Abstract
Chronic obstructive pulmonary disease (COPD), which comprises emphysema and chronic bronchitis resulting from prolonged exposure to cigarette smoke (CS), is a major public health burden with no effective treatment. Emphysema is also associated with pulmonary hypertension, which can progress to right ventricular failure, an important cause of morbidity and mortality among patients with COPD. Nuclear erythroid 2 p45 related factor-2 (Nrf2) is a redox-sensitive transcription factor that up-regulates a battery of antioxidative genes and cytoprotective enzymes that constitute the defense against oxidative stress. Recently, it has been shown that patients with advanced COPD have a decline in expression of the Nrf2 pathway in lungs, suggesting that loss of this antioxidative protective response is a key factor in the pathophysiological progression of emphysema. Furthermore, genetic disruption of Nrf2 in mice causes early-onset and severe emphysema. The present study evaluated whether the strategy of activation of Nrf2 and its downstream network of cytoprotective genes with a small molecule would attenuate CS-induced oxidative stress and emphysema. Nrf2+/+ and Nrf2−/− mice were fed a diet containing the potent Nrf2 activator, 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole (CDDO-Im), while being exposed to CS for 6 months. CDDO-Im significantly reduced lung oxidative stress, alveolar cell apoptosis, alveolar destruction, and pulmonary hypertension in Nrf2+/+ mice caused by chronic exposure to CS. This protection from CS-induced emphysema depended on Nrf2, as Nrf2−/− mice failed to show significant reduction in alveolar cell apoptosis and alveolar destruction after treatment with CDDO-Im. These results suggest that targeting the Nrf2 pathway during the etiopathogenesis of emphysema may represent an important approach for prophylaxis against COPD.
Keywords: chronic obstructive pulmonary disease, oxidative stress
Chronic obstructive pulmonary disease (COPD), comprised of emphysema and chronic bronchitis, represents a major public health concern as it is currently the fourth-leading cause of death in the United States (1). Emphysema, defined as irreversible destruction of the alveoli, is associated with inflammation in the airways and lung parenchyma. Emphysema is also associated with pulmonary hypertension, which results from destruction of the capillary network that is embedded in the alveolar walls. Pulmonary hypertension leads to cor pulmonale, which is an alteration of the structure and function of the right ventricle (RV) that results from pulmonary hypertension. In ≈3–5% of patients, cor pulmonale can progress to RV failure, which contributes significantly to COPD-mediated mortality (2, 3). The primary risk factor for COPD is cigarette smoke (CS), which accounts for ≈80–90% of COPD cases worldwide.
Oxidative stress induced by CS plays a critical role in the development of COPD. Markers of oxidative stress are elevated in both the lungs (4) and serum (5, 6) of COPD patients, and enhanced oxidative stress leads to heightened inflammation and alveolar cell apoptosis (7). A central hypothesis to explain the development of emphysema is the supposition that inflammation promotes a protease/antiprotease imbalance (8), which leads to enhanced elastolytic activity. The elevated elastin degradation and reactive oxygen species (ROS) generated from the protease/antiprotease imbalance in inflammatory cells may perpetuate a positive feedback loop, which promotes further inflammation and cell death. This loop may explain why ex-smokers have persistent inflammation and alveolar destruction for several years after quitting (9).
Although substantial progress has been made in understanding many of the molecular mechanisms underlying COPD, this knowledge has not translated into effective therapies. To date, antioxidant therapies, such as N-acetylcysteine (NAC), have failed to improve lung function or quality of life (10). Other therapies that target inflammation, such as corticosteroids and anti-TNF-α monoclonal therapy (infliximab), yield limited improvements on lung function (11–14). It is apparent that approaches reliant on stoichiometric scavenging of oxidants or targeting the action of individual cytokines are not effective therapeutic strategies.
Only 15% of smokers develop emphysema, which suggests that there are genetic determinants of sensitivity to emphysema. Recent studies from our laboratory and others demonstrate that the transcription factor nuclear erythroid 2 p45 related factor-2 (Nrf2) is a key determinant of COPD susceptibility (15–17). Nrf2 is a member of the basic leucine zipper (bZIP) family of transcription factors that share a conserved cap “n” collar domain (18, 19). Nrf2 functions as a critical mediator of an adaptive response to counteract oxidative stress. Under basal conditions, Nrf2 is tethered to its inhibitor Keap1, which facilitates its ubiquitination and proteolytic degradation in the cytoplasm. However, in the presence of electrophilic and/or oxidative stresses, Nrf2 dissociates from Keap1 and translocates to the nucleus, where it binds to other partner proteins and activates the coordinate expression of a large number of antioxidative and electrophile detoxification genes (20). Highlighting the importance of this pathway in COPD, lung tissues and alveolar macrophages from COPD patients exhibit decreased Nrf2 pathway activation, compared with those from healthy smokers (15–17). Furthermore, Nrf2−/− mice develop increased alveolar destruction, and increased oxidative damage, apoptosis, and inflammation, relative to Nrf2+/+ mice, in response to chronic CS (21, 22). This finding suggests that targeting the Nrf2 pathway could have important clinical benefits for patients with COPD.
We have previously described that the synthetic triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole (CDDO-Im) acts as a potent activator of Nrf2 signaling in vitro and in vivo (23–26). Furthermore, a close chemical congener of CDDO-Im, namely CDDO-methyl ester, is currently in phase II clinical trial for treatment of cancer (http://clinicaltrials.gov), which suggests that CDDO-Im has potential to be a viable therapy. In our present study, we tested whether targeting the Nrf2 pathway with CDDO-Im, resulting in transcriptional induction of antioxidative pathways, can attenuate pathological lung damage and emphysema in mice after chronic CS exposure. We have shown that CDDO-Im mitigates the development of emphysema and its associated pathobiology; moreover, this agent has beneficial systemic effects on pulmonary hypertension and systolic and diastolic function of the RV.
Results
CDDO-Im Reduces Alveolar Destruction After Exposure to CS in an Nrf2-Dependent Manner.
C57BL/6J Nrf2+/+ and Nrf2−/− mice were exposed to CS for 6 months, at which time detailed lung morphological measurements were made. CS-induced airspace enlargement was assessed by measuring the mean linear intercept (MLI), which is an index of alveolar size, and alveolar destruction was assessed by measuring surface–volume ratio (S/V), which is an index of septal loss, using computer-assisted stereologic measurements. MLI in Nrf2+/+ mice exposed to CS increased by 17.3% and S/V decreased by 13.7% when compared with air-exposed controls (P < 0.01), indicating that chronic CS exposure significantly induced airspace enlargement (Table 1). Furthermore, the CS-mediated alveolar destruction observed in Nrf2−/− mice was significantly greater than in Nrf2+/+ mice, after CS exposure (P < 0.05) (Table 1). One month of CS exposure was not sufficient to significantly increase MLI in Nrf2+/+ or Nrf2−/− mice (data not shown). The aggregate of these data demonstrates that Nrf2−/− mice have increased susceptibility to CS-induced lung damage.
Table 1.
Effect of CDDO-Im on lung morphometry after chronic exposure to CS
| Mice | CDDO-Im, mg/kg | MLI, μm |
S/V (×1,000) |
||||
|---|---|---|---|---|---|---|---|
| Air | CS | % Increase | Air | CS | % Increase | ||
| Nrf2+/+ | 0 | 39.4 ± 1.0 | 46.2 ± 0.3* | 17.3 | 50.1 ± 1.4 | 43.3 ± 0.7* | −13.7 |
| 60 | 39.4 ± 2.0 | 41.4 ± 0.6† | 5.1 | 49.3 ± 1.3 | 48.2 ± 1.0† | −2.1 | |
| 90 | 39.8 ± 0.7 | 42.8 ± 0.6† | 7.3 | 49.3 ± 2.7 | 46.4 ± 0.9† | −6.0 | |
| Nrf2−/− | 0 | 42.3 ± 0.7 | 52.2 ± 1.4*‡ | 23.5 | 45.6 ± 1.2 | 38.7 ± 1.4*‡ | −15.1 |
| 60 | 42.5 ± 0.5 | 52.8 ± 1.3* | 24.1 | 45.4 ± 0.9 | 38.9 ± 0.7* | −14.3 | |
| 90 | 43.0 ± 0.3 | 50.7 ± 1.2* | 18.1 | 44.0 ± 1.4 | 38.2 ± 1.6* | −13.3 | |
N = 4–6 per group. P < 0.05 was considered significant by Student's two-tailed t test.
*CS-exposed Nrf2+/+ and Nrf2−/− mice exhibited significant airspace enlargement relative to air-exposed controls.
†Nrf2+/+ mice treated with CDDO-Im exhibited significant improvement relative to Nrf2+/+ without CDDO-Im after CS exposure.
‡Nrf2−/− mice exhibited significant airspace enlargement relative to Nrf2+/+ after CS exposure.
Earlier studies established that the Nrf2 activator CDDO-Im exerted a pronounced pharmacodynamic action in the lungs of mice, leading to enhanced Nrf2 transcriptional activity, after oral administration (24). To determine whether CDDO-Im protected against CS-mediated pulmonary pathological damage and emphysema, mice were treated with CDDO-Im (60 or 90 mg/kg diet) throughout the 6-month CS exposure. There was no significant alteration in weight gain with chronic feeding of CDDO-Im (data not shown). Nrf2+/+ mice that were treated with CDDO-Im had significant improvement in both MLI and S/V (P < 0.05) when compared with vehicle-treated mice after exposure to CS (Table 1). No dose response between 60 and 90 mg/kg was observed with regards to the lung protective effects; however, the maximally effective dose could be <60 mg/kg. In Nrf2−/− mice, neither dose of CDDO-Im significantly improved MLI or S/V. Therefore, the effects of CDDO-Im are mediated in an Nrf2-dependent manner.
CDDO-Im Reduces Pulmonary Hypertension and RV Function After Exposure to CS.
To address whether CS impaired RV function in these mice, we examined heart function by 2D echocardiography, tissue Doppler analysis, and pressure–volume loop catheterization. CS exposure did not result in any significant alterations to the left ventricle (data not shown). However, CS exposure increased RV pressure and caused significant impairments to RV diastolic and systolic functions (Table 2). In Nrf2+/+ and Nrf2−/− mice, CS caused a significant increase (45.5% and 42.5%, respectively) in RV end-systolic pressure (RVESP), compared with air-exposed controls (Table 2). This increased pressure was associated with a significant impairment in RV ejection fraction (RVEF), demonstrating decreased RV contractility (Table 2). CS exposure also caused prolongation of the isovolumetric relaxation time (IVRT), demonstrating the presence of diastolic dysfunction (Table 2). Compared with Nrf2+/+ mice, Nrf2−/− mice had significantly decreased RVEF and elongated IVRT, indicating that RV function in Nrf2−/− mice is more adversely affected by CS than in Nrf2+/+ mice.
Table 2.
Effect of CDDO-Im on end-systolic pressure, ejection fraction, and IVRT of the RV after chronic exposure to CS
| Mice | CDDO-Im, mg/kg | Pressure, RVESP, mmHG |
Systolic function, RVEF, % |
Diastolic function, IVRT, msec |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Air, | CS | Increase, % | Air | CS | Increase, % | Air, msec | CS, msec | Increase, % | ||
| Nrf2+/+ | 0 | 22.4 ± 0.9 (7) | 32.6 ± 1.3 (13)* | 45.5 | 67.7 ± 2.9 (7) | 56.3 ± 2.9 (8)* | −16.8 | 19.6 ± 2.3 (7) | 26.0 ± 1.3 (6)* | 32.7 |
| 90 | 25.2 ± 1.2 (6) | 26.3 ± 0.8 (7)† | 4.4 | 63.2 ± 1.2 (8) | 66.2 ± 3.1 (7)† | 4.7 | 21.9 ± 1.1 (7) | 23.0 ± 1.9 (5) | 5.0 | |
| Nrf2−/− | 0 | 23.6 ± 0.5 (5) | 34.2 ± 1.4 (5)* | 42.5 | 63.5 ± 1.9 (5) | 36.8 ± 3.8 (8)*‡ | −42.0 | 20.5 ± 0.5 (6) | 34.8 ± 1.4 (5)*‡ | 69.8 |
| 90 | 24.7 ± 0.6 (6) | 30.5 ± 2.5 (3) | 23.5 | 64.6 ± 1.3 (7) | 44.4 ± 3.1 (3) | −31.3 | 22.0 ± 0.9 (6) | 27.3 ± 1.2 (3) | 24.1 | |
Number of mice per group is listed in parentheses. P < 0.05 was considered significant by Student's two-tailed t test.
*CS-exposed Nrf2+/+ and Nrf2−/− mice exhibited significant cardiac impairment relative to air-exposed controls.
†CDDO-Im caused significant improvement in Nrf2+/+ mice, relative to Nrf2+/+ without CDDO-Im after CS exposure.
‡Nrf2−/− mice had a significant impairment relative to Nrf2+/+ after CS exposure.
CDDO-Im prevented the decrement of all 3 parameters of RV heart function in Nrf2+/+ mice (Table 2) to levels that were comparable to those in air-exposed mice. CDDO-Im treatment resulted in slight, but statistically insignificant, improvements in heart function in Nrf2−/− mice. Therefore, CDDO-Im improved heart function in a largely Nrf2-dependent manner, which correlates with the reduction in airspace enlargement observed in the lungs of mice treated with CDDO-Im.
Transcriptional Induction of Nrf2-Target Genes by CDDO-Im.
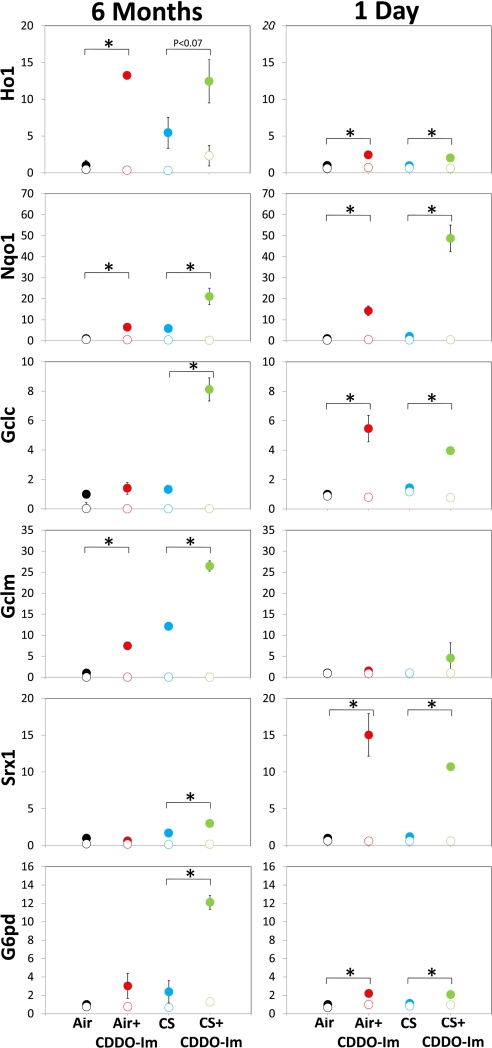
Transcriptional activity of Nrf2 is characterized by up-regulation of expression of >100 genes, many of which have antioxidative functions (21). To determine whether CDDO-Im induced the expression of Nrf2 target genes after chronic CS exposure, we examined gene expression of several known Nrf2-dependent genes in the lungs after 6 months of CS exposure. Lungs were harvested 18 h after the final air/CS exposure. In general, Nrf2 target genes in CS-exposed mice were elevated, compared with air-exposed mice (Fig. 1). CS-exposed Nrf2+/+ mice treated with CDDO-Im exhibited higher expression of several Nrf2 target genes, compared with CS alone. As expected, CDDO-Im did not induce Nrf2-dependent gene expression in Nrf2−/− mice. This observation demonstrates that CDDO-Im enhances the Nrf2-dependent antioxidative response in chronic CS-exposed mice.
Fig. 1.
Relative fold induction of Nrf2-target gene expression. Mice were exposed to air/CS for either 5 h/day for 6 months (Left) or 1 6-h exposure (Right). CDDO-Im (90 mg/kg) was administered in the 6-month group via diet and to the 1-day group by gavage before air/CS exposure. Lungs from mice were harvested 18 h after final exposure, and gene expression of Nrf2-target genes was analyzed by TaqMan. Solid circles represent Nrf2+/+ mice, and open circles represent Nrf2−/− mice. Expression of each gene was normalized to GAPDH, and Nrf2+/+ air was set to 1. Nrf2-target genes analyzed were: heme oxygenase 1 (Ho1), NAD(P)H:quinone oxidoreductase 1 (Nqo1), glutamate cysteine-ligase catalytic subunit (Gclc), glutamate cysteine-ligase modifier subunit (Gclm), sulfiredoxin 1 (Srx1), and glucose-6-phosphate dehydrogenase (G6pd). n = 3 per group. *, P < 0.05 by Student's 1-tailed t test.
We also examined gene expression 18 h after an acute (1 day) CS exposure. The acute CS-exposed mice did not exhibit increased Nrf2-target gene expression compared with air-exposed mice (Fig. 1). However, among CS-exposed mice, mice treated with CDDO-Im had elevated Nrf2-dependent gene expression. Thus, CDDO-Im activates Nrf2-dependent responses after both acute and chronic exposures to CS.
CDDO-Im Reduces CS-Induced Apoptosis and Oxidative Stress in the Lungs.
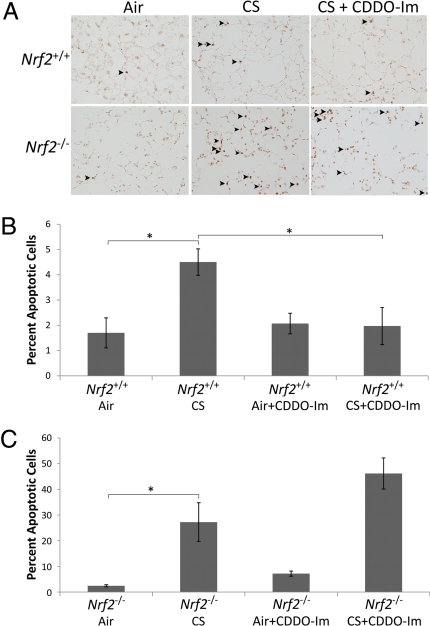
Emphysema has been linked with alveolar cell apoptosis and enhanced oxidative stress (27). Therefore, we investigated whether CDDO-Im-mediated induction of cytoprotective pathways led to decreased oxidative stress and apoptosis in Nrf2+/+ mice. Exposure to CS for 6 months led to an increase in the number of TUNEL-positive cells in both Nrf2+/+ and Nrf2−/− mice. However, this increase was 6-fold greater in Nrf2−/− mice, compared with Nrf2+/+ mice (Fig. 2). One month of CS exposure was not sufficient to increase TUNEL staining in either Nrf2+/+ or Nrf2−/− mice (data not shown). Furthermore, the increase in apoptosis after 6 months of CS corresponded with an increase in cell proliferation in Nrf2+/+ but not in Nrf2−/− mice (Fig. S1). In Nrf2+/+ mice, treatment with CDDO-Im reduced the number of alveolar apoptotic cells to levels observed in air-exposed mice, which was associated with a significant decrease in cellular proliferation. However, CDDO-Im did not reduce the number of apoptotic or proliferative cells in Nrf2−/− mice, after CS exposure. Therefore, the apoptotic profile mirrored the effects of CDDO-Im treatment on lung morphometry, suggesting that apoptosis closely parallels CS-induced alveolar destruction.
Fig. 2.
Treatment with CDDO-Im reduced CS-induced apoptosis in the lungs of Nrf2+/+ mice. (A) Representative images of lung sections from Nrf2+/+ mice stained for TUNEL. (Magnification: 100×.) (B and C) Quantification of TUNEL-positive cells (per 100 DAPI-positive cells) is shown for Nrf2+/+ (B) and Nrf2−/− (C) mice that received either CDDO-Im or control diet. For each mouse, a minimum of 5 fields were captured. n ≥ 4 per group. *, P < 0.05 by Student's 2-tailed t test.
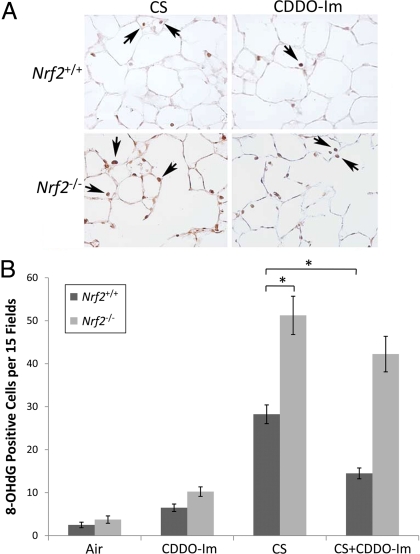
We also assessed whether CDDO-Im reduced oxidative damage after 6 months of CS exposure. To determine the extent of oxidative damage, lungs were stained for the DNA adduct, 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-OHdG). Although CS caused increased 8-OHdG staining in both Nrf2+/+ and Nrf2−/− mice, the level of oxidative damage was significantly greater in Nrf2−/− mice (Fig. 3). CDDO-Im treatment significantly reduced oxidative damage in Nrf2+/+, but not Nrf2−/− mice after chronic CS exposure. One month of CS exposure led to only subtle increases in 8-OHdG staining in Nrf2+/+ mice (data not shown).
Fig. 3.
Treatment with CDDO-Im reduced CS-induced oxidative stress in the lungs of Nrf2+/+ mice. (A) Representative images of immunohistochemical staining for 8-OHdG in lung sections of Nrf2+/+ and Nrf2−/− mice exposed to chronic CS and treated with either CDDO-Im or control diet. (Magnification: 200×.) (B) Quantification of 8-OHdG-positive cells. n = 4 per group. *, P < 0.05 by Student's 2-tailed t test.
We also measured oxidative stress after acute exposure to CS. Mice were exposed to CS for 1 day, and levels of the major antioxidant tripeptide, glutathione, was measured in the lungs. After exposure to CS, glutathione levels decreased significantly in both Nrf2+/+ and Nrf2−/− mice (Fig. S2A). CDDO-Im significantly elevated glutathione levels in both air- and CS-exposed Nrf2+/+ mice, compared with their respective controls (Fig. S2A). In CS-exposed Nrf2−/− mice, CDDO-Im caused a slight increase in glutathione concentration compared with untreated mice. However, the glutathione concentration remained below the level observed in Nrf2−/− air-exposed mice. These results suggest that CDDO-Im induces a protective antioxidant response that is absent in Nrf2−/− mice. Glutathione levels after 1 month of CS exposure were comparable to those observed after 1 day of CS exposure (Fig. S2B).
Discussion
Our findings demonstrate that Nrf2−/− mice have increased alveolar destruction after exposure to chronic CS. The enhanced susceptibility of Nrf2−/− mice to CS is consistent with previous studies in both humans and mice (15–17, 21, 22). The decreased Nrf2 activity in the lungs and macrophages of COPD patients, and the increased susceptibility of Nrf2−/− mice to CS-mediated emphysema, led us to hypothesize that activation of Nrf2 would attenuate the lung destruction caused by CS. We showed in this study that transcriptional induction of Nrf2-regulated antioxidative genes by the small-molecule activator, CDDO-Im, reduced alveolar destruction, lung apoptosis, and oxidative stress imposed by CS exposure.
Cor pulmonale is a common complication of emphysema, and it is closely associated with COPD-mediated mortality. In this study, CDDO-Im reduced RV pressure and improved RV contractility and relaxation. Thus, CDDO-Im can reduce alveolar destruction and improve a major cardiac determinant of COPD-related mortality. Furthermore Nrf2−/− mice exhibited significantly reduced RV function, compared with Nrf2+/+ mice, despite similar RV pressures. This result suggests that Nrf2 is a determinant of RV function, and it is direct in vivo evidence demonstrating that Nrf2 activity impacts cardiac physiology.
CDDO-Im is an exceptionally potent activator of the Nrf2 pathway, exhibiting effects at high picomolar to low nanomolar concentrations in vitro (23, 26). However, at higher concentrations, CDDO-Im has been shown to activate other signaling pathways (25). Although CDDO-Im is a pleiotropic molecule with multiple activities, our studies clearly demonstrate that, at the doses used in this study, CDDO-Im primarily acted in an Nrf2-dependent manner, because CDDO-Im did not significantly reduce indicators of emphysema in Nrf2−/− mice after chronic exposure to CS.
CS is a potent generator of reactive oxygen and nitrogen species, which cause damage to nucleotides, proteins, and lipids. This macromolecular damage, in turn, leads to elevated apoptosis and inflammation. In this study, we showed that CDDO-Im significantly reduced oxidative stress and apoptosis in the lungs of CS-exposed Nrf2+/+ mice, but not in Nrf2−/− mice. However, CDDO-Im did not reduce the number of inflammatory cells in bronchoalveolar lavage fluid and lung parenchyma in CS-exposed Nrf2+/+ mice (Fig. S3). Our previous studies have shown that CDDO-Im can attenuate expression of neutrophilic cytokines and chemokines after acute challenge with LPS (28) or Con A (29).
In response to oxidative stress, Nrf2 coordinately up-regulates the expression of a large cohort of antioxidative and xenobiotic detoxication genes and activates enzymes involved in regeneration of glutathione, thioredoxin, and NADPH. The success of CDDO-Im in preventing the development of emphysema may be caused by its activation of multiple Nrf2-dependent genes. Other antioxidant and antiinflammatory therapies target single antioxidative genes or cytokines, which may limit their effectiveness. Reduced Nrf2 activity in patients with advanced COPD lends support to the importance of an antioxidant-coordinated response in the pathogenesis of CS-induced alveolar destruction (15–17). Furthermore, although most current therapies target inflammation, treatment with CDDO-Im did not reduce inflammation, suggesting that cytoprotection of lung parenchyma from oxidative stress is sufficient to protect against emphysema.
In this study, CDDO-Im was delivered concurrently with CS exposure. This treatment strategy suggests that CDDO-Im could have beneficial effects as a secondary prophylactic that could delay or prevent progression of COPD. Our results provide promise for future clinical trials that target the Nrf2 pathway in active or passive smokers with COPD.
Materials and Methods
Animals and Treatments.
Nrf2+/+ and Nrf2−/− C57BL/6J mice were housed under controlled conditions for temperature and humidity, using a 12-h light/dark cycle. At 10 weeks of age, mice were exposed to CS for 5 h/day, 5 days/week, for 6 months, using a TE-10 smoking machine (Teague Enterprises) and 2R4F reference cigarettes (University of Kentucky, Tobacco Research Institute, Lexington). Chamber atmosphere was monitored for total suspended particles and carbon monoxide, with concentrations of 90 mg/m3 and 350 ppm, respectively. For alveolar morphometry, mice were fed with CDDO-Im (60 or 90 mg/kg diet) throughout the entire duration of CS exposure. For all subsequent experiments, mice were treated with the 90 mg/kg diet dose. All experimental protocols were performed in accordance with the standards established by the U.S. Animal Welfare Acts, as set forth in National Institutes of Health guidelines and in the Policy and Procedures Manual of the Johns Hopkins University Animal Care and Use Committee.
For studies involving acute CS exposures, mice were gavaged with CDDO-Im (13.5 mg/kg body weight) or vehicle at −48, − 24, and 0 h before being exposed to air/CS for 6 h. The dose of CDDO-Im was equivalent to the 90 mg/kg dietary level given for chronic exposure studies. CDDO-Im was synthesized as described (30) and obtained from Reata Pharmaceuticals.
Lung Morphometry.
Eighteen hours after the final CS exposure, lungs were inflated with 0.6% agarose at a constant pressure of 25 cm H2O, as described (31). Lungs were fixed for 24 h in 10% buffered formalin and embedded in paraffin. Sections (5 μm) were stained with H&E, and 15 representative images were captured at 100× magnification. MLI and S/V ratio were determined by using a macro designed with MetaMorph software (Molecular Devices).
Heart Physiology.
RVEF was determined in conscious mice by 2D echocardiography, using a Sequoia Acuson C256 (Siemens Medical Solutions USA) ultrasound machine, equipped with a 15-MHz linear transducer. RVEF was determined by the Simpson method using the apical 4-chamber view. IVRT was measured from the septal wall at the mitral annulus using tissue Doppler imaging. RVESP was determined in anesthetized mice with an SPR-839 4 electrode pressure-volume catheter (Millar Instruments) placed through the RV in the open chest and positioned along the longitudinal axis to record chamber volume by impedance and pressure by micromanometry.
TUNEL Assay.
Apoptotic cells were quantified in the lung parenchymal tissue with the TdT-FragEL DNA Fragmentation Detection Kit (Calbiochem) according to the manufacturer's instructions. Lung sections were stained with Vectashield mounting medium for fluorescence (Vector Laboratories). The number of apoptotic (TUNEL positive) cells was quantified by using the Elements software package (Nikon Instruments). The software determined the number of TUNEL-positive cells through intensity and size of 3,3′-diaminobenzidine staining in each nucleus. Nuclei were identified by double labeling with DAPI.
Oxidative Stress Markers.
The occurrence of oxidative stress in the lung sections was assessed with an anti-8-OHdG antibody (QED Biosciences), followed by staining using the Vectastain Universal ABC-alkaline phosphatase kit (Vector Laboratories). The number of 8-OHdG positive cells in each image was counted manually at 100× magnification.
For determination of glutathione concentration, lungs were harvested from mice immediately after 6-h CS exposure. Glutathione levels in lungs were quantified as described (32). Briefly, lungs were lysed in buffer containing 0.25 M sucrose, 10 mM Tris·HCl, and 1 mM EDTA. Protein was precipitated by adding sulfosalicylic acid to a final concentration of 6.5%, followed by incubation on ice for 10 min and centrifugation at 2,000 × g for 15 min. Total intracellular glutathione was measured by using the glutathione reductase-5,5′-dithiobis(2-nitrobenzoic acid) recycling assay at 412 nm. Total intracellular glutathione levels were determined by quantifying the intracellular glutathione levels and dividing by the protein concentration. The data are expressed as nmol glutathione per mg protein.
Gene Expression.
Total RNA was isolated from lungs 18 h after the last CS exposure, using TRIzol reagent (Invitrogen), and cDNA was generated by using Multiscribe reverse transcriptase (Applied Biosystems). Gene expression was measured using assays on demand probe sets (Applied Biosystems), and reactions were analyzed by using the ABI 7000 Taqman system.
Inflammation.
Inflammation was measured in lung tissues of chronically exposed mice by immunohistochemical staining of macrophages, using the Mac3 antibody (BD Biosciences as described by the manufacturer. Inflammation was measured in bronchoalveolar lavage fluid, as described (21).
Statistical Analyses.
Student's t test was used to determine statistical significance between each group. Values are presented as means ± standard error.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grant HL081205 (to S.B.), National Heart, Lung, and Blood Institute Specialized Centers of Clinically Oriented Research Grant P50HL084945, National Cancer Institute Grant CA78814 (to M.B.S.), and CA94076 (to T.W.K.), National Heart, Lung, and Blood Institute Grant RO1HL66554 (to R.M.T.), the Flight Attendant Medical Research Institute (S.B.), a Maryland Cigarette Restitution Fund research grant (to S.B.), the National Foundation for Cancer Research (M.B.S.), Reata Pharmaceuticals (M.B.S.), National Institute on Environmental Health Sciences Grants P50ES015903, and ES03819. T.E.S. and D.J.B. are supported by National Institute on Environmental Health Sciences Training Grant ES07141, and M.S.Y. is supported by a PhRMA Foundation predoctoral fellowship. H.C.C. is supported by the Bernard A. and Rebecca S. Bernard Foundation.
Footnotes
Conflict of interest statement: M.B.S. is receiving grant support from Reata Pharmaceuticals.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804333106/DCSupplemental.
References
- 1.Heron MP. National Vital Statistics Reports. 5. Vol 56. Hyattsville, MD: National Center for Health Statistics; 2007. Deaths: Leading causes for 2004; pp. 1–96. [PubMed] [Google Scholar]
- 2.MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part One. Am J Respir Crit Care Med. 1994;150:833–852. doi: 10.1164/ajrccm.150.3.8087359. [DOI] [PubMed] [Google Scholar]
- 3.Han MK, McLaughlin VV, Criner GJ, Martinez FJ. Pulmonary diseases and the heart. Circulation. 2007;116:2992–3005. doi: 10.1161/CIRCULATIONAHA.106.685206. [DOI] [PubMed] [Google Scholar]
- 4.Rahman I, et al. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:490–495. doi: 10.1164/rccm.2110101. [DOI] [PubMed] [Google Scholar]
- 5.Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med. 1996;154:1055–1060. doi: 10.1164/ajrccm.154.4.8887607. [DOI] [PubMed] [Google Scholar]
- 6.Sahin U, et al. Lipid peroxidation and glutathione peroxidase activity in chronic obstructive pulmonary disease exacerbation: Prognostic value of malondialdehyde. J Basic Clin Physiol Pharmacol. 2001;12:59–68. doi: 10.1515/jbcpp.2001.12.1.59. [DOI] [PubMed] [Google Scholar]
- 7.MacNee W, Rahman I. Is oxidative stress central to the pathogenesis of chronic obstructive pulmonary disease? Trends Mol Med. 2001;7:55–62. doi: 10.1016/s1471-4914(01)01912-8. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro SD. The pathogenesis of emphysema: The elastase:antielastase hypothesis 30 years later. Proc Assoc Am Physicians. 1995;107:346–352. [PubMed] [Google Scholar]
- 9.Willemse BW, et al. Effect of 1-year smoking cessation on airway inflammation in COPD and asymptomatic smokers. Eur Respir J. 2005;26:835–845. doi: 10.1183/09031936.05.00108904. [DOI] [PubMed] [Google Scholar]
- 10.Decramer M, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): A randomized placebo-controlled trial. Lancet. 2005;365:1552–1560. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- 11.Rennard SI, et al. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:926–934. doi: 10.1164/rccm.200607-995OC. [DOI] [PubMed] [Google Scholar]
- 12.Pauwels RA, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking: European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med. 1999;340:1948–1953. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- 13.Burge PS, et al. Randomized, double-blind, placebo-controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: The ISOLDE trial. Br Med J. 2000;320:1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Group LHSR. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1902–1909. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- 15.Malhotra D, et al. Decline in NRF2 regulated antioxidants in COPD lungs due to loss of its positive regulator DJ-1. Am J Respir Crit Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Suzuki M, et al. Down-regulated NF-E2-related factor 2 in pulmonary macrophages of aged smokers and COPD patients. Am J Respir Cell Mol Biol. 2008;39:673–682. doi: 10.1165/rcmb.2007-0424OC. [DOI] [PubMed] [Google Scholar]
- 17.Goven D, et al. Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax. 2008;63:916–924. doi: 10.1136/thx.2007.091181. [DOI] [PubMed] [Google Scholar]
- 18.Mohler J, Mahaffey JW, Deutsch E, Vani K. Control of Drosophila head segment identity by the bZIP homeotic gene cnc. Development. 1995;121:237–247. doi: 10.1242/dev.121.1.237. [DOI] [PubMed] [Google Scholar]
- 19.Wakabayashi N, et al. Protection against electrophile and oxidant stress by induction of the phase 2 response: Fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci USA. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 21.Rangasamy T, et al. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iizuka T, et al. Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema. Genes Cells. 2005;10:1113–1125. doi: 10.1111/j.1365-2443.2005.00905.x. [DOI] [PubMed] [Google Scholar]
- 23.Dinkova-Kostova AT, et al. Extremely potent triterpenoid inducers of the phase 2 response: Correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci USA. 2005;102:4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yates MS, et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther. 2007;6:154–162. doi: 10.1158/1535-7163.MCT-06-0516. [DOI] [PubMed] [Google Scholar]
- 25.Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7:357–369. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- 26.Liby K, et al. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005;65:4789–4798. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 27.Rahman I. Oxidative stress in pathogenesis of chronic obstructive pulmonary disease: Cellular and molecular mechanisms. Cell Biochem Biophys. 2005;43:167–188. doi: 10.1385/CBB:43:1:167. [DOI] [PubMed] [Google Scholar]
- 28.Thimmulappa RK, et al. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-imidazolide. Biochem Biophys Res Commun. 2006;351:883–889. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osburn WO, et al. Genetic or pharmacologic amplification of Nrf2 signaling inhibits acute inflammatory liver injury in mice. Toxicol Sci. 2008;659:31–39. doi: 10.1093/toxsci/kfn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liby K, et al. The synthetic triterpenoid CDDO-imidazolide suppresses STAT phosphorylation and induces apoptosis in myeloma and lung cancer cells. Clin Cancer Res. 2006;12:4288–4293. doi: 10.1158/1078-0432.CCR-06-0215. [DOI] [PubMed] [Google Scholar]
- 31.Kasahara Y, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.