Abstract
Characterization of the molecular pathways that are required for the viability and maintenance of self-renewing tumor-initiating cells may ultimately lead to improved therapies for cancer. In this study, we show that a CD133+/CD44+ population of cells enriched in prostate cancer progenitors (PCaPs) has tumor-initiating potential and that these progenitors can be expanded under nonadherent, serum-free, sphere-forming conditions. Cells grown under these conditions have increased in vitro clonogenic and in vivo tumorigenic potential. mRNA expression analysis of cells grown under sphere-forming conditions, compared with long-term monolayer cultures, revealed preferential activation of the PI3K/AKT signaling pathway. PI3K p110α and β-protein levels were higher in cells grown under sphere-forming conditions, and phosphatase and tensin homolog (PTEN) knockdown by shRNA led to an increase in sphere formation as well as increased clonogenic and tumorigenic potential. Similarly, shRNA knockdown of FoxO3a led to an increase in tumorigenic potential. Consistent with these results, inhibition of PI3K activity by the dual PI3K/mTOR inhibitor NVP-BEZ235 led to growth inhibition of PCaPs. Taken together, our data strongly suggest that the PTEN/PI3K/Akt pathways are critical for prostate cancer stem-like cell maintenance and that targeting PI3K signaling may be beneficial in prostate cancer treatment by eliminating prostate cancer stem-like cells.
Keywords: FoxO3a, PI3Kinase, prostate cancer progenitors
About 40% of men with localized prostate cancer suffer from a relapse after initial therapy (1). The majority of these men will then undergo androgen ablation therapy, but most patients will suffer from tumor progression to hormone-refractory cancer (2). One possible explanation for the initial positive response to therapy followed by androgen-refractory disease is that although current therapies eliminate the bulk of the tumor, they fail to eliminate cancer stem cells (CSCs) or tumor-initiating cells (TICs). In fact, it has been argued that many cancers are maintained in a hierarchical organization of rare CSCs, rapidly dividing cells, and differentiated tumor cells; the CSCs are not only a renewable source of tumor cells but are also a source of tumor resistance leading to tumor recurrence, metastasis, and tumor progression. Support for this hypothesis came with the identification of TICs in leukemia in 1994 (3) and, subsequently, in a variety of cancers, including solid tumors (4, 5). In addition, cancer cell lines have been shown to harbor cancer stem-like cells and are a promising model for CSC research because these progenitors can be readily expanded under anchorage-independent (sphere formation) serum-free conditions (6–8).
Several putative stem cell populations have been identified in the prostate gland and are characterized by the cell surface markers CD44, CD133, and α2β1highintegrin (9, 10). Prostate cancer progenitors (PCaPs) expressing these cell surface markers make up a subset of prostate cancer cells that are self-renewing, differentiate into heterogeneous tumors, and are highly tumorigenic in immunodeficient mice (9, 10). In this study, we enriched for PCaP populations expressing CD44 and CD133 cell surface markers from prostate cancer cell lines by growing them as spheres in anchorage-independent serum-free media (progenitor media conditions). These progenitors were further functionally defined by increased self-renewal capacity and tumorigenicity. Although the PI3K pathway is a key regulator of prostate cancer progression, little is known about the role of this pathway in PCaP maintenance. In this study, we observed phosphatase and tensin homolog (PTEN)/PI3K/Akt1/FoxO3a signaling to be important for PCaP survival and proliferation. Furthermore, this study suggests that PI3K pathway inhibitors that target cancer stem-like cell populations could prove beneficial for the treatment of prostate cancer.
Results and Discussion
Enrichment of Prostate Cancer Stem-Like Populations Under Sphere-Forming Conditions.
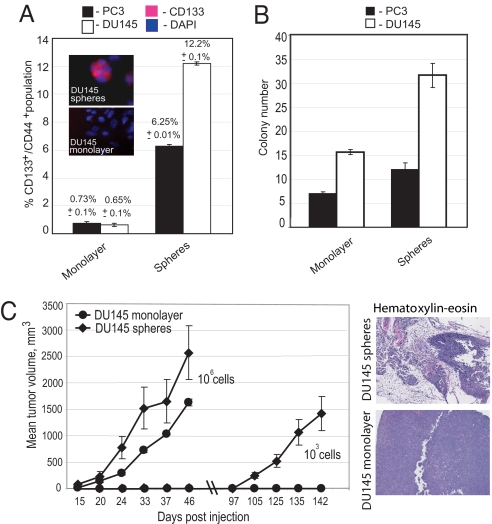
CD133+/CD44+ populations, which are enriched in PCaPs (9, 10), have increased tumor-initiating potential [supporting information (SI) Fig. S1A]. However, the frequency of PCaPs under long-term culture conditions is very low. Therefore, in this study, sphere-forming conditions were used to enrich for PCaPs in prostate cancer cell lines expressing the cell surface markers CD44 and CD133. Whereas prostate cancer cell lines have less than 1% PCaPs under long-term monolayer culture conditions, flow cytometry analysis of the prostate cancer cell lines PC3 and DU145 showed significant enrichment of these progenitors when grown under sphere-forming conditions (>6–12%; Fig. 1A). A similar frequency of progenitors was also observed in other prostate cancer cell lines (Fig. S1B). PCaPs enriched from DU145 and PC3 prostate cancer cells have increased clonogenic potential when grown in soft agar—a 2-fold enrichment was observed for cells grown under sphere-forming conditions compared with monolayer conditions (Fig. 1B). To test the tumorigenic potential of cells grown under sphere-forming conditions with enriched progenitors, we s.c. injected DU145 cells grown under sphere and monolayer conditions at varying cell numbers into nonobese diabetic (NOD)/SCID mice. The sphere-derived cells showed significantly higher tumorigenicity than the cells grown in monolayer conditions, and mice that were injected with 106 cells grown under monolayer and sphere-forming conditions grew tumors. However, 2 of 3 mice inoculated with 1,000 sphere-derived cells developed tumors, whereas no tumors were observed from 1,000 monolayer cultured cells (Fig. 1C), consistent with the increased frequency of TICs under the sphere-forming condition. Three of 3 NOD/SCID mice injected with either monolayer or sphere-derived cells (in each case, both 105 or 106 cells) developed tumors (Table 1), but tumors derived from monolayer-cultured cells were smaller than sphere-derived tumors. In addition, sphere-derived tumors grew faster, and on dissociation, they gave rise to secondary tumors with high efficiency (tumors in 3 of 3 mice when 1,000 cells were injected), whereas comparable tumors derived from monolayer-cultured cells did not (0 of 3 mice) (Fig. S1C). We observed that the secondary tumors derived from spheres (from 1,000 cells) grew faster than the primary tumors, and we attribute this to in vivo selection of increased TIC populations in secondary tumors. Histological analysis indicates that tumor heterogeneity was maintained in tumors derived from cells grown under sphere conditions but not in tumors derived from monolayer cultures (Fig. 1C). We observed both acinar type and ductal differentiation in sphere-derived tumors, and a fused gland pattern was also more frequent in sphere-derived tumors. We attribute this increased heterogeneity to an increased frequency of PCaPs, with progenitors having the ability to differentiate into different lineages of prostate gland.
Fig. 1.
Enrichment of the prostate CSC-like population under sphere-forming conditions. (A) Flow cytometry analysis showed significant enrichment of the CD133+/CD44+ population under sphere-forming conditions compared with monolayer conditions for DU145 and PC3 cells (P < 0.001). Insets show representative immunocytochemical staining for CD133 in DU145 spheres and monolayer cells. (B) Spheres have higher colony formation ability as compared with cells grown under monolayer conditions. The cells were plated on 0.2% agarose with serum-free EBM supplemented with B27, insulin, FGF, and EGF (see Materials and Methods). The colonies were calculated relative to 1,000 cells after 3 weeks of plating. (C) Tumors were produced more efficiently by the cells grown under sphere-forming conditions as compared with the cells grown under monolayer conditions. A total of 106 or 103 cells embedded in a collagen matrix were injected s.c. into NOD/SCID mice. H&E staining showed that tumors originating from the cells grown under monolayer conditions are composed of uniform cells, whereas tumors obtained from cells grown under sphere-forming conditions are highly heterogeneous.
Table 1.
Tumorigenicity of DU145 prostate cancer cells grown under sphere and monolayer conditions
| Conditions | No. of tumors/no. of mice injected |
||
|---|---|---|---|
| With 106 cells | With 105 cells | With 103 cells | |
| DU145 monolayer | 3/3 | 3/3 | 0/3 |
| DU145 spheres | 3/3 | 3/3 | 2/3 |
Gene Expression Profiles of PCaPs.
To identify genes that are preferentially expressed in cells grown under sphere conditions, we carried out microarray analysis of gene expression in DU145 and PC3 cells by using Affymetrix U133 arrays. A total of 1,266 genes were differentially expressed (by ≥2-fold) in cells cultured under sphere versus monolayer conditions. Among these 1,262 genes, 975 genes were common to both cell lines, 545 genes were ≥2-fold up-regulated, and 430 genes were ≥2-fold down-regulated (Fig. S1D). Several genes were chosen (Fig. S1E and Table S1) for quantitative RT-PCR confirmation, and all had similar gene expression patterns as the microarray. All 975 genes with annotations are presented in Table S2. To shed light on the sphere-specific signaling pathways, all 975 genes identified in the microarray that were common to both cell lines and differentially expressed in sphere and monolayer conditions were subjected to pathway analysis by using ingenuity pathway analysis. Functional clustering of these differentially regulated genes revealed that diverse biological processes, including the primary metabolic processes, cell division, cell death, development, growth, signal transduction, cell proliferation, cell cycle, cell transport, and motility (Fig. S1F), differ between cells grown under sphere and monolayer conditions. Several genes involved in negative regulation of the cell cycle were up-regulated in cells grown under sphere conditions. This finding suggests that spheres contain a larger percentage of quiescent cells than cells grown under monolayer conditions. (Table S2).
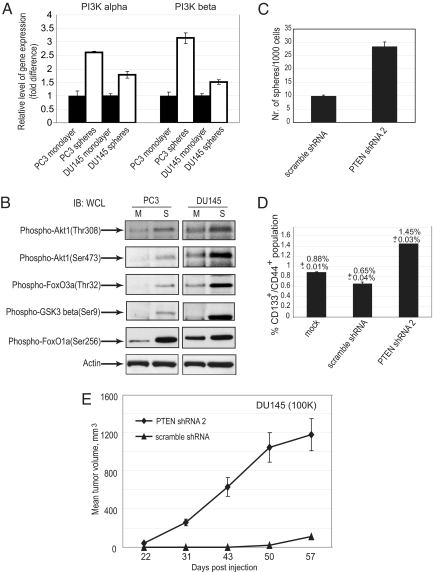
An overlay of canonical pathways identified PTEN/PI3K signaling as one of the top 10 networks, which was represented by ≈25 differentially regulated genes (Fig. S1F). Although the PI3K pathway is an integrator of multiple inputs during tumorigenesis and a known key regulator of prostate cancer progression, angiogenesis, and metastasis (11–15), little is known about the role of this pathway in PCaP maintenance. To confirm differential expression levels of PI3K pathway members in cells grown under sphere versus monolayer conditions, we measured both message and protein levels of PI3K p110α- and β-subunits in DU145 and PC3 cells. Cells grown under sphere-forming conditions showed increased levels of both subunits when compared with monolayer cells (Fig. 2A). Western blot analysis showed significant levels of phosphorylation of downstream effectors of the PI3K signaling pathway, particularly Akt, GSK3β, FoxO1a, and FoxO3a, corresponding to activation of the PI3K signaling pathway (Fig. 2B). PI3K pathway activation was independent of androgen receptor status as determined by Western blot analysis of sphere-derived cells from androgen receptor-positive and -negative cells (data not shown). Taken together, these findings suggest an important role for PI3K signaling in the survival and proliferation of PCaPs.
Fig. 2.
Activation of PI3K pathway in cells grown under sphere versus monolayer conditions. (A) Cells grown under sphere-forming conditions showed increased transcription and translation levels of the p110α and p110β catalytic subunits of PI3K. (B) Western blot analysis showed an increase in phosphorylation of Akt1, FoxO1a, FoxO3a, and GSK3β in the cells grown under sphere-forming conditions. (C) PTEN knockdown in DU145 cells showed an increase in sphere-forming ability (P < 0.02). For the sphere formation assay, PTEN knockdown DU145 cells and control DU145 cells were plated in six-well low-attachment plates at 5,000 cells per well in triplicate and grown in serum-free EBM supplemented with B27 insulin, FGF, and EGF for 1 week. The sphere numbers were calculated relative to 1,000 plated cells. (D) PTEN depletion in DU145 cells increased the CD133+CD44+ population by 2.2-fold (P < 0.006) in comparison with nontargeting shRNA (scrambled shRNA). (E) DU145 PTEN knockdown cells showed higher tumorigenic potential than control DU145 cells (P < 0.02).
PTEN Loss Leads to Increased Cancer Progenitors with High Clonogenic and Tumorigenic Potential.
To investigate the role of PI3K signaling in PCaPs further, we initiated a series of experiments aimed at modulating PI3K pathway activation. Deletion of PTEN, which negatively regulates PI3K signaling, has been shown to expand prostatic stem/progenitor cell subpopulations and to promote tumor initiation in murine models of prostate cancer (16). It was also recently reported that Pten deletion causes the generation of transplantable leukemia-initiating cells and causes depletion of normal hematopoietic stem cells (17). The expression data cited previously suggest that PTEN plays a role in determining the clonogenic and tumorigenic potential of human PCaP cells. To test this hypothesis, PTEN levels were knocked down by validated shRNA1 and shRNA2 in PTEN-positive DU145 prostate cancer cells. As expected, an increase in Akt phosphorylation (>80% by Western blot analysis for shRNA2; Fig. S2A) was observed on stable knockdown of PTEN by shRNA (PTEN expression was reduced >80% by Western blot analysis; Fig. S2A). We then measured the self-renewal capacity of PCaPs and observed a 3-fold increase in the sphere-forming ability of PTEN knockdown DU145 cells (Fig. 2C). Consistent with the increase in sphere-forming ability, we also observed an increase in the fraction of CD44+/CD133+ populations within the PTEN knockdown DU145 cells (Fig. 2D). [PTEN knockdown cells grown under sphere-forming conditions also showed an increase in the CD44+/CD133+ population compared with scrambled shRNA control cells (Fig. S2B).] The increase in PCaPs suggested that these cells may have higher tumorigenicity in vivo. To test this hypothesis, a tumorigenicity assay was performed by injecting 100,000 DU145 stably knocked-down PTEN cells into NOD/SCID mice. The PTEN knockdown DU145 cells showed higher tumorigenicity than WT DU145 cells (Fig. 2E). This observation that stable knockdown of PTEN by shRNA leads to a significant increase of PCaPs in DU145 cells is consistent with the report that loss of PTEN leads to expansion of leukemic stem cells (17). The tumorigenic potential of PTEN knockdown cells was also higher compared with control DU145 cells, thus providing further evidence for role of PTEN on the tumor initiation potential and maintenance of stem-like cell populations in prostate cancers.
FoxO3a Plays an Important Role in the Maintenance of PCaPs.
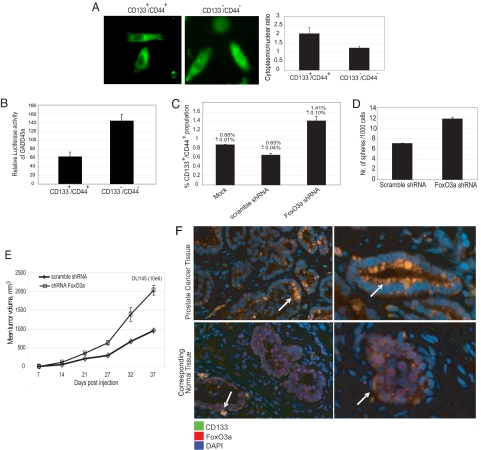
PI3K signaling regulates transcription through the forkhead family of transcription factors (FoxO) by phosphorylating conserved serine/threonine residues, which, in turn, control FoxO cellular localization. Activation of the PI3K-AKT signaling cascade leads to phosphorylation of FoxOs by Akt, resulting in cytoplasmic sequestration of the protein followed by degradation (18, 19). Transcriptionally active FoxOs affect a wide range of biological processes, including cell cycle arrest, apoptosis, DNA repair, antioxidative stress, and longevity (20–22). Among the members of FoxO, FoxO3a has been shown to be important for the maintenance of an unlimited lifespan of hematopoietic stem cells (23). Consistent with activated PI3K signaling, we observed an increase in FoxO3a phosphorylation in DU145 and PC3 cells grown under sphere-forming conditions (Fig. 2B). Moreover, FoxO3a protein cellular localization analysis revealed a decrease in the nuclear fraction of FoxO3a in FACS-sorted CD133+/CD44+ DU145 cells relative to CD133−/CD44− cells (Fig. 3A). We also evaluated the activity of FoxO3a in PCaPs by measuring the levels of the FoxO3a transcriptional target growth arrest and DNA damage 45-alpha (GADD45a). Expression of GADD45a has been shown to be positively regulated by FoxO3a (22). FACS-sorted CD133+/CD44+ and CD133−/CD44− DU145 cells were transiently transfected with a GADD45a luciferase reporter plasmid. In accordance with the cellular localization data, luciferase levels were lower in CD133+/CD44+ cells (Fig. 3B).
Fig. 3.
FoxO3a is a regulator of stem-like populations within prostate cancer cells. (A) FoxO3a protein cellular localization analysis revealed an increase in the nuclear fraction of FoxO3a in CD133−/CD44− cells compared with CD133+/CD44+ cell-enriched PCaPs. Representative microscopy images of PC3 CD133+/CD44+ and CD133−/CD44− cells with stably introduced FoxO3a-GFP; 20 cells per group were used to measure the cytoplasmic/nuclear ratio by using computerized image analysis. (B) FACS-sorted CD133+/CD44+ and CD133−/CD44− DU145 cells were transiently transfected with the GADD45-luc reporter vector. Luciferase activity was measured after 48 h. Luciferase assay data were normalized to the cell viability. The luciferase levels were low in CD133+/CD44+ PCaPs compared with CD133−/CD44− cells (P < 0.004). (C) FoxO3a knockdown in DU145 cells showed a 2-fold increase in the CD133+/CD44+-enriched PCaP population compared with scrambled shRNA-transduced control DU145 cells (P < 0.03). (D) FoxO3a knockdown in DU145 cells showed an increase in sphere-forming ability. (E) DU145 FoxO3a knockdown cells showed high tumorigenic potential in vivo (P < 0.02). (F) Primary human low-grade and high-grade prostate tumors consist of CD133+ cells with higher levels of phosphorylated (Ser-253) FoxO3a. The basal cells in the corresponding normal tissue are also CD133+ and FoxO3a+.
To establish the role of FoxO3a in the maintenance of PCaPs, we used shRNA to knock down FoxO3a in DU145 cells and measured CD133+/CD44+ populations. We observed a 2-fold increase of the CD133+/CD44+-enriched PCaPs in FoxO3a knockdown (1.41%) cells compared with scrambled shRNA-transduced control DU145 cells (0.65%; Fig. 3C) (FoxO3a expression was reduced >80%, by RT-PCR; Fig. S3A). Consistent with this result, there was an increase in sphere-forming ability in FoxO3a knockdown DU145 cells compared with control cells (Fig. 3D). We also observed an increase in clonogenic capacity of FoxO3a knockdown DU145 cells (Fig. S3B). In agreement with the increased sphere-forming ability and clonogenicity, FoxO3a knockdown DU145 cells also showed higher tumorigenic potential compared with scrambled control cells. A total of 106 FoxO3a knockdown DU145 cells and scrambled shRNA-transduced control DU145 cells were injected s.c. into NOD/SCID mice, and a 2-fold increase was observed in the tumorigenicity of FoxO3a knockdown cells (Fig. 3E). Taken together, these data suggest that in PCaPs, inactive FoxO3a is important for maintenance of cancer progenitors as a result of activated PI3K signaling. Deletion of FoxO3a led to an increase in the clonogenic, self-renewal, and tumorigenic capacity of prostate CSCs. This increase is likely partially attributable to decreased expression of GADD45a, a nuclear protein involved in the maintenance of genomic stability, DNA repair, and suppression of cell growth. In addition to GADD45a, several other genes regulated by FoxO3a (Trail, p21, p27, and p130) had decreased expression in PCaPs (Fig. S4) and may also play role in the maintenance of cancer progenitors. To translate these findings to human prostate tumors, we immunostained paraffin-embedded human primary low-grade and high-grade prostate tumors with CD133 and FoxO3a. We observed that tumors composed of CD133+ cells had high levels of phosphorylated (Ser-253) FoxO3a (Fig. 3F)
Inhibition of PI3K Pathway Leads to a Decrease in PCaP Survival.
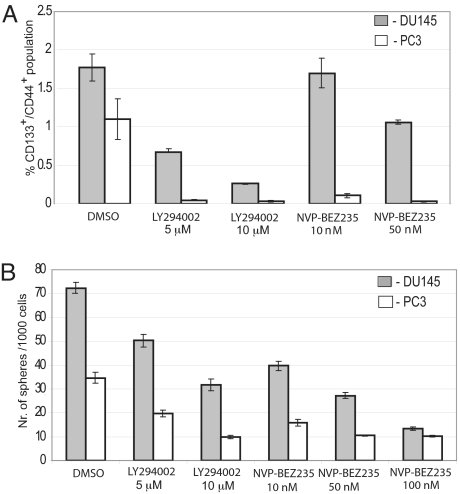
To provide additional evidence that the PI3K pathway is important for stem-like cell maintenance and survival, we tested the effect of 2 PI3K pathway modulators, LY294002 and NVP-BEZ235 (24), on the survival of PCaPs (CD44+/CD133+) within prostate cancer cell lines. PC3 and DU145 cells were treated with LY294002 (IC50 = 10 μM) or NVP-BEZ235 (IC50 = 20 nM) at 5, 10, and 20 μM or 10, 50, and 100 nM, respectively, for 3 days in serum-free epithelial growth medium. As expected, there was strong inhibition of Akt phosphorylation and consequent nuclear localization of FoxO3a in response to LY294002 and NVP-BEZ235 in all tested cell lines (Fig. S5 A and B). The PTEN-positive DU145 and PTEN-negative PC3 cells treated with inhibitors and control cells treated with DMSO were then subjected to flow cytometry analysis to determine CD44+/CD133+ populations. As shown in Fig. 4A, 5 and 10 μM LY294002 or 10 and 50 nM NVP-BEZ235 had a significant effect on the viability of PCaPs in multiple prostate cancer cell lines. Treatment of PTEN-positive DU145 cells with 10 μM LY294002 or 50 nM BEZ235 decreased the CD44+/CD133+ population more than 7-fold and 2-fold, respectively, when compared with DMSO-treated control cells. A similar effect was seen in PTEN-negative PC3 cells (Fig. 4A). To determine whether inhibition of the PI3K pathway has any effect on sphere formation, PC3 and DU145 cells were treated with 5 and 10 μM LY294002 or 10, 50, and 100 nM BEZ235 and sphere formation ability was measured. Treatment of PC3 and DU145 cells with 50 μM LY294002 or 100 nM BEZ235 leads to a ≥2.5-fold decrease in sphere-forming ability compared with DMSO-treated cells (Fig. 4B). In addition, we assayed differential inhibition of CD133+/CD44+ and CD133−/CD44− cells by LY294002 and observed a significant inhibition of double-positive cells compared with double-negative cells for both DU145 and PC3 cells (Fig. S5B). These data show that inhibition of the PI3K pathway has a significant effect on maintenance of PCaPs in vitro and suggest that the combination of cytotoxic agents and a PI3K inhibitor may lead to enhanced tumor regression in vivo through ablation of both bulk tumor and progenitor cells.
Fig. 4.
Inhibition of the PI3K pathway decreases survival of PCaPs. (A) Treatment with the PI3K pathway modulators LY294002 and NVP-BEZ235 decreases the CD133+/CD44+ population within prostate cancer cells in a dose-dependent manner. PC3 and DU145 cells were grown in serum-free EBM with supplements and treated with the indicated concentration of LY294002 or NVP-BEZ235 or with DMSO. On the third day, the cells were subjected to flow cytometry analysis. (B) Prostate cancer cells treated with the PI3K inhibitors LY294002 and NVP-BEZ325 showed a decrease in sphere formation capacity compared with DMSO-treated cells. PC3 and DU145 cells were plated in six-well low-attachment plates at 5,000 cells per well in serum-free EBM with supplements and treated with the indicated concentration of LY294002 or NVP-BEZ235 or with DMSO. The sphere numbers were calculated relative to 1,000 plated cells after 1 week.
Conclusion
To identify signaling pathways in PCaPs, we performed gene expression profiling experiments and subsequent pathway analysis, which showed that the PTEN/PI3K network plays an important role in the maintenance of PCaPs. Our results are in agreement with recently published data from Zhou et al. (25), showing that activation of the PI3K/PTEN pathway in breast cancer stem-like cells is required for CSC viability and maintenance. In addition to having increased protein levels of PI3K subunits p110α and p110β, cells grown under sphere conditions also had increased phosphorylation levels of several signaling molecules involved in PI3K signaling, including Akt1, GSK3β, FoxO1a, and FoxO3a. Inhibition of the PI3K pathway by LY290042 and NVP-BEZ235 led to a relative decrease in CD133+/CD44+ stem-like populations in prostate cancer cell lines. We also observed a significant decrease in the clonogenic potential of these progenitors. Our findings are consistent with previous data showing that PI3K signaling plays an important role in acute malignant leukemia and breast CSCs (26, 27). In these studies, it was shown that PI3K/mTOR/PTEN signaling was critical for cancer progenitor survival and proliferation, as confirmed by pathway-specific inhibitors, selected gene knockdown, and in vivo tumorigenicity assay.
Consistent with PI3K results and the reported activities of PTEN, we observed that on knockdown of PTEN by shRNA, there was a significant increase in PCaPs in prostate cancer cell lines. The tumorigenic potential of PTEN knockdown cells was also higher compared with control cells, thus providing further evidence for a role of PTEN on the tumor initiation potential and maintenance of stem-like cell populations in prostate cancers. We also demonstrated that inhibition of FoxO3a nuclear translocation led to increased clonogenic and tumorigenic potential of prostate cancer cells attributable to an increase in PCaPs. Consistent with the role of FoxO factors in promoting cell cycle arrest at the G1/S and G2/M boundaries, the expression of active forms of FoxO3a up-regulates several genes, including GADD45a, which is involved in DNA repair; Trail, which is involved in apoptosis; and genes involved in the cell cycle, such as p21, p27, and p130, that may play a role in CSC maintenance (28). Taken together, these results suggest that FoxO3a may negatively regulate prostate cancer stem-like cells, which agrees with other observations suggesting that FoxO proteins inhibit CSC maintenance and promote cell differentiation. For example, FoxO3a has been shown to induce differentiation of Bcr-Abl–transformed cells through transcriptional down-regulation of ID1 (29). In addition, the conditional deletion of FoxOs in specific tissues has demonstrated lineage-restricted tumorigenesis (30). Further analysis of FoxO3a modulation may lead to the development of novel points of therapeutic intervention in prostate cancer.
Materials and Methods
Cell, Reagents, and Animals.
DU145 and PC3 (prostate cancer cells) were obtained from the American Type Culture Collection and cultured in the recommended medium containing 10% FBS. NOD.CB17-Prkdc(SCID) mice were obtained from Jackson Laboratories and maintained under standard conditions according to institutional guidelines. The antibodies used were anti-Akt(pan), phospho-Akt (Ser-473), phospho-Akt (Thr-308), PTEN, and ubiquitin (mAb; Cell Signaling); FoxO1 and phospho-FoxO1 (Ser-256) (polyclonal antibody; Cell Signaling); phospho-GSK-3beta (Ser-9), FoxO3a, phospho-FoxO3a (Thr-32), and FoxO3a (pAb; Santa Cruz Biotechnology); β-actin (mAb; Sigma); CD133 (directly conjugated with phycoerythrin, clone 293C3; Miltenyi Biotech Ltd); CD44v6 (directly conjugated with allophycocyanin, clone C44–26; BD PharMingen); and rabbit IgG, HRP-linked whole Ab, mouse IgG, and HRP-linked whole Ab (GE Healthcare). The antibodies used for immunofluorescence studies on paraffin-embedded primary human tumor samples were CD133 (Ab-19898; Abcam), Alexafluor 488 and 594 (A11008 and A21207; Invitrogen), and phospho-FoxO3a (Ser-253; Genway Biotech; 18785210166). PI3K inhibitor LY294002 was purchased from LC Laboratories, and PI3K inhibitor NVP-BEZ235 was obtained from Novartis.
Spheres Culture.
Single cells were plated at 1,000 cells/mL on 10-cm low-attachment dishes with an ultralow attachment surface (Fisher Scientific Co.). Cells were grown in serum-free epithelial basal medium (EBM; with bicarbonate and phenol red; Cambrex) supplemented with 4 μg/mL insulin (Sigma), B27, 20 ng/mL EGF, and 20 ng/mL basic FGF (Invitrogen). Spheres were collected after 7 to 14 days, and protein was extracted for Western blotting or dissociated with Accutase (Innovative Cell Technologies, Inc. ) for expansion. The cells obtained from dissociation were sieved through a 40-μm filter and analyzed by flow cytometry.
Flow Cytometry Analysis of CD44 and CD133 Expression.
For flow cytometry, cells were dissociated with Accutase (Innovative Cell Technologies, Inc.) and washed twice in the staining solution containing Ca2+- and Mg2+-free PBS with 1 mM EDTA, 25 mM Hepes (pH 7.0) (Gibco BRL), and 0.5% FBS. Cells were stained live in the staining solution containing conjugated anti-CD44 and anti-CD133 antibody (50 min at 4°C). Samples were analyzed on a BD LSR II flow cytometer (Becton Dickinson Immunocytometry Systems). A minimum of 500,000 viable cell events were collected per sample. For sorting, 2 × 107 cells were processed for CD44 and CD133 multicolor staining, along with appropriate negative controls and single-color positive controls. The CD44+/CD133+ and CD44−/CD133− populations were sorted out on a BD FACS Diva cell sorter (Becton Dickinson Immunocytometry Systems).
In Vivo Tumorigenicity Experiments.
DU145 xenograft tumors were established by using early-passage cells and maintained in NOD.CB17-Prkdc(SCID) mice. For s.c. tumor development, 100 μL of collagen-embedded cells was injected s.c. into 5- to 8-week-old NOD.CB17-Prkdc(SCID) mice. The mice were observed for 3–5 months for appearance and development of tumors.
Microarray Analysis.
Total RNA was isolated from cell pellets by using the RNeasy kit (Qiagen). Sample preparation for GeneChip analysis was carried out according to the protocol detailed by Affymetrix. Briefly, first and second cDNA strands were synthesized; double-stranded cDNA was in vitro transcribed by using the Affymetrix 3′ amplification kit; and the resulting cRNA was purified, fragmented, and hybridized to oligonucleotide arrays (Human Genome U133 Plus 2.0 Array; Affymetrixcatalog no. 900467) representing more than 47,000 transcripts. Arrays were processed by using standard Affymetrix protocols. The Affymetrix Hybridization control kit and PolyA RNA control kit were used for the hybridizations. Probe values from image files were condensed to probe sets by using the gcRMA package from Bioconductor and the R program (31). The data set was unlogged, and the median was scaled to a target intensity of 100.
Soft Agar Colony Formation Assay.
To examine anchorage-independent growth, a cell suspension (2 × 104 cells/mL) in 0.7 mL of 0.2% low-melting SeaPlaque CTG agarose (Cambrex Bio Science Rockland, Inc.) with EBM was overlaid into 12-well plates containing a 0.5% agar base. All samples were plated in triplicate. The plates were incubated at 37°C in a humidified incubator for 21 days.
DNA Constructs.
The vectors encoding the FLAG-tagged WT FoxO3a and FLAG-tagged FoxO3a with the triple mutation T32A, S253A, S315A were provided by Dr. Michael Greenberg (Children's Hospital, Boston, MA). WT and mutant FoxO3a were inserted into pMIEG3 vector for retroviral expression. Briefly, the FoxO3a ORF and pMIEG3 backbone (except the IRES region) were amplified by using primers with the following sequences: GAG CTG GGT GCC AGG CAT GGC CAC AAC CAT GG, GGT GCC TCT GCC ATG GTG GCG AAT TCC CGG ATC C (for pMIEG3 backbone amplification) and CCA TGG TTG TGG CCA TGC CTG GCA CCC AGC TC, GGA TCC GGG AAT TCG CCA CCA TGG CAG AGG CAC C (for FoxO3a ORF amplification). FoxO3a and pMIEG3 PCR products were mixed, treated with DpnI for 2 h at 37°C, and used for transformation of competent DH5α cells. Recombinant plasmids were verified by sequencing and delivered into Phoenix cells (Orbigen) by using the calcium phosphate transfection method. Retrovirus production and transduction of PC3 and DU145 cells were performed according to the Orbigen recommendations.
Statistical Analysis.
Results of soft agar colony formation assays, flow cytometry analyses, cell proliferation assays, and in vivo tumorigenicity assays were analyzed by paired t test. A probability value of <0.05 was regarded as statistically significant.
Supplementary Material
Acknowledgments.
We thank Vasyl Lukiyanchuk for his support with DNA cloning procedures, James Watson for his help in histological staining, and Christopher Trussel for his technical assistance with flow cytometry. We thank Drs. Michael Greenberg (Children's Hospital, Boston, MA) and Sandra Schmidt (The Scripps Research Institute, La Jolla, CA) for the gift of plasmids. We thank Drs. Charles Cho, Quinn Deveraux, Michael Cooke, and Christian Schmedt for critical reading of the manuscript. This study was supported by Susan G. Komen for the Cure Grant PDF0707903 (to A.D.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE10832).
This article contains supporting information online at www.pnas.org/cgi/content/full/0810956106/DCSupplemental.
References
- 1.Grubb RL, III, Kibel AS. Prostate cancer: Screening, diagnosis and management in 2007. Mo Med. 2007;104:408–413. [PubMed] [Google Scholar]
- 2.Kasper S, Cookson MS. Mechanisms leading to the development of hormone-resistant prostate cancer. Urol Clin North Am. 2006;33:201–210. doi: 10.1016/j.ucl.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Lapidot T, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 4.Singh SK, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 5.Jordan CT. Cancer stem cell biology: From leukemia to solid tumors. Curr Opin Cell Biol. 2004;16:708–712. doi: 10.1016/j.ceb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Wei C, Guomin W, Yujun L, Ruizhe Q. Cancer stem-like cells in human prostate carcinoma cells DU145: The seeds of the cell line? Cancer Biol Ther. 2007;6:763–768. doi: 10.4161/cbt.6.5.3996. [DOI] [PubMed] [Google Scholar]
- 7.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA. 2004;101:781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dontu G, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patrawala L, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 10.Richardson GD, et al. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2005;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 11.Wu Z, McRoberts KS, Theodorescu D. The role of PTEN in prostate cancer cell tropism to the bone micro-environment. Carcinogenesis. 2007;28:1393–1400. doi: 10.1093/carcin/bgm050. [DOI] [PubMed] [Google Scholar]
- 12.Pourmand G, et al. Role of PTEN gene in progression of prostate cancer. Urol J. 2007;4:95–100. [PubMed] [Google Scholar]
- 13.Fang J, Ding M, Yang L, Liu LZ, Jiang BH. PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis. Cell Signalling. 2007;19:2487–2497. doi: 10.1016/j.cellsig.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shukla S, et al. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int J Cancer. 2007;121:1424–1432. doi: 10.1002/ijc.22862. [DOI] [PubMed] [Google Scholar]
- 15.Le Page C, Koumakpayi IH, Alam-Fahmy M, Mes-Masson AM, Saad F. Expression and localization of Akt-1, Akt-2 and Akt-3 correlate with clinical outcome of prostate cancer patients. Br J Cancer. 2006;94:1906–1912. doi: 10.1038/sj.bjc.6603184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, et al. Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc Natl Acad Sci USA. 2006;103:1480–1485. doi: 10.1073/pnas.0510652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tothova Z, Gilliland DG. FoxO transcription factors and stem cell homeostasis: Insights from the hematopoietic system. Cell Stem Cell. 2007;16:140–152. doi: 10.1016/j.stem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 19.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120(Pt 15):2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 20.Huang H, Tindall DJ. FOXO factors: A matter of life and death. Future Oncol. 2006;2:83–89. doi: 10.2217/14796694.2.1.83. [DOI] [PubMed] [Google Scholar]
- 21.Burgering BM, Kops GJ. Cell cycle and death control: Long live Forkheads. Trends Biochem Sci. 2002;27:352–360. doi: 10.1016/s0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- 22.Furukawa-Hibi Y, Kobayashi Y, Chen C, Motoyama N. FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid Redox Signal. 2005;7:752–760. doi: 10.1089/ars.2005.7.752. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto K, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Maira SM, et al. Identification and characterization of NVP-BEZ235 a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1–13. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 25.Zhou J, et al. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci USA. 2007;104:16158–16163. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martelli AM, et al. Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia. 2006;20:911–928. doi: 10.1038/sj.leu.2404245. [DOI] [PubMed] [Google Scholar]
- 27.Dillon RL, White DE, Muller WJ. The phosphatidyl inositol 3-kinase signaling network: Implications for human breast cancer. Oncogene. 2007;26:1338–1345. doi: 10.1038/sj.onc.1210202. [DOI] [PubMed] [Google Scholar]
- 28.Tran H, et al. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–534. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- 29.Birkenkamp KU, et al. FOXO3a induces differentiation of Bcr-Abl-transformed cells through transcriptional down-regulation of Id1. J Biol Chem. 2007;282:2211–2220. doi: 10.1074/jbc.M606669200. [DOI] [PubMed] [Google Scholar]
- 30.Paik JH, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128(2):309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.