Abstract
Members of the poxvirus family have been investigated for their applications as vaccines and expression vectors and, more recently, because of concern for their potential as biological weapons. Vaccinia virus, the prototypic member, evolves through multiple forms during its replication. Here, we show a surprising way by which vaccinia hijacks coatomer for early viral biogenesis. Whereas coatomer forms COPI vesicles in the host early secretory system, vaccinia formation bypasses this role of coatomer, but instead, depends on coatomer interacting with the host KDEL receptor. To gain insight into the viral roles of these two host proteins, we have detected them on the earliest recognized viral forms. These findings not only suggest insights into early vaccinia biogenesis but also reveal an alternate mechanism by which coatomer acts.
Keywords: COPI, morphogenesis
Vaccinia virus initiates replication in discrete regions of the host cytoplasm, known as viral factories. Here, viral DNA and proteins first assemble into a core particle, which then undergoes membrane wrapping to become an immature virus (IV). The IV then undergoes extensive membrane transformation to become a mature virus (MV), which then acquires additional layers of membrane from the host trans-Golgi network to become a wrapped virion (WV). Upon transport to the host periphery, the WV fuses with the plasma membrane to become extracellular viruses. Events that lead to MV formation have been intensely investigated, because the MV is the first, most abundant infectious form generated. MV formation also entails a complex process of membrane morphogenesis, which has intrigued not only virologists but cell biologists over the years (1–3).
As viruses often hijack host factors, an intriguing possibility is that host coat proteins, which are well known to act in membrane morphogenesis to form intracellular transport vesicles from host compartmental membrane (4), participate in early viral biogenesis. However, none has been identified thus far. Yet, another issue relates to the origin of the earliest viral membrane, which is needed for the acquisition of viral infectivity. A compelling way to demonstrate a host origin for this viral membrane would be the detection of host integral membrane proteins on early viral forms, but none has been detected thus far.
We became interested in these key issues of vaccinia biogenesis through an initial serendipitous detection of coatomer on vaccinia virus. Pursuing this finding, we find that coatomer is important for early viral formation, and this role is linked to another host protein, the KDEL receptor (KDELR). Further elucidation of how these two proteins play interlinking roles in vaccinia formation suggests insights into viral biogenesis and how coatomer can be regulated.
Results
In the course of using vaccinia virus as an expression vector, we observed by ImmunoGold EM that β-COP, a subunit of coatomer (4), was on the virus (data not shown). Because this initial detection occurred in the context of viral-mediated overexpression of a host protein, we first sought to ascertain the presence of coatomer on wild-type virus that did not encode for any foreign protein. By ImmunoGold EM, we detected β-COP on intracellular infectious forms of the WR strain, MV and WV (Fig. 1A). Subsequently, EM morphometry revealed that β-COP on intracellular viruses was enriched by ≈4-fold over that on the host Golgi, where coatomer is normally concentrated (Table 1). Thus, because a subunit of coatomer was being specifically enriched on the virus, we next sought to determine functionally whether it had a role in vaccinia replication.
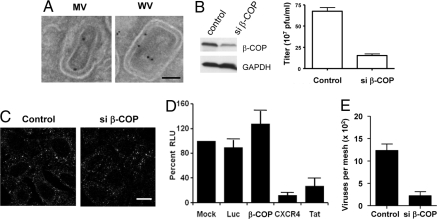
Fig. 1.
Coatomer acts in vaccinia formation. (A) HeLa cells were infected with the WR strain of vaccinia virus for 16 h and then examined by EM with ImmunoGold labeling for β-COP. (Scale bar: 50 nm.) (B) HeLa cells, either treated with siRNA against β-COP for 48 h or mock-treated, were then infected with WR virus for 24 h. The vaccinia replication assay was then performed, with the mean and standard error from 3 experiments shown (P < 0.01). Gel shows the level of β-COP comparing siRNA-treated versus mock-treated HeLa cells. (C) The surface pool of TfR on HeLa cells, either treated with siRNA or mock-treated, was labeled with fluorescently tagged Tf, and then tracked for internalization into early endosomes. (Scale bar: 10 μm.) (D) TZM-bl cells were transfected with siRNAs against different targets, β-COP, CXCR4, Tat, or Luciferase as control, or mock-treated. After infection with HIV-IIIB, β-galactosidase activity was measured, which was expressed as relative light units (RLU), and then normalized to the condition of mock treatment. The mean with standard deviation from 3 experiments is shown. (E) HeLa cells, either treated with siRNA against β-COP for 48 h or mock-treated, were then infected with WR virus for 24 h. Intracellular viral forms were then purified from infected cells, followed by EM examination for intact viruses. Quantitation was performed from 10 randomly selected images, and then expressed as mean with standard error (P < 0.01).
Table 1.
β-COP is highly enriched on vaccinia viral membrane
| Golgi | Vaccina | Endosome/lysome | Plasma membrane |
|---|---|---|---|
| 20 ± 5 | 80 ± 12 | <0.1 | <0.1 |
The density of gold particles labeling for β -COP per 10 μm membrane length was determined by morphometry. In each case, 100 μ m was measured for all compartments in 4 independent sections.
An initial key hurdle was that coatomer had been shown to be essential for eukaryotic cell viability from mammals to yeast (5, 6). Thus, we first sought to define a condition of partially perturbing coatomer that would allow us to detect a potential role for coatomer in vaccinia replication and yet not result in global cellular dysfunction. When HeLa cells were treated with siRNA against β-COP for 48 h, we observed partial reduction in endogenous β-COP (Fig. 1B). When cells were subsequently infected with WR virus for 24 h in this condition, we found that viral replication was reduced by ≈4-fold (Fig. 1B).
To show that this reduction upon the partial crippling of coatomer was not caused by generalized cellular dysfunction, we assessed two distinct parameters. With respect to a host process, we examined clathrin-mediated endocytosis, which did not involve coatomer (4). Tracking the internalization of transferrin (Tf) receptor (TfR) through fluorescently-labeled Tf bound to surface TfR, we found that TfR internalization was not significantly affected (Fig. 1C). We also examined the replication of HIV and found that this viral process was not significantly affected (Fig. 1D). Thus, we concluded that we had achieved a condition of partially perturbing coatomer without leading to generalized cellular dysfunction.
We next considered that the standard vaccinia replication assay measured both viral formation and viral infectivity (7). Thus, we sought to assess viral formation more directly by purifying intracellular viruses from infected cells, and then quantifying for intact viruses by EM examination. Partial silencing of β-COP resulted in a ≈5-fold reduction in intracellular viruses detected (Fig. 1E).
As β-COP is a subunit of coatomer (4), a prediction was that coatomer as an entire complex participated in viral formation. To confirm this idea, we also examined a mutant CHO cell line that had been characterized to express a temperature-sensitive form of ε-COP, which became misfolded at the nonpermissive temperature, resulting in the degradation of the entire coatomer complex (6). To overcome a technical hurdle that vaccinia virus could not replicate in CHO cells because of the lack of a specific host-range factor, we used a recombinant virus that expressed a requisite factor from cowpox to enable vaccinia replication in CHO cells (8). Infection of the mutant CHO cells followed by shift to nonpermissive temperature resulted in vaccinia replication being reduced by ≈5-fold (Fig. 2A). This result was also obtained by partially crippling coatomer, as Western blot analysis revealed residual level remaining (Fig. 2A).
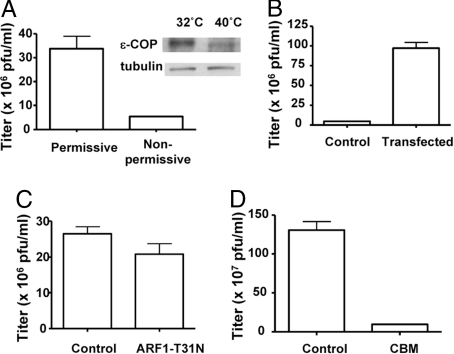
Fig. 2.
Characterizing how coatomer acts in vaccinia formation. (A) A CHO cell line that expressed a temperature-sensitive mutant of ε-COP was incubated either at permissive or nonpermissive temperature for 12 h, and then infected with a recombinant virus (vP30CP77, capable of replicating in CHO cells) for another 24 h. The vaccinia replication assay was then performed, with the mean and standard error from 3 experiments shown (P < 0.01). Gel shows the level of ε-COP, comparing permissive versus nonpermissive temperatures. (B) The mutant CHO cell line that expressed a temperature-sensitive mutant of ε-COP was stably transfected with wild-type ε-COP. After shifting to the nonpermissive temperature for 12 h followed by infection with the recombinant virus (vP30CP77) for 24 h, the vaccinia replication assay was then performed, with the mean and standard error from 3 experiments shown (P < 0.01). (C) HeLa cells, transiently transfected with ARF1-T31N for 48 h or mock-treated, were infected with WR virus for 24 h. The vaccinia replication assay was then performed, with the mean and standard error from 3 experiments shown (P = 0.15). (D) HeLa cells were infected with WR virus for 24 h, with CBM treatment (or mock treatment with vehicle alone) given at the start of the infection. The vaccinia replication assay was then performed, with the mean and standard error from 3 experiments shown (P < 0.01).
However, the use of temperature shift introduced another caveat. Increased temperature that would perturb coatomer was also predicted to enhance viral replication and thereby blunting the inhibitory effect seen by this perturbation. Thus, we stably expressed wild-type ε-COP in the mutant CHO cell line, and then compared this stable transfectant with control cells that only expressed mutant ε-COP at the nonpermissive temperature. By this comparison, we found that viral replication was reduced by ≈16-fold (Fig. 2B). This level of reduction was greater than that seen above by transiently transfecting siRNA and was likely explained by the limitation of the latter approach in uniformly targeting a cell population by transient transfection.
As the results thus far allowed us to conclude that coatomer participated in vaccinia formation, we next sought to gain further insight into its role. In this respect, whereas ARF1 is a key regulator of coatomer for host COPI vesicle formation (9), an earlier vaccinia study had shown that MV formation was not affected by treating cells with brefeldin A (BFA) (10), which would have perturbed ARF1-regulated COPI transport (9). Thus, these observations suggested the possibility that viral usage of coatomer may be independent of host COPI transport. However, we also noted that multiple guanine nucleotide exchange factors (GEFs) had been identified to activate ARF1 in a BFA-insensitive manner (9). Thus, to explore whether the virus still used an ARF1-dependent mechanism to recruit coatomer by usurping a BFA-insensitive GEF, we overexpressed a mutant ARF1 (T31N) that had been shown to act dominantly in preventing ARF1 activation (11). In this setting, vaccinia replication was not significantly affected (Fig. 2C). As control, we confirmed that approximately half of the cell population exhibiting disrupted Golgi (Fig. S1), reflecting the dominant negative effect of this ARF1 mutant (11). Thus, we concluded that viral usage of coatomer likely bypassed the requirement for ARF1 activation.
Searching for an alternate mechanism by which the virus hijacked coatomer, we noted coatomer interacted with transmembrane cargo proteins through specific sequence motifs in the cytoplasmic domain of these host proteins. A major set of these interactions could be feasibly screened by an in vivo approach using a pharmacologic agent, 1,3-cyclohexanebis[methylamine] (CBM), which disrupted the interaction of coatomer with proteins that contained di-basic residues (12). When infected cells were treated with CBM, vaccinia replication was reduced by ≈15-fold (Fig. 2D). Similar effects were also seen by assessing for viral formation more directly, by purifying and then quantifying for the level of intact viruses from infected cells (Fig. S2). Notably, we used a dose of CBM (2 mM) that was shown to be relatively specific in disrupting the binding of coatomer to di-basic residues (12). However, because such a dose of CBM was predicted not to disrupt the binding of coatomer with its targets completely (12), this circumstance further highlighted the practical hurdle in providing a completely quantitative assessment for the degree by which coatomer was important for viral formation.
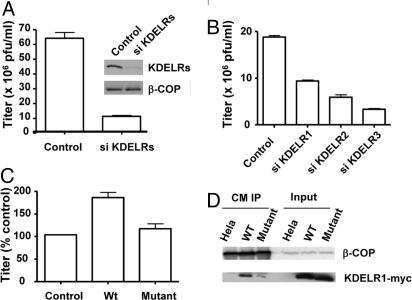
Nevertheless, the effect of CBM suggested that COPI cargo proteins could act as an alternate mechanism by which vaccinia bypassed ARF1 to recruit coatomer. Because one class of cargo, known as the KDELR, had been shown to regulate COPI transport in addition to being a passenger this pathway (13), we next examined its potential role in vaccinia biogenesis. Multiple isoforms of the human KDELR exist and are encoded by 3 genes. Targeting all 3 human genes in HeLa cells by a mixture of siRNAs, we first confirmed that the total cellular level of KDELR was reduced, and then found that vaccinia replication was also reduced (by ≈7-fold; Fig. 3A). Targeting individual isoforms of the KDELR led to lesser reductions (Fig. 3B). Nevertheless, this latter finding suggested a way for us to determine whether its interaction with the KDELR was important for the role of coatomer in viral formation. Previously, mutation of the di-basic residues in KDELR1 had been shown to reduce its interaction with coatomer (14). Thus, we generated HeLa cells that stably expressed either this mutant KDELR1 or the wild-type counterpart, with both also modified to be resistant to siRNA against endogenous KDELR1. Upon the depletion of endogenous KDELR1 by siRNA treatment that followed vaccinia infection, we found that, whereas stable expression of the modified wild-type KDELR1 rescued the effect of siRNA treatment, stable expression of the modified mutant KDELR1 did not (Fig. 3C). Coprecipitation studies on the infected cell lysate confirmed that the mutant KDELR1 had significantly reduced interaction with coatomer (Fig. 3D). Thus, we concluded that both coatomer and KDELR were important for vaccinia formation with their interaction likely linking their roles.
Fig. 3.
Interaction with the KDELR is important for the viral role of coatomer. (A) HeLa cells, either treated with a combination of siRNAs that targets all human KDELR genes for 48 h or mock-treated, were infected with WR virus for 24 h. The vaccinia replication assay was then performed, with the mean and standard error from 3 experiments shown (P < 0.01). Gel shows protein levels in whole-cell lysate by immunoblotting for proteins indicated. (B) HeLa cells, either treated with siRNAs against individual KDELR genes as indicated for 48 h or mock-treated, were infected with WR virus for 24 h. The vaccinia replication assay was then performed, with the mean and standard error from 3 experiments shown. (C) HeLa cells, stably transfected with either a siRNA-resistant form of wild-type KDELR1 or a mutant KDELR1 defective in binding to coatomer or untransfected as control, were treated with siRNA against native KDELR1 for 48 h. Cells were then infected with WR virus for 24 h. The vaccinia replication assay was then performed to obtain a mean with standard error from 3 experiments. Values are normalized to that of control cells. (D) HeLa cells treated with siRNA against KDELR1, stably expressing wild-type KDELR1, mutant KDELR1, or untransfected as control, were infected with virus for 24 h. Coatomer was then immunoprecipitated from cells from the different conditions followed by immunoblotting for the proteins indicated.
To gain further insight into how their roles may be linked, we next examined viral formation by ImmunoGold EM. This goal was initially complicated by the asynchronous nature of viral replication, with MV and WV forms predominating under steady-state conditions. To overcome this technical hurdle, we took advantage of rifampin, a drug shown to act reversibly in blocking viral assembly before IV formation. Upon rifampin washout, early viral assembly could be examined more readily because of a more synchronized process (15). Infecting cells in the presence of rifampin followed by EM examination after 30 min of drug washout, we detected β-COP on partially assembled IVs (Fig. 4A), suggesting that coatomer acted at the earliest stage of viral membrane morphogenesis. Consistent with this possibility, we did not detect an obvious accumulation of any particular assembling viral form when coatomer was perturbed (data not shown).
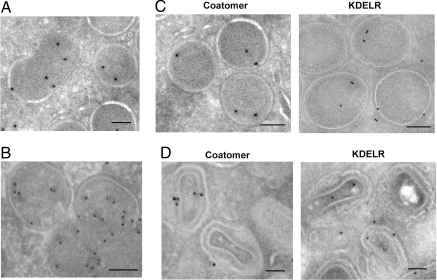
Fig. 4.
Distributions of coatomer and the KDELR on early viral forms. (A) HeLa cells were treated with rifampin and then infected with WR virus for 14 h. After washing out rifampin for 30 min, infected cells were examined by EM with ImmunoGold labeling for β-COP. (B) Cells were infected, treated, and then examined in the same manner as described in A, except ImmunoGold labeling was performed for KDELR. (C) Cells were infected, treated, and then examined in the same manner as described in A. ImmunoGold labeling was performed for either β-COP or KDELR. (D) Cells were infected, treated, and then examined in the same manner as described in A. ImmunoGold labeling was performed for either β-COP or KDELR. (Scale bars: 50 nm.)
ImmunoGold labeling for the KDELR revealed a similar distribution, which was initially observed by using the anti-KDEL antibody to detect endogenous KDELRs (data not shown). However, because the anti-myc antibody gave a greater level of specific labeling, and tagging did not affect the function of the KDELR (13), we also confirmed these initial results by using the anti-myc antibody to detect KDELR1-myc that had been stably expressed in HeLa cells (Fig. 4B). Tracking the fate of β-COP and KDELR1-myc in later stages of viral assembly, we observed similar labeling on assembled IV (Fig. 4C) and MV forms (Fig. 4D).
We also noted that gold particles that tracked β-COP and KDELR appeared to mark not only the outer limiting membrane of viral forms, but also their internal space. Moreover, both host proteins existed in larger amorphous-appearing areas, from which early assembling IV forms arose (Fig. 5A). Thus, because the KDELR is a transmembrane protein, an intriguing possibility was that these amorphous areas contained lipids that eventually became incorporated internally into early viral forms. However, because these amorphous areas did not appear to have typical features of organized membrane by transmission EM, we also considered a possibility that the KDELR existed as protein aggregates in these areas, based on the precedence that certain pathologic conditions could lead to transmembrane proteins accumulating as protein aggregates (16). To distinguish between these two possibilities, we noted that lipids would have significantly lighter buoyant density than proteins. Thus, we performed density gradient analysis on infected cell homogenate, derived from the condition of rifampin washout above, when we had observed KDELR and coatomer to exist mainly on incompletely assembled early viral forms. Upon equilibrium centrifugation, we found that the KDELR in both infected and uninfected cells floated from the bottom of such density gradients (Fig. 5B). In contrast, a chimeric form of mutant huntingtin with polyglutamine expansion, which had been documented to form protein aggregates (16), remained at the bottom of such a gradient (Fig. 5C). Moreover, to determine whether a sufficient level of the KDELR existed on viral structures for detection in the buoyant density gradient, we also performed quantitative ImmunoGold EM. This analysis revealed that ≈36% of KDELR resided on host structures (ER and Golgi), whereas 64% resided on viral structures (Fig. S3). Thus, the level of the KDELR on viral structures predicted that we would have detected the KDELR to behave as protein aggregates by using the buoyant density gradient. Instead, the lack of such detection led us to conclude that the internal space of the assembling early viral forms, as marked by the KDELR, contained lipids.
Fig. 5.
KDELR resides in assembling early viral structures that contain lipids. (A) Cells were infected, treated, and then examined in the same manner as described in Fig. 4, with ImmunoGold labeling for either β-COP or KDELR. (Scale bar: 50 nm.) (B) Infected cells subjected to rifampin washout, as described in Fig. 4, were homogenized and then loaded at the bottom of a sucrose density gradient. After equilibrium centrifugation, fractions from the gradient were immunoblotted for KDELR. (C) HeLa cells transiently transfected with the mutant huntingtin protein tagged by GFP were analyzed in a sucrose density gradient as described in B.
Discussion
We have identified two host proteins, coatomer and the KDELR, to act in vaccinia viral formation. Unlike viral proteins whose expression can be completely deleted to determine whether their roles are absolutely required, such an undertaking for coatomer is unfeasible, because it is fundamental for eukaryotic cell viability from yeast to mammals (5, 6). Thus, we have taken care to perturb coatomer in limited ways and have sought other ways to assess how coatomer could be important in vaccinia formation. Coatomer is the core component of the COPI complex, which represents one of the best-characterized coat proteins (4). Even though ARF1 is critical for this host function of coatomer (9), our findings suggest that the virus bypasses ARF1 in hijacking coatomer for early viral replication. Searching for an alternate mechanism by which coatomer is recruited for viral usage, we find that its interaction with the KDELR is important. The elucidation of this interaction sheds insight into how these two host factors participate in early viral formation.
A major question in vaccinia biogenesis that has not been conclusively resolved is the origin of early viral membrane. Even though initial studies suggest the possibility of de novo synthesis, more recent evidence suggests a host origin (17, 18). However, because this evidence is indirect, our finding that a host integral membrane protein, the KDELR, resides in early viral forms now provides more compelling support for a host origin of early viral membrane. Further characterization of the KDELR on the early viral forms has helped to resolve yet another issue regarding the biogenesis of the vaccinia IV form. A process of early viral membrane encirclement has been suggested, for which the hydrophobic core of the lipid bilayer at the two “open” ends of the encircling IV membrane is proposed to be exposed before the final sealing (3). However, such a situation is predicted to be thermodynamically unfavorable. Our characterization of the KDELR, which concludes lipids to exist in the amorphous viral material that eventually becomes incorporated into the IV, now suggests a reconciling mechanism, by predicting that the open ends of the encircling IV membrane are surrounded by a more hydrophobic environment than previously suspected.
We also note that the KDELR is normally transported between the host ER and Golgi (13). Thus, at first glance, transport pathways that operate between these two host compartments would be presumed to deliver host membrane to viral factories to form assembling viruses. However, a previous study has concluded that MV formation is not affected by perturbing the small GTPase Sar1p, which initiates host COPII transport from the ER (19). Similarly, because perturbing the small GTPase ARF1, either by BFA (10) or the expression of a dominant negative mutant (this study), has no significant effect on viral replication, host COPI transport is also predicted not to be important for early viral formation. Instead, we have found that the virus uses a novel mechanism of recruiting coatomer, which substitutes a critical host regulatory mechanism (ARF1) with another (KDELR). Thus, through a KDELR-related mechanism, the virus likely has recruited coatomer to form a novel transport mechanism for delivery of host membrane to early viral forms. Such a mechanism is also supported by a recent finding that the host Golgin-97 acts in early vaccinia formation (20). Golgins are now recognized as molecular tethers for the docking of host transport vesicles with their target compartment. However, because the host transport pathways of the early secretory system are not important for early viral formation (10, 19), how can Golgin-97 act in viral formation? Our proposal that vaccinia has hijacked coatomer to form novel transport carriers suggests that Golgin-97 may also be hijacked to act as molecular tethers in targeting host membrane (marked by the KDELR) from the early secretory system to viral factories.
Coat proteins have been well characterized to possess two major activities, membrane remodeling and cargo sorting (4). Thus, the detection of coatomer in the earliest stages of viral biogenesis suggests the possibility that coatomer may also participate in the reorganization lipids in the amorphous viral material and possibly recruit other proteins (viral and/or host) to aid in this process. Along this line, it is interesting to note that a “lipid droplet” hypothesis has been proposed for how the encircling of IV membrane is achieved (3), and coatomer has been shown recently to play a key role in lipid droplet formation (21). Thus, because the elucidation of pathogen biology has often uncovered insights into mechanisms of host proteins, more detailed studies in the future on the role of coatomer in vaccinia biogenesis have the additional prospect of contributing to the understanding of a growing number of unconventional cellular roles recently implicated for coatomer, including cytokinesis (22), peroxisome formation (23), and lipid droplet formation (21).
Materials and Methods
Chemicals, Cells, and Viruses.
CBM (Acros Organics) was used at 2 mM, and rifampin (Sigma) was used at 100 μg/mL. HeLa cells used for primary vaccinia infections were maintained in DMEM. BSC-1 cells used for quantifying viral titer were maintained in MEM-α. The mutant CHO cell line that expressed a temperature sensitive mutant of ε-COP (ldlF2; a kind gift from Monty Krieger, Massachusetts Institute of Technology, Cambridge) was maintained in Ham's F-12 medium. A modified ldlF2 cell line that also stably expressed wild-type ε-COP (a kind gift from John F. Presley, McGill University, Quebec, and Jennifer Lippincott-Schwartz, National Institutes of Health, Bethesda) was maintained in RPMI medium 1640. A HeLa cell line that stably expressed KDELR-myc has been described (24). All media were supplemented with 10% FBS, glutamine, and gentamicin. The WR strain of vaccinia virus and a recombinant virus that expressed a host range factor allowing propagation in CHO cells (vP30CP77) were amplified and titrated as described (7). Culturing of TZM-b1 cells and propagation of HIV-IIIB have been described (25).
Antibodies.
Mouse antibodies have been described (14, 26), including anti-β-COP (M3A5), anti-coatomer (CM1A10), anti-Myc (9E10), and anti-tubulin. Rabbit antibodies have also been described (14, 26), including anti-β-COP, anti-ε-COP, anti-giantin, and anti-KDELR (generated against the cytoplasmic tail that has the highest level of conservation among KDELR isoforms). Mouse anti-GFP and rabbit anti-GAPDH antibodies were obtained from Covance. Alexa Fluor 488-conjugated Tf has been described (27).
Plasmids and Transfections.
ARF1-T31N in the mammalian expression vector (pXS) has been described (28). KDELR1-myc in pXS has also been described (24). The mutant huntingtin (103Q) tagged with GFP was obtained from David Rubinsztein (Addenbrooke's Hospital, Cambridge, U.K.). siRNA-resistant forms of KDELR1, wild type and mutant [with di-basic residues mutated to di-serines (14)], were generated by using QuikChange (Strategene) by altering the underlined nucleotides within the following sequence: GTTCAAGGCTACGTACGAT.
Silencing by siRNA.
RNA oligonucleotides targeting human β-COP (5′-GGAUCACACUAUCAAGAAA), Tat (5′- CUGCUUGUACCAAUUGCUAUU), luciferase (5′-CGCCTGAAGTCTCTGATTAA), and human KDELR1 (5′-GUUCAAAGCUACUUACGAU), KDELR2 (5′-ACACAUCUAUGAAGGUUAU), and KDELR3 (5′-GGUACCAGACUGAGAAUUU) were obtained from Dharmacon. RNA oligonucleotides targeting CXCR4 were obtained as a SmartPool (Dharmacon).
Vaccinia Assays.
The vaccinia viral replication assay and purification of intracellular viruses were performed as described (7). Rifampin treatment followed by washout was performed as described (15). Vaccinia infection times and statistical significance expressed as P (using Student's t test) are detailed in the figure legends.
HIV Replication Assay.
Experiments were performed as described (25), with the following modifications: oligonucleotides for siRNAs (50 nM final concentration) were transfected into 2,500 TZM-bl cells in a 96-well format. After 48 h of siRNA-mediated knockdown, the medium was removed and the cells were treated with HIV-IIIB (multiplicity of infection 0.5). After another 48 h, cells were treated with Gal-Screen chemiluminescence reagent (Applied Biosystems) to assess for Tat-dependent transcription of the stably integrated β-galactosidase reporter gene. These results were normalized to cell viability for identically treated wells, as determined by CellTiter-Glo Luminescent Assay (Promega).
Biochemical and Cellular Assays.
Western blot analysis and protein coprecipitation using whole-cell lysates were performed as described (14). Equilibrium centrifugation using sucrose gradients was performed essentially as described (14), except total cell homogenate rather than Golgi-enriched fraction was loaded at the bottom of the gradient for floatation analysis. TfR internalization assay was performed as described (27).
EM.
ImmunoGold EM examination of cryosections was performed essentially as described (29). Specificity of the antibodies used for ImmunoGold labeling has been established for β-COP (29) and KDELR1-myc (24). Purified viruses were examined by EM using the whole-mount technique, as described (14).
Supplementary Material
Acknowledgments.
We thank Jian Li and Ming Bai for advice and Erik Bos for technical assistance. This work was supported by grants from the National Institutes of Health (to V.W.H. and S.N.I.), Telethon Italy (to A.M.), and the Harvard University Center for AIDS Research (to A.L.B.). S.J.E. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811631106/DCSupplemental.
References
- 1.Condit RC, Moussatche N, Traktman P. In a nutshell: Structure and assembly of the vaccinia virion. Adv Virus Res. 2006;66:31–124. doi: 10.1016/S0065-3527(06)66002-8. [DOI] [PubMed] [Google Scholar]
- 2.Sodeik B, Krijnse-Locker J. Assembly of vaccinia virus revisited: De novo membrane synthesis or acquisition from the host? Trends Microbiol. 2002;10:15–24. doi: 10.1016/s0966-842x(01)02256-9. [DOI] [PubMed] [Google Scholar]
- 3.Heuser J. Deep-etch EM reveals that the early poxvirus envelope is a single membrane bilayer stabilized by a geodetic “honeycomb” surface coat. J Cell Biol. 2005;169:269–283. doi: 10.1083/jcb.200412169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMahon HT, Mills IG. COP and clathrin-coated vesicle budding: Different pathways, common approaches. Curr Opin Cell Biol. 2004;16:379–391. doi: 10.1016/j.ceb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Hosobuchi M, Kreis T, Schekman R. SEC21 is a gene required for ER to Golgi protein transport that encodes a subunit of a yeast coatomer. Nature. 1992;360:603–605. doi: 10.1038/360603a0. [DOI] [PubMed] [Google Scholar]
- 6.Guo Q, Vasile E, Krieger M. Disruptions in Golgi structure and membrane traffic in a conditional lethal mammalian cell mutant are corrected by epsilon-COP. J Cell Biol. 1994;125:1213–1224. doi: 10.1083/jcb.125.6.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Earl PL, Cooper N, Wyatt LS, Moss B, Caroll MW. Preparation of cell cultures and vaccinia virus stocks. In: Ausubel FM, et al., editors. Current Protocols in Molecular Biology. New York: Wiley; 1998. pp. 16.16.11–16.16.13. [Google Scholar]
- 8.Ramsey-Ewing A, Moss B. Restriction of vaccinia virus replication in CHO cells occurs at the stage of viral intermediate protein synthesis. Virology. 1995;206:984–993. doi: 10.1006/viro.1995.1021. [DOI] [PubMed] [Google Scholar]
- 9.D'Souza-Schorey C, Chavrier P. ARF proteins: Roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 10.Ulaeto D, Grosenbach D, Hruby DE. Brefeldin A inhibits vaccinia virus envelopment but does not prevent normal processing and localization of the putative envelopment receptor P37. J Gen Virol. 1995;76:103–111. doi: 10.1099/0022-1317-76-1-103. [DOI] [PubMed] [Google Scholar]
- 11.Dascher C, Balch WE. Dominant inhibitory mutants of ARF1 block endoplasmic reticulum to Golgi transport and trigger disassembly of the Golgi apparatus. J Biol Chem. 1994;269:1437–1448. [PubMed] [Google Scholar]
- 12.Hu T, Kao CY, Hudson RT, Chen A, Draper RK. Inhibition of secretion by 1,3-cyclohexanebis(methylamine), a dibasic compound that interferes with coatomer function. Mol Biol Cell. 1999;10:921–933. doi: 10.1091/mbc.10.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoe T, Lee AJ, van Donselaar E, Peters PJ, Hsu VW. Modulation of intracellular transport by transported proteins: Insight from regulation of COPI-mediated transport. Proc Natl Acad Sci USA. 1998;95:1624–1629. doi: 10.1073/pnas.95.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang JS, et al. ARFGAP1 promotes the formation of COPI vesicles, suggesting function as a component of the coat. J Cell Biol. 2002;159:69–78. doi: 10.1083/jcb.200206015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sodeik B, Griffiths G, Ericsson M, Moss B, Doms RW. Assembly of vaccinia virus: Effects of rifampin on the intracellular distribution of viral protein p65. J Virol. 1994;68:1103–1114. doi: 10.1128/jvi.68.2.1103-1114.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravikumar B, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 17.Punjabi A, Traktman P. Cell biological and functional characterization of the vaccinia virus F10 kinase: Implications for the mechanism of virion morphogenesis. J Virol. 2005;79:2171–2190. doi: 10.1128/JVI.79.4.2171-2190.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Husain M, Weisberg AS, Moss B. Existence of an operative pathway from the endoplasmic reticulum to the immature poxvirus membrane. Proc Natl Acad Sci USA. 2006;103:19506–19511. doi: 10.1073/pnas.0609406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husain M, Moss B. Evidence against an essential role of COPII-mediated cargo transport to the endoplasmic reticulum-Golgi intermediate compartment in the formation of the primary membrane of vaccinia virus. J Virol. 2003;77:11754–11766. doi: 10.1128/JVI.77.21.11754-11766.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alzhanova D, Hruby DE. A host cell membrane protein, golgin-97, is essential for poxvirus morphogenesis. Virology. 2007;362:421–427. doi: 10.1016/j.virol.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Y, et al. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008;453:657–661. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skop AR, Liu H, Yates J, 3rd, Meyer BJ, Heald R. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 2004;305:61–66. doi: 10.1126/science.1097931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lay D, Grosshans BL, Heid H, Gorgas K, Just WW. Binding and functions of ADP-ribosylation factor on mammalian and yeast peroxisomes. J Biol Chem. 2005;280:34489–34499. doi: 10.1074/jbc.M503497200. [DOI] [PubMed] [Google Scholar]
- 24.Aoe T, et al. The KDEL receptor, ERD2, regulates intracellular traffic by recruiting a GTPase-activating protein for ARF1. EMBO J. 1997;16:7305–7316. doi: 10.1093/emboj/16.24.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brass AL, et al. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319:921–926. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- 26.Yang JS, et al. Key components of the fission machinery are interchangeable. Nat Cell Biol. 2006;8:1376–1382. doi: 10.1038/ncb1503. [DOI] [PubMed] [Google Scholar]
- 27.Dai J, et al. ACAP1 promotes endocytic recycling by recognizing recycling sorting signals. Dev Cell. 2004;7:771–776. doi: 10.1016/j.devcel.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Peters PJ, et al. Overexpression of wild-type and mutant ARF1 and ARF6: Distinct perturbations of nonoverlapping membrane compartments. J Cell Biol. 1995;128:1003–1017. doi: 10.1083/jcb.128.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kweon HS, et al. Golgi enzymes are enriched in perforated zones of golgi cisternae but are depleted in COPI vesicles. Mol Biol Cell. 2004;15:4710–4724. doi: 10.1091/mbc.E03-12-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.