Abstract
GTF2I and GTF2IRD1 encoding the multifunctional transcription factors TFII-I and BEN are clustered at the 7q11.23 region hemizygously deleted in Williams-Beuren syndrome (WBS), a complex multisystemic neurodevelopmental disorder. Although the biochemical properties of TFII-I family transcription factors have been studied in depth, little is known about the specialized contributions of these factors in pathways required for proper embryonic development. Here, we show that homozygous loss of either Gtf2ird1 or Gtf2i function results in multiple phenotypic manifestations, including embryonic lethality; brain hemorrhage; and vasculogenic, craniofacial, and neural tube defects in mice. Further analyses suggest that embryonic lethality may be attributable to defects in yolk sac vasculogenesis and angiogenesis. Microarray data indicate that the Gtf2ird1 homozygous phenotype is mainly caused by an impairment of the genes involved in the TGFβRII/Alk1/Smad5 signal transduction pathway. The effect of Gtf2i inactivation on this pathway is less prominent, but downregulation of the endothelial growth factor receptor-2 gene, resulting in the deterioration of vascular signaling, most likely exacerbates the severity of the Gtf2i mutant phenotype. A subset of Gtf2ird1 and Gtf2i heterozygotes displayed microcephaly, retarded growth, and skeletal and craniofacial defects, therefore showing that haploinsufficiency of TFII-I proteins causes various developmental anomalies that are often associated with WBS.
Keywords: embryonic, GTF2I, GTF2IRD1
The TFII-I transcription factors represent a versatile protein family with broad functional activities (1–4). The GTF2IRD1 and GTF2I paralogs encoding BEN and TFII-I proteins, respectively, are clustered at the 7q11.23 region hemizygously deleted in Williams-Beuren syndrome (WBS), a disorder characterized by distinctive facial features, mental disability, and growth retardation (5, 6). The protein products of the syntenic Gtf2i and Gtf2ird1 on murine chromosome 5 share considerable sequence homology within a repeated domain, which is involved in sequence-specific DNA-binding properties (7–10). Comparative sequence analysis of the TFII-I family members suggests that these genes have evolved from a single ancestor via duplication and divergence (7, 11). Our previous studies revealed that products of Gtf2ird1 and Gtf2i are expressed very early in mouse development (12–14). Although biochemical properties of these factors have been analyzed over the past few years, little is known about their physiologic role during embryogenesis. To define the individual function of TFII-I proteins, we have generated mouse lines mutant for Gtf2ird1 and Gtf2i alleles and provide evidence that TFII-I family factors are crucial in various aspects of mouse development.
Results and Discussion
Multiple Embryonic Defects in Gtf2ird1−/− and Gtf2i−/− Embryos.
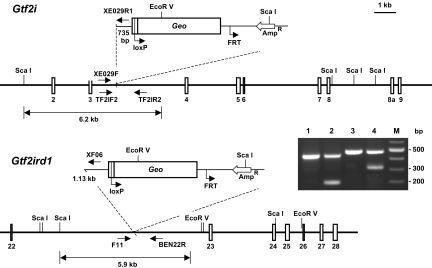
To elucidate the biological functions of Gtf2ird1 and Gtf2i during mammalian embryonic development, we generated 4 mutant mouse lines. One line, designated as Gtf2ird1XE465, was derived from a gene-trap clone carrying the LacZ-neo insertion in the 22nd intron of Gtf2ird1. Three Gtf2i mutant lines were generated carrying independent insertions within intron 2 (Gtf2iRR105), intron 3 (Gtf2iXE029), and intron 9 (Gtf2iXC455) of the Gtf2i locus (Fig. 1). In all 4 gene-trap lines, the LacZ-neo insertion was upstream of the functional nuclear localization signal of BEN and TFII-I. The rationale to use these lines was that the β-galactosidase–fused polypeptides are not able to translocate to the nucleus, therefore creating a KO condition in these mice. The gene-trap clones were injected into blastocysts to produce mice with the inactivated Gtf2ird1 and Gtf2i alleles. All 3 Gtf2i lines produced the same embryonic defects, indicating that disruption of the Gtf2i allele causes these phenotypic manifestations. Therefore, in all our following studies to characterize Gtf2i, we used embryos mainly derived from the XE029 line. The Gtf2ird1XE465 and Gtf2iXE029 lines were maintained on a mixed C57BL/6 and 129Sv background.
Fig. 1.
Inactivation of Gtf2ird1 and Gtf2i using the gene-trap strategy (http:/www.genetrap.org/). Mutant mice were produced from gene-trap lines carrying the β-Geo insertion within intron 22 (clone Gtf2ird1XE465) or intron 3 (Gtf2iXE029). Geo, lacZ-neo insertion; NLS, nuclear localization signal. Animals were genotyped by PCR with primers specific for Gtf2i (TF2IF2/TF2IR2 and XE029F/XE029R1) and Gtf2ird1 (F11/BEN22R/XF06). The WT Gtf2i allele gives a 437-bp band (line 1), whereas the Gtf2iXE029 allele displays a 200-bp product (line 2). Similarly, Gtf2ird1-specific primers amplify a 479-bp fragment in WT DNA (line 3) and an additional 320-bp band from the Gtf2ird1XE465 gene-trapped allele (line 4). Positions of primers used in PCR are shown in the diagram.
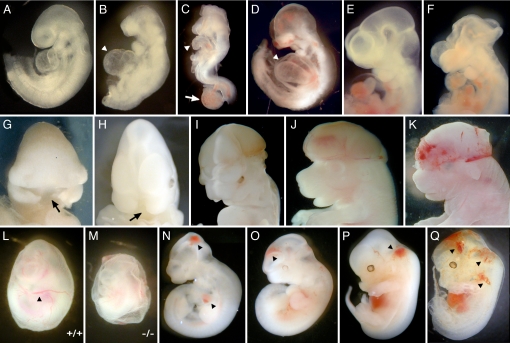
Most mice heterozygous for the Gtf2ird1 and Gtf2i alleles appeared to be normal, based on their external appearance and fertility. F1 heterozygous mice were intercrossed to produce Gtf2ird1−/− and Gtf2i−/− embryos. No homozygotes were recovered at weaning among 367 and 272 offspring of different heterozygous intercrosses of Gtf2ird1 and Gtf2i, respectively. These results indicated that loss of either the Gtf2ird1 or Gtf2i allele causes embryonic lethality. Gtf2ird1−/− and Gtf2i−/− embryos appeared to be delayed in development by 12 to 24 h based on their body size and die between embryonic day (E) 8.5 and E12.5. A large number of homozygous embryos at E8.5 did not initiate axial turning, reflecting retarded development. In some mutant embryos, we observed abnormal development of the allantois, which appeared swollen and failed to fuse to the chorion (Fig. 2C). At E9.5 and E10.5, surviving Gtf2ird1−/− and Gtf2i−/− embryos show growth retardation, dilated pericardial sacs with arrested heart looping, hypoplasia of branchial arches, and small frontonasal primordia (Fig. 2 B–D). Many embryos at this stage were already resorbed. A few embryos were found with midfacial clefts (Fig. 2 G and H). Embryonic hemorrhage of the mutant embryos was apparent at E9.5 (Fig. 2D). The incidence and severity of the hemorrhage increased during development, and at E12.5, over 60% of embryos suffered serious bleeding (Fig. 2 K, N–Q). Mutant embryos displayed varying degrees of intraembryonic bleeding in the head, neck, heart, and back areas. Blood vessels in the yolk sac of Gtf2ird1−/− embryos were poorly developed, resulting in an obviously pale yolk sac compared with that of WT littermates (Fig. 2 L and M). The mutant embryos themselves were pale and showed arrested growth at E9.5, suggesting the possibility of defective hematopoiesis in both the embryo body and the yolk sac attributable to defects in blood vessel formation.
Fig. 2.
Phenotypes of Gtf2ird1−/− (B, F–H, M, and N) and Gtf2i−/− (C, D, I–K, and O–Q) embryos at E9.5–12.5 (I–K). (B–D) Mutants are much smaller compared with a WT littermate (A), and some are resorbed. The morphology of the cranial region of mutants is distinct from that of WT littermates; they lack prominent mesencephalic and telencephalic vesicles. Homozygotes have a small first branchial arch (BA) and a reduced second BA. The dilated pericardial sacs are highlighted by arrowheads. (C) Malformation of the allantois in an E8.5 embryo is shown by a white arrow. The allantois appeared short and swollen and failed to fuse to the chorion in mutant embryos. Gtf2ird1−/− (F–H) and Gtf2i−/− (I–K) embryos showed open neural tube defects (exencephaly) and midfacial clefts (black arrow). The embryo in K has hemorrhaging in the head. The WT E10 embryo is shown in E. (L and M) Yolk sac angiogenesis defects in E10.5 Gtf2ird1−/− embryos. Gross morphology of yolk sac of WT and mutant embryos at E10.5. The mutant yolk sac exhibits a pallid appearance because of the vast reduction in circulating erythrocytes. Large blood vessels and extensive branching of the vessels can be seen in the control yolk sac (black arrowhead in L), but there is absence of an organized yolk sac vasculature in the mutant embryo, although blood islands are readily detectable. (N–Q) Vascular defects in E10.5 and E12.5 Gtf2ird1−/− and Gtf2i−/− embryos. Mutant embryos with hemorrhage in the head, the neck, and the pericardial cavity are shown by black arrowheads.
An open anterior neural tube (exencephaly) was displayed by ≈15% of Gtf2i+/− and 2% of Gtf2ird1+/− mice (Fig. 4B). In homozygous embryos, this number is larger: 60% of Gtf2i−/− embryos have exencephaly compared with 9% of Gtf2ird1−/− embryos (Fig. 2 C, F, I–K). Normally, the neural tube starts to close along the ventral midline around E8.5–9.0 and is completed by E9.5. This process of neurulation is a complex task, which involves cell migration as well as extensive neuroectoderm proliferation and/or cell death. At E9.5, either Gtf2i or Gtf2ird1 homozygotes displayed open and everted cranial neural folds. In most cases, closure defects occurred at the forebrain/midbrain boundary and extended rostrally to the anterior neuropore, caudally to the cervical/hindbrain boundary, or both (Fig. 2 F, I–K).
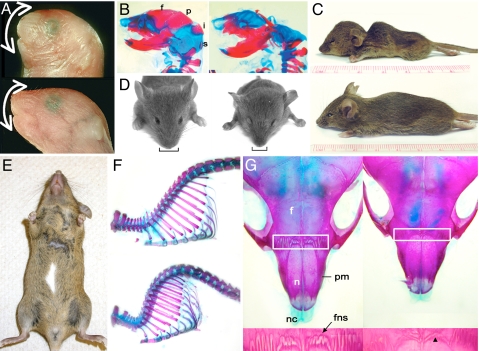
Fig. 4.
Reduced growth, exencephaly, and craniofacial and pigmentation defects in heterozygous animals. (A and B) Lateral views of WT and mutant embryos at E18. (A, C, and D) Subset of Gtf2ird1+/− mice displayed craniofacial defects (A, Top), indicated by the white arrow. (A, Bottom) The WT control is also shown. (B) Defects in the cranial vault in Gtf2i heterozygotes. Skeletal preparations of a WT mouse (Left) and a Gtf2i+/− littermate (Right). The mutant mouse is missing the frontal (f), parietal (p), interparietal (i), and supraoccipital (s) bones. (E) Pigmentation defects in Gtf2ird1+/− animals. A ventral white patch (melanocyte defects) is noticeable on the belly of some of the heterozygous mice. (C) Lateral view of Gtf2ird1+/− mouse at 4 weeks showing kyphosis, an increased backward curvature of the spine in the mutant (Top) compared with the WT littermate (Bottom). (D) Frontal view of the same mice showing the hydrocephalus, the characteristic domed head (a wider but longitudinally shorter skull) and lateral displacement of the ears, and a short narrow snout (black bracket) in the Gtf2ird1+/− mouse (Right) compared with the WT littermate (Left). (F and G) Skeletal staining of Gtf2ird1+/− mouse to show kyphosis, the curvature of the spine (F, Bottom), compared with the WT littermate (F, Top) and the defective frontonasal suture (G, Right). (G) Dorsal view of the snout. The white box marks the frontonasal suture, and the frontopremaxillary suture area is shown in detail in the panel below. The black arrow indicates the control frontonasal suture (Left) and the black arrowhead marks the defective frontonasal suture in the heterozygote (Right). The mutant is a different mouse from what is shown in C and D. f, frontal bone; fns, frontonasal suture; n, nasal bone; nc, nasal cartilage; pm, premaxillary bone.
Expression Pattern of Gtf2ird1 and Gtf2i.
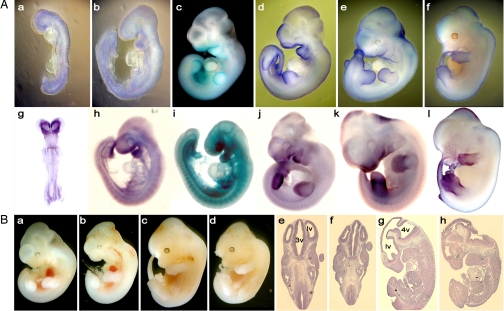
To understand the cause of embryonic defects, we compared the expression of Gtf2ird1 and Gtf2i using whole-mount in situ hybridization and LacZ staining of gene-trap embryos (Fig. 3A). We confirmed the findings of previous immunohistological studies that protein products of Gtf2ird1 and Gtf2i exhibit dynamic expression during embryogenesis (13, 14). In E8.5 embryos, the majority of Gtf2ird1 transcripts are in the rostral parts of developing embryo (Fig. 3A g). Transcripts are also detected in somites and primitive streak. At E9.5, Gtf2ird1 displays a more distinctive pattern of expression, with a high level in the neural crest derivatives, including the frontonasal area, branchial arches, and dorsal root ganglia (Fig. 3A h, i). Additional expression was detected in the neural tube, limb buds, and tail. From E10.5 until E12.5, Gtf2ird1 expression is maintained in the regions of frontonasal primordia, downregulated in somites (Fig. 3 A j–l), and increased in the tail tips and appendages. Gtf2i exhibits a more diffused expression pattern in comparison to Gtf2ird1 (Fig. 3A a–f).
Fig. 3.
Morphological analysis of the head in mutant embryos and expression of Gtf2i and Gtf2ird1. (A) Expression of Gtf2i (a–f) and Gtf2ird1 (g–l) was detected by the β-galactosidase staining and whole-mount in situ hybridization of developing embryos. (B) Brain defects in Gtf2ird1 embryos at E11.5 (b) and E12.5 (d, f, and h) are compared with WT embryos (a, c, e, and g), respectively. (e–h) Parasagittal sections of WT and Gtf2ird1+/− embryos. (b, d, f, and h) Mutant brain shows bitemporal narrowing. lv, lateral ventricle; 3v, third ventricle; 4v, fourth ventricle.
A Subset of Gtf2ird1+/− and Gtf2i+/− Embryos Demonstrate Bitemporal Narrowing and Growth, Skeletal, Craniofacial, and Pigmentation Defects.
In a small subset of Gtf2ird1+/− and Gtf2i+/− embryos at E11.5 and E12.5, the head is smaller (Fig. 3B b, d, f, h), with signs of abnormal brain development compared with that of control embryos. Given that hemizygosity in these genes are causal to a WBS phenotype, we investigated these mice further. The parasagittal histological sections revealed bitemporal narrowing in mutant embryos (Fig. 3B f, h). About 15% of Gtf2ird1+/− mice displayed craniofacial defects (Fig. 4 A and D). ≈11% of Gtf2ird1+/− mice and 10% of Gtf2i+/− mice were significantly smaller than their WT littermates despite being viable and fertile (Fig. 4 C and D). The body weight was 65–75% of the normal weight of adult mice. To evaluate the growth potential of newborn pups, we analyzed WT and Gtf2ird1+/− mice over several months. Heterozygotes grew significantly slower than control pups, indicating poor growth rate (not shown). We also observed pigmentation defects in some heterozygous embryos, albeit at a low frequency. The lack of pigment was observed as white patches of variable size on the belly (Fig. 4E).
Among other abnormalities in Gtf2ird1+/− animals, we observed hydrocephalus and kyphosis, a pronounced arched spine (Fig. 4C). The skeletal staining confirmed that the dorsal “hump” of heterozygous animals resulted from an increased backward curvature of the spine (Fig. 4F). There were no apparent differences between the skulls of mutant and normal adult mice except for the small size and defects in the bones associated with the snout in heterozygotes. The frontonasal suture located between the nasal and frontal bones was abnormal, lacking the degree of interdigitation observed in the corresponding WT sutures (Fig. 4G). In contrast, other sutures of the cranial vault looked normal.
Microarray Studies Revealed Altered Gene Expression Pattern in Gtf2ird1−/− and Gtf2i−/− Embryos.
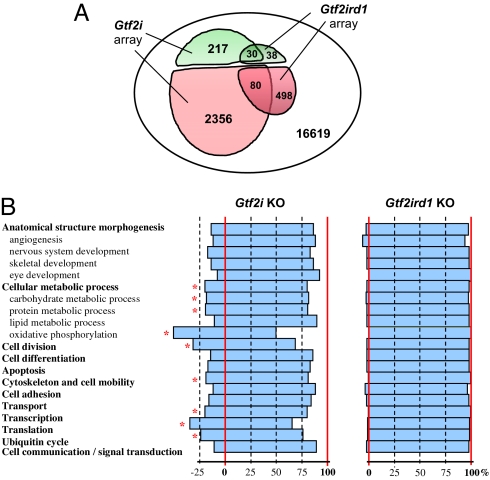
In an effort to identify possible molecular changes caused by the absence of TFII-I proteins, we compared the gene expression profiles of WT and mutant E9.5 embryos by microarray analysis. A total of 217 upregulated and 2,356 downregulated genes with more than 1.5-fold difference were identified in the Gtf2i embryonic array (Fig. 5A). Numbers of genes that changed their expression in the Gtf2ird1 embryonic array were considerably lower: 38 upregulated and 498 downregulated genes. It is remarkable that activities of a substantial portion of these genes showed similar changes in both arrays (Fig. 5B) despite the difference in total numbers of affected genes between Gtf2ird1−/− and Gtf2i−/− embryos, with the exception that only 7 genes were upregulated in the Gtf2i array but downregulated in the Gtf2ird1 array. Gene sorting according Gene Ontology biological functions (15) revealed another difference between Gtf2i and Gtf2ird1 embryonic arrays (Fig. 5B). Namely, downregulated genes in the Gtf2i array are statistically significantly overrepresented by many genes involved in core biological processes such as oxidative metabolism, cell division, transcription, translation, ubiquitin cycle, reorganization of cytoskeleton, and cell motility. In contrast, Gtf2ird1 loss of function did not result in any appreciable clustering of affected genes in these functional categories.
Fig. 5.
Microarray functional profiling with Gene Ontology (GO). (A) Number of genes up-regulated (green) and downregulated (red) and their overlapping sets among total genes represented on the OMM16K chip. (B) GO functional enrichment analysis. Changes are shown as percentages of downregulated genes calculated from total number of genes with the same GO represented on the chip. Statistically significant enrichments (P < 0.05) are indicated by an asterisk.
TFII-I Transcription Factors Regulate TGFβRII/Alk1/Smad5 and Vegfr2 Signal Transduction Cascades.
Defects in establishing a proper cardiovascular system is a frequent cause of early embryonic lethality at midgestation (16), which might explain the observed phenotype of our mutant embryos. Therefore, we analyzed the expression of specific genes that are indispensable in early vasculogenesis and angiogenesis.
Vascular defective embryos described in the literature can be generally divided into 2 groups based on their phenotypes in murine models. One group has an early defect in the development of both hematopoietic and endothelial cells as a result of disruption of the Flk1/vascular endothelial growth factor receptor (VEGFR)/ligand system (17), whereas the other shows abnormal angiogenesis as a consequence of a broken TGFβRII/Alk1/Smad5 signal transduction cascade but maintains intact hematopoietic potential (18).
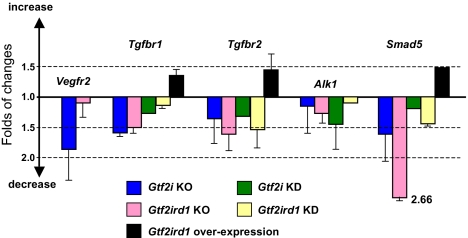
The VEGFR2 (Vegfr2/Kdr/Flk1) functions as the primary mediator of vascular endothelial growth factor activation in endothelial cells. The critical role of VEGFR2 in development was revealed by targeted disruption of this gene in mice (19): yolk-sac blood islands were absent at 7.5 days, organized blood vessels could not be observed in the embryo or yolk sac at any stage, and hematopoietic progenitors were severely reduced. It has been shown that promoter of the VEGFR2 gene is bound and activated by ectopically expressed TFII-I and counterregulated by BEN/TF2IRD1 in pulmonary artery endothelial cells (20, 21). Consistent with these cell-based observations, our current results indicate significant downregulation of the Vegfr2 gene in Gtf2i mutant embryos but not in Gtf2ird1 embryos (Fig. 6).
Fig. 6.
TFII-I transcription factors regulate TGFβRII/Alk1/Smad5 and Vegfr2 signal transduction cascades. Changes in expression of genes involved in early vasculogenesis and angiogenesis in Gtf2i and Gtf2ird1 KO embryos are based on microarray data, Gtf2i and Gtf2ird1 knockdown data were obtained by quantitative (q) RT-PCR after posttranscriptional silencing of Gtf2i or Gtf2ird1 in ES cells by gene-specific siRNAs, and Gtf2ird1 overexpression results are based on qRT-PCR data obtained after doxycycline induction of Gtf2ird1 in a Tet-on ES cell line. Error bars represent the SD calculated from 3 or 2 biological repeats.
It is important to note that phenotypes of Gtf2i−/− and Gtf2ird1−/− were similar to each other but different from the Vegfr2 KO phenotype. Although the vessels in the yolk sac failed to form a robust network, Gtf2i or Gtf2ird1 mutants contained red blood cells and had enlarged embryonic blood vessels (Fig. 2). This phenotype strongly resembles Smad5−/− mutants (22) as well as phenotypes resulting from inactivation of genes for receptors involved in activation of Smad5: Tgfbr1/Alk5 (23), Tgfbr2 (24, 25), and Alk1 (26). In line with this phenotypic observation, our microarray results show a dramatic decrease in the expression of Smad5 and significant downregulation of both TGFβ receptor genes: Tgfbr1 and Tgfbr2 (Fig. 6).
Because the TGFβ pathway is one of the signaling cascades that is initiated during embryonic stem (ES) cell differentiation, we used in vitro differentiating mouse ES cells to test our hypothesis that the TFII-I family of transcription factors is involved in regulation of TGFβRII/Alk1/Smad5 signal transduction. We performed posttranscriptional silencing (knockdown) of Gtf2i or Gtf2ird1 in ES cells by gene-specific siRNAs and observed downregulation of Smad5, Tgfbr1, and Tgfbr2, which mirrors the KO effects (Fig. 6). Conversely, doxycycline induction of Gtf2ird1 overexpression results in a considerable increase in mRNA levels for Smad5, Tgfbr1, and Tgfbr2 in our Tet-on ES cell line (Fig. 6).
Our current studies revealed the importance of TFII-I family transcription factors during mouse embryonic development. The majority of Gtf2ird1−/− and Gtf2i−/− embryos die by E10.5. Gross anatomical analysis indicated that these homozygotes die from multiple developmental defects. Most mutant embryos develop to the gastrulation stage but die before and during midgestation, exhibiting severe defects in the embryonic and extraembryonic vasculature. The finding that disruption of Gtf2ird1 or Gtf2i causes embryonic lethality is consistent with the expression patterns of these genes. Gtf2ird1 and Gtf2i display dynamic expression during both fetal and postnatal development (12–14). The fact that Gtf2ird1−/− and Gtf2i−/− embryos can survive until midgestation might be explained by the presence of maternal proteins that supported early stages of embryonic development before implantation. Alternatively, some functional redundancy may exist between Gtf2ird1 and Gtf2i. The incomplete penetrance of Gtf2ird1−/− and Gtf2i−/− phenotypes such as neural tube and craniofacial defects could be explained by these variables. These results also indicate that Gtf2i cannot compensate for the loss of Gtf2ird1 activity or vice versa at later stages of development. A subset of mice haploinsufficient for Gtf2ird1 or Gtf2i displayed various brain, growth, craniofacial, neural, frontonasal suture, and skeletal defects, indicating that a dose of TFII-I proteins is critical during embryonic development. These observations also suggest that both alleles of Gtf2ird1 and Gtf2i are necessary for normal embryonic growth and development. Taken together, we conclude that TFII-I transcription factors are indispensable during mouse embryonic development.
The results reported here are different from those for the recently described transgenic, knock-in and KO Gtf2ird1 mice that are viable and have either a mild craniofacial or behavioral phenotype (27–29). However, comprehensive identification of transcription start sites by Cap Analysis Gene Expression, together with our preliminary data on alternative splicing in the beginning of Gtf2ird1 (data not shown), suggests that targeted disruption of the first coding exons can leave the possibility for expression of an alternative but functional protein isoform. In such case, the residual expression of Gtf2ird1 in the previously described mouse lines could explain observed discrepancies in phenotypes. In addition, the mouse background used in our studies versus the earlier studies may play a significant role in phenotypic manifestations.
The characteristic facial appearance and dental problems in individuals with WBS (5, 6) suggest that some critical genes within the deleted region may be involved in neural crest development. Our earlier work as well as studies by other investigators has shown that murine Gtf2ird1 and Gtf2i are expressed in embryonic precursors to many structures affected in WBS, including neural crest–derived tissues (12–14, 27). Here, we show that mice heterozygous for these genes are often growth retarded and exhibit hypoplasia of the mandible and other craniofacial defects. There are several reports in which spinal defects, scoliosis, and kyphosis were detected in WBS (30, 31). Collectively, these results suggest that haploinsufficiency for GTF2IRD1 and GTF2I could be responsible for some of the skeletal and craniofacial pathogenesis observed in patients with WBS.
In line with the complexity of the observed phenotypes, microarray data revealed changes in the expression of a wide variety of genes in both Gtf2i and Gtf2ird1 mutants. It is of note that in the Gtf2i array, the number of affected genes was considerably higher than in the Gtf2ird1 array (Fig. 5), and in contrast to the Gtf2ird1 response genes, the vast majority of the Gtf2i response genes were associated with basal cellular processes (Fig. 5). However, a more dramatic shift of general gene expression in Gtf2i homozygotes does not necessarily mean a less significant effect of the Gtft2ird1 loss of function in developing embryos, because changes in the expression of some selected genes are equally strong in both Gtf2i and Gtf2ird1 arrays. Conversely, expression changes of the largest group of genes in the Gtf2i array (genes involved in oxidative phosphorylation) are relatively low, indicating that these changes are possibly indirect. Although the microarray results suggest a broad and more basic function for Gtf2i in comparison to Gtf2ird1, Gtf2ird1 is likely to be important and indispensable in more specific developmental processes. A notable overlap between Gtf2i and Gtf2ird1 affected genes is consistent with the phenotypic similarity of Gtf2i and Gtf2ird1 mutants.
In summary, our studies show the importance of the Gtf2i and Gtf2ird1 genes that encode the TFII-I family transcription factors. The inactivation of each individual locus causes mouse embryonic lethality by midgestation, which may be attributable to defects in yolk sac vasculogenesis and angiogenesis, although the precise molecular effects caused by the inactivation of these genes may be different. We speculate that in Gtf2ird1 homozygous embryos, the phenotype is mainly caused by an impairment of the genes involved in the TGFβRII/Alk1/Smad5 signal transduction pathway. In contrast, the effect of Gtf2i inactivation on this pathway is less prominent, but deterioration of VEGFR signaling is most likely to exacerbate the severity of the Gtf2i mutant phenotype. In addition to the homozygous phenotype, a small subset of Gtf2i or Gtf2ird1 heterozygotes exhibits microcephaly, retarded growth, and skeletal and pigmentation defects, suggesting that even haploinsufficiency of these alleles may cause significant anomalies in developing mouse embryos. Further analysis of the mutant embryos, along with validation of target genes of TFII-I family factors, will undoubtedly lead to uncovering of other signaling pathways and corresponding biological processes that these transcription factors control.
Materials and Methods
Information about the targeting vector, gene-trap clones, PCR probes, protocols, generation of mutant mouse lines, siRNA knockdown, and Tet-on system is available from the authors on request. Whole-mount in situ staining, skeletal preparations, and histology were carried out by standard methods (32). Mouse embryos for expression microarray studies were obtained at the age of E9.5. Total RNA was isolated with RNeasy columns (Qiagen). The RNA integrity and quality for each embryonic sample were ensured by electrophoresis, using the Agilent Bioanalyzer 2100 (Agilent Technologies). Two mutant and 2 WT embryonic RNAs from each female mouse (total of 4 mutant and 4 WT embryos from 2 different female mice) were pooled for the expression microarray analysis. The mouse 16K microarray slide (OMM16K) was the same platform that we used in our recent studies of the expression profiling of BEN and TFII-I in mouse embryo fibroblast cells (33, 34). Two micrograms of total RNA was used for each hybridization experiment. The 3DNA Array 900 kit (Genisphere) designed to provide increased sensitivity for small quantities of RNA was used for labeling reactions. The labeling, hybridization, and scanning were done by the Keck facility at Yale University. Scanning was performed with a GenePix 4000A scanner (Axon Instruments), and the acquired images were analyzed with GENEPIX PRO 5.1 (Molecular Devices). Microarray functional profiling with Gene Ontology was performed using FatiGO+ and FatiScan software (http://babelomics.bioinfo.cipf.es/) (15). Quantitative PCR was carried out on an ABI PRISM 7300 detection system. We used predeveloped TaqMan gene expression assays for quantitative detection of Gtf2i (Mm00494841_ml), Gtf2ird1 (Mm00465654_ml), Smad5 (Mm01341687_g1), Tgfbr1 (Mm00436971_ml), and Tgfbr2 (Mm00436978_ml) and TaqMan Gene Expression Master Mix (Applied Biosystems). Data from triplicates are expressed as normalized expressions by using the delta-delta-Ct calculation method (35) and the Gapdh reference gene (Mm99999915_g1).
Acknowledgments.
This work was supported by National Institutes of Health grants K02DE18412 and R01DE017205 (to D.B.) and R01HD046034 (to A.L.R).
Footnotes
The authors declare no conflict of interest.
References
- 1.Roy AL. Signal-induced functions of the transcription factor TFII-I. Biochim Biophys Acta. 2007;1769:613–621. doi: 10.1016/j.bbaexp.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashworth T, Roy AL. Cutting edge: TFII-I controls B cell proliferation via regulating NF-kappaB. J Immunol. 2007;178:2631–2635. doi: 10.4049/jimmunol.178.5.2631. [DOI] [PubMed] [Google Scholar]
- 3.Hakre S, et al. Opposing functions of TFII-I spliced isoforms in growth factor-induced gene expression. Mol Cell. 2006;24:301–308. doi: 10.1016/j.molcel.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Caraveo G, van Rossum DB, Patterson RL, Snyder SH, Desiderio S. Action of TFII-I outside the nucleus as an inhibitor of agonist-induced calcium entry. Science. 2006;314:122–125. doi: 10.1126/science.1127815. [DOI] [PubMed] [Google Scholar]
- 5.Morris CA, Mervis CB. Williams syndrome and related disorders. Annu Rev Genomics Hum Genet. 2000;1:461–484. doi: 10.1146/annurev.genom.1.1.461. [DOI] [PubMed] [Google Scholar]
- 6.Bellugi U, Lichtenberger L, Mills D, Galaburda A, Korenberg JR. Bridging cognition, the brain and molecular genetics: Evidence from Williams syndrome. Trends Neurosci. 1999;22:197–207. doi: 10.1016/s0166-2236(99)01397-1. [DOI] [PubMed] [Google Scholar]
- 7.Makeyev AV, et al. GTF2IRD2 is located in the Williams-Beuren syndrome critical region 7q11.23 and encodes a protein with two TFII-I-like helix-loop-helix repeats. Proc Natl Acad Sci USA. 2004;101:11052–11057. doi: 10.1073/pnas.0404150101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayarsaihan D, et al. Genomic organization of the genes Gtf2ird1, Gtf2i, and Ncf1 at the mouse chromosome 5 region syntenic to the human chromosome 7q11.23 Williams syndrome critical region. Genomics. 2002;79:137–143. doi: 10.1006/geno.2001.6674. [DOI] [PubMed] [Google Scholar]
- 9.Vullhorst D, Buonanno A. Characterization of general transcription factor 3, a transcription factor involved in slow muscle-specific gene expression. J Biol Chem. 2003;278:8370–8377. doi: 10.1074/jbc.M209361200. [DOI] [PubMed] [Google Scholar]
- 10.Vullhorst D, Buonanno A. Multiple GTF2I-like repeats of general transcription factor 3 exhibit DNA binding properties. Evidence for a common origin as a sequence-specific DNA interaction module. J Biol Chem. 2005;280:31722–31731. doi: 10.1074/jbc.M500593200. [DOI] [PubMed] [Google Scholar]
- 11.Hinsley TA, Cunliffe P, Tipney HJ, Brass A, Tassabehji M. Comparison of TFII-I gene family members deleted in Williams-Beuren syndrome. Protein Sci. 2004;13:2588–2599. doi: 10.1110/ps.04747604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayarsaihan D, Ruddle FH. Isolation and characterization of BEN, a member of the TFII-I family of DNA-binding proteins containing distinct helix-loop-helix domains. Proc Natl Acad Sci USA. 2000;97:7342–7347. doi: 10.1073/pnas.97.13.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayarsaihan D, et al. Expression of BEN, a member of TFII-I family of transcription factors, during mouse pre- and postimplantation development. Gene Expr Patterns. 2003;3:579–589. doi: 10.1016/s1567-133x(03)00118-2. [DOI] [PubMed] [Google Scholar]
- 14.Enkhmandakh B, Bitchevaia N, Ruddle FH, Bayarsaihan D. The early embryonic expression of TFII-I during mouse preimplantation development. Gene Expr Patterns. 2004;4:25–28. doi: 10.1016/s1567-133x(03)00155-8. [DOI] [PubMed] [Google Scholar]
- 15.Al-Shahrour F, et al. BABELOMICS: A systems biology perspective in the functional annotation of genome-scale experiments. Nucleic Acids Res. 2006;34:472–476. doi: 10.1093/nar/gkl172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ihle JN. The challenges of translating knockout phenotypes into gene function. Cell. 2000;102:131–134. doi: 10.1016/s0092-8674(00)00017-9. [DOI] [PubMed] [Google Scholar]
- 17.Carmeliet P, Collen D. Transgenic models in angiogenesis and cardiovascular disease. J Pathol. 2000;190:387–405. doi: 10.1002/(SICI)1096-9896(200002)190:3<387::AID-PATH595>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 18.Goumans MJ, Mummery C. Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol. 2000;44:253–265. [PubMed] [Google Scholar]
- 19.Shalaby F, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Patterson C. The human KDR/flk-1 gene contains a functional initiator element that is bound and transactivated by TFII-I. J Biol Chem. 1999;274:3207–3214. doi: 10.1074/jbc.274.5.3207. [DOI] [PubMed] [Google Scholar]
- 21.Jackson TA, Taylor HE, Sharma D, Desiderio S, Danoff SK. Vascular endothelial growth factor receptor-2: Counter regulation by transcription factors, TFII-I and TFII-IRD1. J Biol Chem. 2005;280:29856–29863. doi: 10.1074/jbc.M500335200. [DOI] [PubMed] [Google Scholar]
- 22.Yang X, et al. Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development. 1999;126:1571–1580. doi: 10.1242/dev.126.8.1571. [DOI] [PubMed] [Google Scholar]
- 23.Larsson J, et al. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J. 2001;20:1663–1673. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oshima M, Oshima H, Taketo MM. TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol. 1996;179:297–302. doi: 10.1006/dbio.1996.0259. [DOI] [PubMed] [Google Scholar]
- 25.Goumans MJ, et al. Transforming growth factor-beta signalling in extraembryonic mesoderm is required for yolk sac vasculogenesis in mice. Development. 1999;126:3473–3483. doi: 10.1242/dev.126.16.3473. [DOI] [PubMed] [Google Scholar]
- 26.Oh SP, et al. Activin receptor-like kinase 1 modulates TFG-beta signaling in the regulation of angiogenesis. Proc Natl Acad Sci USA. 2000;97:2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer SJ, et al. Expression of Gtf2ird1, the Williams syndrome-associated gene, during mouse development. Gene Expr Patterns. 2007;7:396–404. doi: 10.1016/j.modgep.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Tassabehji M, et al. GTF2IRD1 in craniofacial development of humans and mice. Science. 2005;310:1184–1187. doi: 10.1126/science.1116142. [DOI] [PubMed] [Google Scholar]
- 29.Young EJ, et al. Reduced fear and aggression and altered serotonin metabolism in Gtf2ird1-targeted mice. Genes Brain Behav. 2007;7:224–234. doi: 10.1111/j.1601-183X.2007.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osebold WR, King HA. Kyphoscoliosis in Williams syndrome. Spine. 1994;19:367–371. doi: 10.1097/00007632-199402000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Cohen DB, Quigley MR. Thoracolumbar syrinx in association with Williams syndrome. Pediatrics. 2006;118:522–525. doi: 10.1542/peds.2005-2737. [DOI] [PubMed] [Google Scholar]
- 32.Hogan B, Beddington R, Constantini F, Lacy E. Manipulating the Mouse Embryo. A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Lab Press; 1994. [Google Scholar]
- 33.Chimge NO, Mungunsukh O, Ruddle FH, Bayarsaihan D. Expression profiling of BEN regulated genes in mouse embryonic fibroblasts. J Exp Zool. 2007;308:209–224. doi: 10.1002/jez.b.21129. [DOI] [PubMed] [Google Scholar]
- 34.Chimge NO, Mungunsukh O, Ruddle FH, Bayarsaihan D. Gene expression analysis of TFII-I modulated genes in mouse embryonic fibroblasts. J Exp Zool. 2007;308:225–235. doi: 10.1002/jez.b.21134. [DOI] [PubMed] [Google Scholar]
- 35.Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]