, His-4, Thr-24, and Thr-119, and the backbone of Ala-120, are altered at the pivot. Furthermore, the side chains of Thr-2 and Ser-121 hydrogen bond only in the extended conformation (Fig. 1D).
Switch and Pivot Regions.
Invoking the principle of the reversibility of chemical reactions, it is arbitrary in which direction conformational change is traced through the structure. We will begin at the EGF–lectin domain interface and trace allosteric change to the ligand binding site, because in the end, we are interested in how tensile force, acting at this interface, may alter the conformational equilibrium for ligand binding.
To identify regions that shift shape in selectin allostery, the bent and extended P-selectin conformations were superimposed on (i) the lectin domain or (ii) on the EGF domain. Switch regions were defined as residues with Cα atoms that differ >1 Å in position in the lectin domain after superposition on the lectin domain or in the EGF domain after superposition on the EGF domain. Four switch regions are numbered according to their position in the sequence (Fig. 2 A–C); switch2 and switch3 have previously been noted (7). By using the same superpositions, the pivot is defined as the region that varies the least in each superposition (Fig. 1 A and B, Fig. 2, dashed lines, and Fig. 2C, arrows). There are two pivot points, between residues 1 and 2 and at the Cα of residue 119. Remarkably, although Trp-1 is commonly considered part of the lectin domain, it pivots together with the EGF domain (Fig. 2 A and B and Fig. 3).
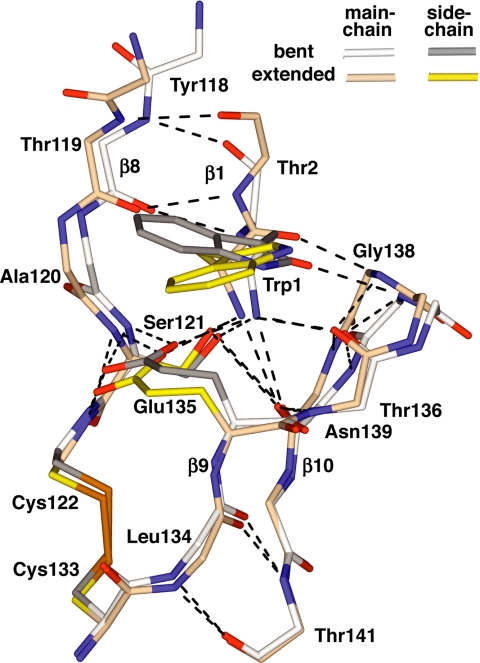
Fig. 3.
The P-selectin pivot region. The backbone and selected side chains are shown after superposition on the EGF domain. Hydrogen bonds are dashed black lines, nitrogens are blue, oxygens are red, and cysteine sulfurs are gold.
Trp-1, the EGF Yoke, the Prybar, and the Fulcrum.
Residue Trp-1 transduces pivoting motion at the interdomain interface into lectin domain allostery. A yoke formed by residues 120–122 in the lectin-EGF linker and residues 135–139 in the turn between β-strands 9 and 10 of the EGF domain (Fig. 3) is rigidified by an unusually large number of hydrogen bonds between the backbones and between the Glu-135 Ser-121 side chains and backbone. Furthermore, a disulfide connects Cys-122 and Cys-133 (Fig. 3). The Trp-1 α-amino group is held in the center of this rigid EGF yoke by a set of 4 hydrogen bonds to side chains of Ser-121 and Glu-135 and backbones of residues 136 and 139. Additionally, the carbonyl oxygen of Trp-1 forms a backbone hydrogen bond to Gly-138 (Fig. 3). Although a pivoting motion of the Trp-1 α-amino group and side chain is apparent when superposition is conducted on the lectin domain (Figs. 1 C and D and 2), little displacement is evident when superposition is based on the EGF domain (Fig. 3). This is a consequence of the maintenance of the extensive hydrogen-bond network that rigidifies the N terminus of the EGF domain and yokes it to Trp-1 during pivoting (Fig. 3).
Because Trp-1 is hydrogen-bonded to and moves with the EGF domain in selectin allostery, it may be considered part of the EGF domain, even though, because of its polypeptide backbone connectivity, it is commonly considered part of the lectin domain. This high degree of structural connectivity through Trp-1 between the lectin and EGF domains is consistent with the requirement of the EGF domain for ligand binding by the lectin domain. Internal deletion of the EGF domain results in loss of ligand binding and either diminution in or loss of mAb reactivity with epitopes in the lectin domain (17, 18).
Together, Trp-1 and the EGF domain form an L-shaped lever, i.e., a pry, with the short and long arms formed by the Trp side chain and the long, N- to C-terminal axis of the EGF domain, respectively (Fig. 2 A and B). The hydrogen bonds between the Trp-1 backbone and the EGF yoke act as welds to strengthen the connection between the two arms of the lever.
A strong fulcrum for this lever is formed by pivot residues 2 and 119, which locate to one end of the β2-β8-β1 sheet and are hydrogen-bonded to one another in adjacent β-strands 1 and 8 (Figs. 2C and 3). The strong hydrogen bond networks in the prybar and the pivot will maintain their structural integrity during force loading and appear to be an important design feature for selectin mechanochemistry.
Switch4 at P-Selectin-Specific Residue Gly-138.
During pivoting, the backbone of switch4 residue Gly-138 moves as a consequence of its hydrogen bond to the Trp-1 carbonyl oxygen. When the bent and extended states are superimposed by using the EGF domain, the Trp-1 N, Cα, C, and O atoms differ in position by 0.5, 0.9, 1.5, and 1.9 Å, respectively. This differential movement in the Trp-1 backbone is imposed by the covalent connection at the pivot to residue 2. In response, the hydrogen-bonded Gly-138 N atom moves 1.1 Å, and the Gly-138 Cα atom moves 1.4 Å, within the EGF domain frame of reference.
In L- and E-selectin, residue 138 is Asn (Fig. 2C). Substitution of the EGF domain of P-selectin into L-selectin greatly enhances rolling adhesiveness, and the sole substitution responsible for this effect is Asn-138 → Gly (10, 11, 13–15). Backbone movement at residue 138 has not previously been noted. It has been speculated that loss of a side-chain hydrogen bond between Asn-138 and Tyr-37 in the Gly-138 mutant would promote flexibility (7), and this idea has been further developed in the sliding-rebinding model (11).
There are two alternative explanations for the markedly enhanced adhesiveness of the Asn-138 → Gly mutant. In the bent conformation, Asn-138 in E-selectin and Gly-138 in P-selectin are in a favored, left-handed α-helix region of the Ramachandran plot (19). However, in extended P-selectin, Gly-138 moves to a region that is favored for Gly, but highly unfavored for non-Gly residues. As described above, the Gly-138 backbone conformation is highly constrained by hydrogen bonds (Fig. 3). Therefore, considerable strain is likely to be introduced into the backbone of Asn-138 in the extended conformations of L-selectin and E-selectin. It follows that the Asn → Gly mutation in L-selectin should markedly stabilize the extended conformation relative to the bent conformation, and thereby increase affinity for ligand. The relative importance of backbone strain at residue 138 and the hydrogen bond between the side chains of Asn-138 and Tyr-37 could be tested by mutating Asn to a non-Gly residue incapable of forming a side-chain hydrogen bond, such as Ala.
A second explanation concerns kinetics rather than equilibria. Rapid equilibration between bent and extended conformational states may be important for stable selectin-mediated rolling (8). The backbone near Asn-138 in E- and L-selectin might adjust to allow Asn-138 to adopt a favorable Ramachandran plot position after extension, and this might involve a substantial activation energy, and slow equilibration between the bent and extended states.
Stiffeners Along the Pathway of Allosteric Flow.
The lectin domain has unusually high Trp and Tyr contents of 5.8% and 10%, compared with the average of 1.3% and 2.7%, respectively. All Trp locate within or adjacent to the shape-shifting pathway (Fig. 2 A and B). The bulky aromatic Trp side chain has no rotatable bonds, and it therefore promotes rigidity in proteins. Trp residues 60 and 62 move in concert with the other residues within the switch2 region (Fig. 2 A and B; and see Fig. 5). These Trp residues act as stiffeners, to prevent damping of the motion within switch2 over the long distance between switch1 and switch3, and to ensure the highly coherent movement of the residues within switch2. Five Trp residues locate directly adjacent to the pathway of conformational change, and brace the nonmoving portion of the domain against shear strain within the lectin domain (Fig. 2 A and B). Furthermore, the Trp-1 side chain imparts rigidity to the short arm of the prybar. Tyr is the second bulkiest amino acid and is also aromatic and relatively rigid and thus, like Trp, acts as a stiffener for the lectin domain.
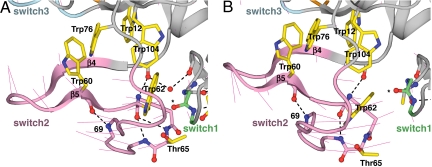
Fig. 5.
The rigid-body movement of the switch2 region. (A) Bent conformation. (B) Extended conformation. See Fig. 4 legend for details.
The two disulfide bonds in the lectin domain are present within the nonmoving portion of the lectin domain, near the boundary with the shape-shifting region. Cys-19 anchors the α1 helix to Cys-117 in the β8 strand close to the pivot (Fig. 2). Cys-90 is at the beginning of β strand 6, the central β-strand of the β3-β6-β7 sheet. Its cross-link to Cys-109 further stabilizes the boundary between shape-shifting and rigid portions of the lectin domain.
Together, the unusually high Trp and Tyr content, the two disulfides, and the two β-sheets help to rigidify the nonmoving portion of the lectin domain so that it can act as a stable platform that supports, and is not perturbed by, the allosteric movements at the pivot and switch regions. Allosteric movements in the lectin domain all occur along one face (Fig. 2 A and B). The stiffeners appear to channel shape-shifting to this relatively limited portion of the lectin domain, keep shape-shifting cohesive, prevent its dissipation, and enable it to travel the long distance from the pivot to the ligand binding site.
Pivoting and the Switch1 Region.
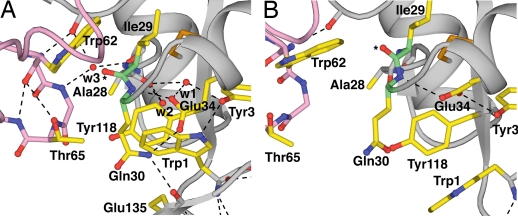
The pivoting motion of Trp-1 demolishes an interface between the switch1 and pivot regions. Trp-1 is buried in a tightly packed region of the lectin–EGF interface in the bent selectin conformation (Fig. 4A). Trp-1 has significant hydrophobic interactions with Tyr-118 and the aliphatic portions of Gln-30 and Glu-34. A network of hydrogen bonds among Gln-30, Glu-34, and Glu-135 stabilizes their packing over Trp-1 (Fig. 4A). In turn, Trp-1 buries another hydrogen bond network that includes Tyr-3, two completely buried water molecules (w1 and w2 in Fig. 4A), and three backbone atoms of the switch1 region. Although these buried waters in the bent conformation of P-selectin have not previously been noted in the literature, their structural importance is emphasized by their conservation in E-selectin (7). Water 2 is notable for stabilizing the interaction between Trp-1 and Tyr-118 by forming hydrogen (polar) bonds to the π elections of both of these aromatic residues (not shown). The pivoting of Trp-1 with the EGF domain disrupts this hydrogen-bond network (Fig. 4B). This disruption contributes to the reorientation of the Ala-28 carbonyl, which hydrogen bonds to both waters in the bent conformation, and to the reorientation of switch1 backbone residues 29 and 30 (Fig. 4). Glu-34, which is largely buried and at the center of this hydrogen-bond network, also reorients and contributes to switch1 movement.
Fig. 4.
The switch1–pivot interface. (A) Bent conformation. (B) Extended conformation. PDB ID codes, chains, superposition on the lectin domain, and coloring are as in Fig. 2. The carbonyl oxygen of Ile-29 is indicated by an asterisk. Water molecules are red spheres. Hydrogen bonds are shown as dashed lines.
As a result of the radical perturbation of the hydrogen bonds at the switch1 backbone, the Ile-29 carbonyl oxygen moves to a new position (indicated by an asterisk in Fig. 4). In this new position, the Ile-29 oxygen would clash with the Trp-62 side chain. Therefore, Trp-62 and with it the switch2 region are pushed away from the switch1 region. The Trp-62 side chain and Ile-29 carbonyl oxygen remain in van der Waals contact in both the bent and extended conformations. Thus, the interface opens up into which the Ala-28 → His substitution is introduced in an accompanying article (8). Residues Trp-1, Ala-28, Gln-30, Thr-65, and Tyr-118 in this interface all experience substantial increases in solvent-accessible surface area.
Rigid-Body Movement of the Switch2 Region.
The switch2 region undergoes rigid-body motion to transmit allostery between the switch1 and switch3 regions. Within the frame of reference of the nonswitch portion of the lectin domain, the 22 residues in the switch2 region move an average of 3.4 Å, between the bent and extended conformations, but only an average of 0.5 Å when the switch2 regions themselves are superimposed. This rigidity of the switch2 body comes from (i) the backbone hydrogen bonds within the β4-β5 sheet of switch2, (ii) backbone hydrogen bonds of Trp-60 and Trp-62 with residues in the Thr-65 to Lys-69 loop, and (iii) a backbone linkage of Trp-62 with the residue preceding the β4 strand that augments the β4-β5 network (Fig. 5). These hydrogen bonds, with one minor exception, are preserved in the two states of the switch2 region. Hydrophobic packing interactions of the Trp-60 and Trp-62 side chains with neighboring side chains within switch2 further contribute to coherent movement of switch2. The swinging, rigid-body motion of switch2 relays allostery over most of the distance between the lectin–EGF interface and the ligand binding site (Fig. 2 A and B).
Switch3 Backbone Remodeling and Ligand Binding.
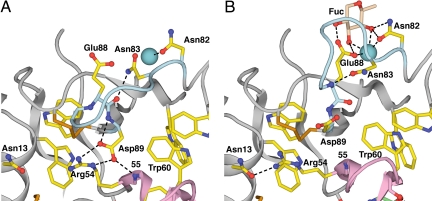
The switch3 region undergoes a large change in backbone conformation, which remodels the ligand binding site. In the bent conformation, the Asp-89 side chain in switch3 is at the center of a well-formed hydrogen-bond network that includes the Asp-89 backbone and the side chain of Arg-54 and backbone of residue 55 in switch2 (Fig. 6A). The displacement of switch2 in the extended conformation pushes Asp-89 toward the Ca2+ ion, and moves Arg-54 out of hydrogen-bonding distance of Asp-89. In the bent conformation, a hydrogen bond of Asn-83 to the backbone carbonyl oxygen of Glu-88 stabilizes a specific conformation of the loop between residues 83 and 88 in switch3 (Fig. 6A). In the extended conformation, reorientation at the Glu-88–Asp-89 backbone caused by switch2 displacement results in an Asn-83 side-chain hydrogen bond to the Glu-88 backbone nitrogen, stabilizing a radically different conformation of the Asn-83–Glu-88 loop in switch3 (Fig. 6B). In the extended conformation, the Glu-88 side chain reorients to coordinate the metal ion and hydrogen bond to 2 hydroxyl oxygens of the fucose residue in sialyl LewisX, thereby increasing the affinity of the extended selectin conformation for ligand (Fig. 6B).
Fig. 6.
The remodeling of the switch3 region. (A) Bent conformation. (B) Extended conformation. The switch2 and switch3 backbones are colored pink and light blue, respectively. The Ca2+ (or Sr2+) ion is a large teal sphere. See Fig. 4 legend for other details.
Allostery in Selectins and Force as an Effector.
This study defines the structural basis in selectins for transmission of allostery. The EGF domain interface and the ligand binding site locate on opposite faces of the lectin domain, 30 Å apart, an unusually long distance through which to convey allostery. Although lectin and EGF domains are each widely distributed in extracellular proteins, the Conserved Domain Architecture Retrieval Tool shows that only selectins have lectin and EGF domains in tandem. Furthermore, none of the 12 other subfamilies of C-type lectins in the conserved domain database contains a Trp that aligns with Trp-1 of selectin C-type lectin domains (20). Therefore, the allosteric mechanism described here appears to have evolved uniquely in selectins.
When a leukocyte is tethered through a selectin to the vessel wall, the hydrodynamic drag force acting on the leukocyte exerts a tensile force on the selectin-ligand complex (arrows, Fig. 7), and aligns the bent and extended conformations (Fig. 7 A and B). The mucin-like segment of PSGL-1 and the sushi domains of P-selectin should align in the same direction, because the mucin-like region of PSGL-1 has an extended, highly flexible structure (21), and the joints between the 9 sushi domains are likely to be flexible.
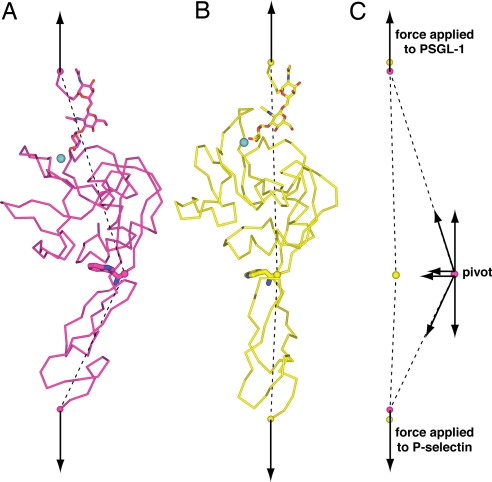
Fig. 7.
Force as an allosteric effector. (A and B) A model of the bent conformation bound to ligand (A) and the extended conformation bound to ligand (B) are shown aligned by tensile forces (arrows) applied to PSGL-1 residue 18 and P-selectin residue 157 (spheres). The dashed lines show the directions of forces exerted on the pivot (for clarity shown as a sphere at the C atom of Trp-1). Also for clarity, for PSGL-1 only residues 16–18 (Cα-trace), the O-linked Thr-16 side chain and 5 carbohydrate residues (stick) are shown in wheat. The Ca2+ ion is shown as a teal sphere, and Trp-1 is shown as large sticks. (C) The force components acting on the pivot.
Force is exerted throughout the length of the receptor/ligand complex, both at the ligand binding site where it enhances dissociation and at the pivot where it enhances extension. The forces acting on the pivot (dashed lines, Fig. 7) are nearly aligned with the applied tensile force in the extended conformation (Fig. 7B), showing that the extended conformation is near to the alignment between the lectin and EGF domains favored by applied force. In contrast, the forces acting on the pivot are diagonal in the bent conformation (Fig. 7A). These diagonal forces acting on the pivot in the bent conformation are each shown decomposed into component vectors in Fig. 7C. One component is parallel to and equal in magnitude to the force applied to the C-terminal residues of PSGL-1 and P-selectin. The other component is perpendicular to the applied force. The perpendicular force components acting on the pivot in the bent conformation (magenta sphere) point toward the pivot in the extended conformation (yellow sphere) (Fig. 7C). Furthermore, the external forces applied to the C-terminal residues of PSGL-1 and P-selectin in the bent conformation (magenta spheres) point to the positions of these residues in the extended conformation (yellow spheres) (Fig. 7C). This is important, because it means that not only does force favor the extended conformation but that force is well aligned with the transition state for conformational change. Thus, tensile force applied to a selectin–ligand complex favors transition of the bent conformation to the extended conformation and disfavors transition of the extended conformation to the bent conformation. This explains how force acts as an allosteric effector to shift the conformational equilibrium to the extended, high-affinity selectin conformation.
Steered molecular dynamics (MD) simulations found that tensile force induced extension at the EGF–lectin domain interface but failed to find associated shape-shifting in the lectin domain (16); however, this result is not surprising. For integrin I domains, moderate success in reproducing transitions seen in crystal structures was only obtained when nonshifting portions of the domain were harmonically restrained, the C-terminal α-helix, which moved further than any other element within the domain, was restrained to remain helical, and partial charges of specific atoms were adjusted (22). Shape-shifting through two domains a distance of 60 Å in selectins is much more complex than through one domain a distance of 20 Å in integrin I domains. Furthermore, a far greater number of hydrogen bonds are remodeled in selectins than integrins, and the backbone rearrangements are more complex. Moreover, MD equilibration of I domains or selectins starting from their higher-energy open or extended conformations has not been shown to lead to transition to their lower-energy closed or bent conformations, respectively, and there is no assurance that such transitions could be found even at biological time scales. One shortcoming is that the electrostatic potentials of MD do not capture the orientation dependence of hydrogen bonds, and this makes selectins, with their long pathway of allostery involving complex hydrogen bond networks, a particularly daunting challenge.
Implications for Selectin Mechanochemistry.
Selectin–ligand complexes can bear forces in the 10- to 100-pN range (3). Many domains retain their native state at these forces (23), and thus it is reasonable to use the selectin crystal structures to approximate the states accessible to force-loaded receptor–ligand complexes (Fig. 7). Tensile force will lower the free energy of the extended state relative to the bent state by approximately FΔx. The above force range and the Δx value of 4 Å (measured at the force-bearing points shown in Fig. 7) yield stabilizations of 0.6–6 kcal/mol, which translate to a shift in equilibrium of 3- to 25,000-fold. Biochemical and crystal studies suggest that basally, selectins are largely in their bent state, and thus selectins will be extended a substantially greater proportion of the time when tensile force is applied. Because both the bent and extended selectin conformations can bind ligand (7), an extended selectin bound to ligand may convert to the bent state before dissociation. Therefore, at all forces, the stabilization of the extended state by force will lower koff below what it would have been otherwise, for a protein in which the conformational equilibrium was unaffected by force. This will moderate the increase in koff as force is applied, and contributes to mechanical stability of selectins (3).
Other mechanochemical consequences of force-induced allostery will depend on details such as the rate of equilibration between bent and extended states relative to the rate of receptor–ligand dissociation, and the relationship between the levels of force that substantially induce state-switching and substantially increase the rate of receptor–ligand dissociation. With certain combinations of these parameters, as force increases, koff may first increase, then decrease (the catch-bond regime), and then increase again (4, 5). For the mechanical stability of selectins, the alignment between applied tensile force and the transition state for conformational change is particularly important so that transition to the high-affinity, extended state may occur with a faster time scale than force-induced rupture of the bond between the selectin and its ligand.
For additional information about methods, see SI Methods.
Supplementary Material
Acknowledgments.
I thank Drs. Larry Shapiro, Wendy Thomas, and William Somers for reviewing this manuscript. This work was supported by National Institutes of Health Grant HL-48675.
Footnotes
The author declares no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810784105/DCSupplemental.
References
- 1.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multi-step paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 2.Chen S, Springer TA. An automatic braking system that stabilizes leukocyte rolling by an increase in selectin bond number with shear. J Cell Biol. 1999;144:185–200. doi: 10.1083/jcb.144.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alon R, Hammer DA, Springer TA. Lifetime of the P-selectin: Carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 1995;374:539. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- 4.Marshall BT, et al. Direct observation of catch bonds involving cell-adhesion molecules. Nature. 2003;423:190–193. doi: 10.1038/nature01605. [DOI] [PubMed] [Google Scholar]
- 5.Evans E, Leung A, Heinrich V, Zhu C. Mechanical switching and coupling between two dissociation pathways in a P-selectin adhesion bond. Proc Natl Acad Sci USA. 2004;101:11281–11286. doi: 10.1073/pnas.0401870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graves BJ, et al. Insight into E-selectin/ligand interaction from the crystal structure and mutagenesis of the lec/EGF domains. Nature. 1994;367:532–538. doi: 10.1038/367532a0. [DOI] [PubMed] [Google Scholar]
- 7.Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLeX and PSGL-1. Cell. 2000;103:467–479. doi: 10.1016/s0092-8674(00)00138-0. [DOI] [PubMed] [Google Scholar]
- 8.Waldron TT, Springer TA. Transmission of allostery through the lectin domain in selectin-mediated cell adhesion. Proc Natl Acad Sci USA. 2008;106:85–90. doi: 10.1073/pnas.0810620105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konstantopoulos K, Hanley WD, Wirtz D. Receptor-ligand binding: “Catch” bonds finally caught. Curr Biol. 2003;13:R611–R613. doi: 10.1016/s0960-9822(03)00529-3. [DOI] [PubMed] [Google Scholar]
- 10.Phan UT, Waldron TT, Springer TA. Remodeling of the lectin/EGF-like interface in P- and L-selectin increases adhesiveness and shear resistance under hydrodynamic force. Nat Immunol. 2006;7:883–889. doi: 10.1038/ni1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lou J, et al. Flow-enhanced adhesion regulated by a selectin interdomain hinge. J Cell Biol. 2006;174:1107–1117. doi: 10.1083/jcb.200606056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Astrof NS, Salas A, Shimaoka M, Chen JF, Springer TA. Importance of force linkage in mechanochemistry of adhesion receptors. Biochemistry. 2006;45:15020–15028. doi: 10.1021/bi061566o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kansas GS, Spertini O, Stoolman LM, Tedder TF. Molecular mapping of functional domains of the leukocyte receptor for endothelium, LAM-1. J Cell Biol. 1991;114:351–358. doi: 10.1083/jcb.114.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kansas GS, et al. A role for the epidermal growth factor-like domain of P-selectin in ligand recognition and cell adhesion. J Cell Biol. 1994;124:609–618. doi: 10.1083/jcb.124.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dwir O, Kansas GS, Alon R. An activated L-selectin mutant with conserved equilibrium binding properties but enhanced ligand recognition under shear flow. J Biol Chem. 2000;275:18682–18691. doi: 10.1074/jbc.M001103200. [DOI] [PubMed] [Google Scholar]
- 16.Lou J, Zhu C. A structure-based sliding-rebinding mechanism for catch bonds. Biophys J. 2007;92:1471–1485. doi: 10.1529/biophysj.106.097048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowen BR, Fennie C, Lasky LA. The Mel 14 antibody binds to the lectin domain of the murine peripheral lymph node homing receptor. J Cell Biol. 1990;110:147–153. doi: 10.1083/jcb.110.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pigott R, Needham LA, Edwards RM, Walker C, Power C. Structural and functional studies of the endothelial activation antigen endothelial leucocyte adhesion molecule-1 using a panel of monoclonal antibodies. J Immunol. 1991;147:130–135. [PubMed] [Google Scholar]
- 19.Davis IW, et al. MolProbity: All-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007;35:W375–W383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchler-Bauer A, et al. CDD: A conserved domain database for interactive domain family analysis. Nucleic Acids Res. 2007;35:D237–D240. doi: 10.1093/nar/gkl951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li F, et al. Visualization of P-selectin glycoprotein ligand-1 as a highly extended molecule and mapping of protein epitopes for monoclonal antibodies. J Biol Chem. 1996;271:6342–6348. doi: 10.1074/jbc.271.11.6342. [DOI] [PubMed] [Google Scholar]
- 22.Jin M, Andricioaei I, Springer TA. Conversion between three conformational states of integrin I domains with a C-terminal pull spring studied with molecular dynamics. Structure (London) 2004;12:2137–2147. doi: 10.1016/j.str.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Carrion-Vazquez M, et al. Mechanical design of proteins studied by single-molecule force spectroscopy and protein engineering. Prog Biophys Mol Biol. 2000;74:63–91. doi: 10.1016/s0079-6107(00)00017-1. [DOI] [PubMed] [Google Scholar]
- 24.Kabsch W, Sander C. Dictionary of protein secondary structure: Pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.