Abstract
To produce a word, the intended word must be selected from a competing set of other words. In other domains where competition affects the selection process, the left inferior frontal gyrus (LIFG) responds to competition among incompatible representations. The aim of this study was to test whether the LIFG is necessary for resolution of competition in word production. Using a methodological approach applying the same rigorous analytic methods to neuropsychological data as is done with neuroimaging data, we compared brain activation patterns in normal speakers (using fMRI) with the results of lesion-deficit correlations in aphasic speakers who performed the same word production task designed to elicit competition during lexical selection. The degree of activation of the LIFG in normal speakers and damage to the LIFG in aphasic speakers was associated with performance on the production task. These convergent findings provide strong support for the hypothesis that the region of cortex commonly known as Broca's area (i.e., the posterior LIFG) serves to bias competitive interactions during language production.
Keywords: aphasia, language production, left inferior frontal gyrus, lexical competition
In 1861, Paul Broca wrote “somewhere in these [frontal] lobes, one or several convolutions holds under their dependence one of the elements essential to the complex phenomenon of speech.” In the ensuing century and a half, investigations of the psychological and neural characterization of the “phenomenon of speech” have flourished. Here, we unite the principal method of Broca's day, the assessment of the relation between lesion location and cognitive impairments, with the primary human neuroscientific tool of the modern era, functional magnetic resonance imaging (fMRI), in a rigorous evaluation of one putative element of speech: conflict resolution.
The need to resolve conflict during speech production is not self-evident; producing speech can feel spontaneous and easy. However, every word produced is susceptible to error. The analysis of speech errors, both the relatively infrequent ones that are made by normal speakers and those that occur with much greater frequency in patients with acquired language disorders, has been a rich source of information about the speech production process. Such analyses have revealed that word selection during production is a naturally competitive process, determined by the relative degree of support for (i.e., activation of) a set of candidate words (1–3). The question addressed in this article is whether a region of the frontal lobes commonly referred to as Broca's area [i.e., the posterior portion of the left inferior frontal gyrus (LIFG)], an area implicated in controlled memory retrieval (4), multiple aspects of language processing (5–8), and competition among linguistic and nonlinguistic representations (9, 10), is necessary for the resolution of conflict among competing lexical representations during word production. We posed this question of both normal and impaired speakers, using a word production task that isolates competition during lexical selection. In Exp. 1, we measured normal speakers' brain activations by using fMRI. In Exp. 2, we mapped performance deficits to lesion locations in participants with aphasia.
Our approach has 3 major methodological strengths. First, we used a well-studied speech production paradigm known to create competition during word selection (described in more detail below). Second, we explored both directions of the mapping between brain and behavior, namely, the effects of competitive speech production on the LIFG (with fMRI) and the effects of the LIFG on competitive speech production (with lesion-deficit analyses). Both “directions” of inference are necessary to understand a system with a many-to-many mapping of structure and function (cf. ref. 11). Lastly, our lesion-deficit analysis combined a region-based approach with a statistical innovation in voxel-based lesion-symptom mapping (12) to search for relations with adequate specificity (low type I error) and sensitivity (low type II error).
Exp. 1: fMRI Analysis of Interference Effects on Normal Word Production
Word selection during production is considered a naturally competitive and automatic process. The selection of a word is determined by the word with the highest activation level compared with other activated words (1–3). We manipulated lexical competition during simple picture naming by varying the context in which the pictures to be named appear, with successive trials depicting either semantically related items (semantically blocked context: e.g., truck, car, bike…) or mixed-category items (mixed context: e.g., truck, foot, dog…) named over 4 cycles. This blocking manipulation, referred to as the “blocked naming paradigm,” results in longer naming latencies to an item appearing in a semantic block than to the same item in a mixed block. This blocking effect has been attributed to competition among lexical items that are simultaneously activated because of their semantic relatedness (13–19), making it ideally suited for our purposes. This simple picture-naming task offers advantages over other tasks in that the same items are named in both high and low competition conditions, error rates are low, it is a pure production task (e.g., picture naming only), effects are robust across participants, and it is well studied in both unimpaired and aphasic populations (13–21).
Sixteen healthy volunteers named the pictures aloud in this blocked naming paradigm while undergoing blood-oxygen-level-dependent (BOLD) imaging. As a control for effects of semantic blocking beyond increased competition (e.g., phonological and/or articulatory processes), we compared semantic blocked naming to a closely-matched naming task, phonological-blocked naming (naming pictures that sound the same vs. those mixed between phonological categories; cf. refs. 14 and 22). Blocking in each paradigm induces opposing behavioral effects (semantic interference, phonological facilitation; see Fig. 1 A and B).
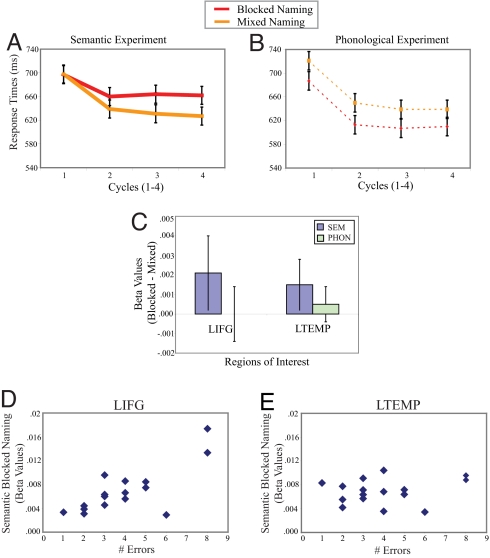
Fig. 1.
fMRI analysis of interference effects on normal word production. (A and B) Naming latencies in semantic and phonological blocked naming paradigms (error bars are 1 SEM). Because naming latencies were not obtained during fMRI scanning, a separate group of 18 volunteers (8 males, 10 females; ages 19–34) participated in a behavioral session so that naming latencies could be used to verify the predicted effects of semantic and phonological blocking with these materials and design. (A) We observed a semantic blocking interference effect (F1 and F2 P < 0.0001) that increased across cycles (interaction F1 P < 0.05, F2 P < 0.0001). (B) In contrast, phonological blocking facilitated naming latencies (F1 and F2 P < 0.0001). (C) fMRI blocking effects (blocked − mixed) in semantic and phonological paradigms, in LIFG and left temporal ROI (y axis reflects activation differences indexed by β values; error bars are 95% confidence intervals). Anatomical regions were defined in each subject by sulcal boundaries. Within each of these anatomical regions, the ROI was further constrained to include only those voxels that were more activated during the first cycle (repetition) of both semantic/phonological and mixed blocks compared with baseline. We used the first cycle to create the functional picture naming ROIs because repetition has been shown to decrease activations in the LIFG and temporal cortex (23). Within each functional–anatomical ROI, we compared activation (indexed by β values) associated with the two naming conditions. (D) The relationship between the numbers of errors produced in semantic blocked naming (x axis) and the difference in signal between semantically blocked naming and the baseline task as indexed by β values (y axis). This relationship was significant in the LIFG ROI (y axis; r = 0.76, P < 0.0001), but not in the left temporal ROI (r = 0.20, P = 0.46).
We examined activity in both the LIFG and a left temporal region (consisting of the left superior and middle temporal gyri), the latter region's involvement suggested by magnetoencephalography evidence in blocked naming (cf. ref. 17). The LIFG was significantly more active during semantically blocked compared with mixed naming [t(1,15) = 2.37, P = 0.03], but was unaffected by phonological blocking (t < 1); see Fig. 1C. A direct comparison of blocking effect sizes for semantic and phonological experiments in the LIFG shows a significant greater involvement of LIFG in semantic blocked naming [t (1,15) = 2.21, P = 0.04]. The left temporal cortex was also significantly more active during semantically blocked compared with mixed naming [t(1,15) = 2.14, P = 0.04], an effect that did not obtain for phonologically-blocked compared with mixed naming [t(1,15) = 1.17, P > 0.20]. However, a direct comparison between blocking effect size in the left temporal cortex for semantic and phonological experiments showed no difference in activation between experiments [t(1,15) = 1.08, P = 0.29].
We also examined the right inferior frontal gyrus as an analogous region of interest (ROI) to the LIFG, and the anterior cingulate cortex because of its role in conflict monitoring in other tasks (24). Neither the anterior cingulate cortex nor the right inferior frontal gyrus was significantly more active in blocked compared with mixed naming in either the semantic or phonological paradigms.
To examine the specificity of the finding from the ROI analyses, we conducted a whole-brain group analysis to see activation across the whole brain. The whole-brain analysis supports the ROI analysis: the LIFG, specifically the pars triangularis in the posterior portion of the LIFG, and the left middle temporal gyrus were significantly more active in semantic blocked naming compared with phonological blocked naming. The whole-brain analysis also revealed that the frontal involvement extended to the left middle frontal gyrus, and additional loci included the right superior temporal gyrus, the insula, and lateral globus pallidus (see SI Text and Table S1).
Error rates across all conditions were low (semantically blocked: 1.2%; semantically mixed: 1.3%; phonologically blocked: 1.1%; phonologically mixed: 1.4%) but sufficiently variable to permit a correlational analysis of individual differences in psychological and neural blocking effects. Errors in semantic blocked naming are attributed to the misselection and production of semantically related competitors (18, 25). Thus, we assumed that a participant's error rate is an index of increased competition during naming. We correlated the number of errors in the semantic blocked condition with the activity in the LIFG, left temporal, right inferior frontal gyrus, and anterior cingulate ROIs. As depicted in Fig. 1D, individuals with a large LIFG response to semantic blocking tended to make more naming errors in the blocked condition (r = 0.76, P < 0.0001); this correlation was not found in the left temporal cortex (r = 0.20, P = 0.46) (Fig. 1E) or the other regions (r < 0.30, P > 0.30). Further, the LIFG correlation was significantly different from the correlations in the other ROIs (Steigler's Z > 2.8, P < 0.01). To rule out the possibility that the LIFG responds as an “error detector,” we also correlated activity in the LIFG with the number of errors produced during the other 3 naming conditions (phonological blocked, semantic, and phonological mixed). We found no significant correlations (r < 0.15, P > 0.70). These analyses suggest that the LIFG responds to competition as measured by selection difficulty (e.g., erroneous naming) during blocked naming.
In summary, both the LIFG and left temporal cortex responded to the semantic blocking manipulation during speech production. This pattern was specific to semantic blocking (i.e., dissociated from phonological blocking) only in the LIFG. Additionally, the magnitude of the semantic blocking effect in the LIFG (but not left temporal cortex) correlated with the number of errors produced. We interpret these neural blocking effects as the result of increased demands to resolve conflict among competing lexical items made active by the blocking manipulation.
Exp. 2: Lesion Analysis of Interference Effects on Impaired Word Production
To assess whether the LIFG is not only involved but also necessary to resolve competition during word production, we mapped lesions in a subset of patients reported in Schnur et al.'s (18) semantic blocked naming experiment (Exp. 2). In 18 patients with poststroke aphasia, Schnur et al. observed a significant semantic blocking effect (phonological blocking was not manipulated), measured in errors; the magnitude of the blocking effect increased across naming cycles. These effects were significantly larger in patients diagnosed with Broca's aphasia, a syndrome characterized by low fluency and agrammatic speech and associated with anterior damage, compared with patients with different aphasia syndromes. However, damage to Broca's area (i.e., the posterior LIFG) is neither necessary nor sufficient to produce Broca's aphasia, so these results do not elucidate the putative role of this region in speech production. Thus, in Exp. 2 we conducted an anatomical study with 12 of 18 patients from Schnur et al. to definitively assess whether lesion location, specifically the LIFG, predicts impairment in resolving competition during naming. The only criterion for participation was the ability and willingness to undergo neuroanatomical scans (12 of 18 patients qualified); behavioral performance varied. Based on Schnur et al.'s findings, we predicted that damage to the LIFG would be associated with an increase in interference at later naming cycles (i.e., as competition among names increases but the capability to resolve the competition is impaired), whereas those with lesions outside the LIFG might show an overall interference effect, but one that did not increase at later cycles (i.e., competition among names increases but is resolved by an intact LIFG). Because these deficit analyses do not suffer from the temporal limitations of BOLD data, in this experiment we could specifically test the association between LIFG and the growth of interference over temporal repetitions.
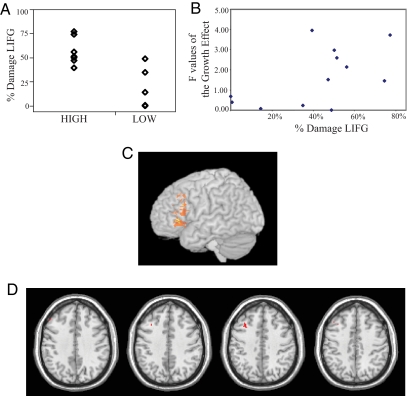
We performed a ROI analysis in which we assessed whether the percentage damage in either the LIFG or the left temporal cortex predicted performance during naming in the blocked naming task (specifically, blocking growth, the increase in the blocking effect across naming cycles). As the relation between degree of damage and behavior is not particularly well characterized by a linear function, we computed linear contrasts of the condition by cycle effect, to classify each patient as having either a large growth effect (i.e., only positive increases in blocking interference across cycles, f > 1; mean = 2.61, n = 7) or a small growth effect (e.g., no increase in blocking interference across cycles, f < 1; mean = 0.27, n = 5). Patients showed a range in behavioral performance (F values 0–3.95, see Table S2). Individuals with a large growth effect had a greater extent of damage in the LIFG than did those with a small growth effect [t(10) = 3.59; P < 0.01] (see Fig. 2A). There was no difference between these 2 groups in the extent of damage in the left temporal ROI [t(10) = 1.44; P > 0.15]. These results support our hypothesis that the LIFG plays a specific role in the regulation of competition that emerges in the semantic blocking paradigm.
Fig. 2.
Lesion analysis of interference effects on impaired word production. (A) In the ROI analyses of lesion-deficit associations, patients who exhibited a large (HIGH) growth of interference across cycles of blocked naming (n = 7) exhibited a greater extent of damage to the LIFG (expressed as a percentage of ROI volume) than did patients with a small (LOW) growth effect [n = 5; t(10) = 3.59; P < 0.01]. [Reproduced with permission from ref. 37 (Copyright 2005, Elsevier).] (B) The correlation (r = 0.56) between the magnitude of the growth effect (individual f value describing the linear increase in semantic blocking across cycles) and the extent of damage to the LIFG. This relationship was not obtained in the left temporal ROI (r = 0.20 P = 0.52). (C) Results of a voxel-based comparison of the lesion locations of patients with a large or small growth effect. The subtraction overlay analysis reveals the number of lesioned voxels in one group that overlap in a location not shared by the other group, across the whole brain. Voxels colored yellow were damaged in 7 of the 7 patients with a large growth effect (100%) and 0 of the 5 patients with a small growth effect (0%); voxels colored orange were damaged in at least 6 of the patients with a large growth effect and in no more than 1 of the patients with a small growth effect. (D) The effect of damage on naming behavior, evaluated at every voxel in which at least 1 patient was represented. To control for type I error (avoiding false positives) across the 62,990 comparisons, while maintaining adequate sensitivity (controlling type II error, or false negatives), we determined the critical threshold via permutation test. Across the brain, we randomly repaired the voxels' associated lesion values and behavioral scores 1,000 times. For each permutation, the maximum statistic observed anywhere in the brain was recorded. The 95th percentile maximum statistic is the threshold at which, when our experimental hypothesis is false, we expect to see even a single more extreme value only 5% of the time. Using this threshold can therefore be said to control the false positive rate at a mapwise corrected α of 0.05 (26). This procedure naturally accounts for the nonindependence of the 62,990 voxels in this sample (because for example, many voxels clustered together in 1 lesioned region will all have identical values of “lesioned”). Permutation testing provides the same kind of control as Bonferroni correction (i.e., control of the familywise error rate), while taking the nonindependence of the observations into account. Shown is the only region containing voxels that surpassed this threshold (t > 5.4): the dorsal region of the LIFG (BA 44).
Correlational analyses support this conclusion. There was no correlation between the magnitude of a patient's growth effect (f value) and that individual's age, months poststroke, overall lesion size, or percentage damage to left temporal cortex (all P > 0.25). In contrast, the correlation between percentage damage to LIFG and the growth effect was marginally significant (r = 0.56, P < 0.06) (see Fig. 2B).
To confirm results from the ROI analysis and assess whether other (nonpredicted) anatomical areas contributed to the behavioral deficit, we performed 2 whole-brain analyses. Using the 2 patient groups described above (high and low growth effects), we compared the lesion distribution between these groups by creating a lesion subtraction map (see Fig. 2C). The results show that the most common area of lesion overlap for the group of patients with a large growth effect is centered in the LIFG. This result was confirmed by the statistical comparison of the behavioral growth effect (again, the f value) at every voxel as a function of that voxel's status (i.e., damaged versus not damaged) in a given individual. We used permutation testing, a nonparametric approach to significance testing that has been applied to solve the multiple comparison problem in functional imaging (statistical comparisons made at every voxel number >60,000) (26). The advantages of permutation testing are multifold, especially in the case of voxel-based statistical mapping (12). The only voxels that were reliably related to the growth effect (at a permutaton-derived threshold of P < 0.05) were located in the dorsal portion of the LIFG (see Fig. 2D).
In summary, 3 different lesion-deficit analysis strategies converged on the result that damage in the LIFG is associated with growth of interference over cycles of semantically blocked naming. That is, although all aphasic speakers will tend to have difficulty with word production, by definition, their ability to resolve competition that arises in the course of language processing appears to depend on the integrity of the LIFG.
Discussion
We opened with a quotation from Paul Broca, in which he proposes a relation between some part of the left frontal cortex and some aspect of speech production. The 2 experiments presented here provide a detailed specification of both aspects of this putative relation. Our findings indicate that although both the LIFG and the left temporal cortex respond to a manipulation that increases conflict among lexical representations competing for selection during word production, only the LIFG is necessary for resolution of heightened competition during blocked naming. These results provide an interpretation of speech errors in some aphasic patients (i.e., those who have damage to the LIFG) and add to the evidence that the prefrontal cortex functions more generally to regulate cognitive processing by biasing competitive interactions among incompatible representations (27).
We are preceded in our attempt to use the semantic blocking paradigm to understand the role of frontal cortex in speech production by 2 case studies. Patient FAS, whose language testing and single-photon emission computed tomography studies were consistent with dysfunction of left frontal regions, made more errors in semantic blocked compared with mixed naming (20). The effects occurred only on the output side (i.e., in naming, but not a comprehension version of the task) and only when naming required lexical selection from semantics (e.g., picture naming, not reading), suggesting the impairment occurred during production at a lexical level. Another patient, BM, also made more errors naming pictures when blocked by semantic category than when pictures were mixed between categories (21). Although lesion localization information was not available, BM presented with right hemiparesis and her language profile was consistent with transcortical motor aphasia, a left anterior aphasia presentation. Their resulting interpretation, that speakers with aphasia consistent with anterior damage have increased difficulty in producing words in situations of high competition, receives rigorous support from our analyses of a larger group of aphasic speakers in the present study. In so doing, we have also illustrated the great potential of voxel-based lesion-symptom mapping, and, unlike previous applications of these methods, we have done so in a modestly-sized group of patients and with a procedure that provides adequate statistical sensitivity and specificity.
The ability to subject neuropsychological data to the same rigorous analytic methods as is done with neuroimaging data, and then to examine direct parallels between the two, stands to substantially increase our potential for understanding the complex mappings of psychological functions onto neural structures. As demonstrated here, this approach successfully localized the mechanism that resolves heightened competition during word production to the neuroanatomical substrate known as Broca's area. Damage to this mechanism may explain the hesitant multiword speech evinced by those described as Broca's aphasics. Thus, this finding opens an exciting line of research into how multiword speech is produced seemingly effortlessly and why it can be so difficult for those with damage to the prefrontal cortex.
Methods
Exp. 1.
Subjects.
We collected fMRI data from 16 volunteers (5 males, 11 females; ages 18–33). All were right-handed and native English speakers, and none reported neurological or neuropsychological illnesses or were on medication. Participants gave informed consent in accordance with policies of the Institutional Review Board (IRB) of the University of Pennsylvania and were paid for their participation.
Materials.
Each of the 12 semantically-blocked sets comprised 6 achromatic line drawings (e.g., coat, dress, glove, hat, skirt, and sock) from the same semantic category (e.g., clothing; other categories were animals, appliances, body parts, food, furniture, nature, plants, roles, shapes, toys, utensils). The same pictures composed 12 semantically-mixed sets of 6 pictures from different categories; for details see ref. 18. For the phonological blocking control, 72 pictures were grouped into 12 phonologically-blocked sets of 6 items that shared the same initial phoneme (e.g., bear, belt, bird, boat, bone, and boot) and phonologically-mixed sets of items with different onsets (phonological and semantic stimuli shared 16 pictures). We refer to a set of 6 semantic, phonological, or mixed experimental pictures named 4 times as a block. Scrambled versions of every picture were created for a baseline task (detection of a horizontal or vertical line in the image).
Procedure.
Each session contained 12 imaging runs, each comprising 8 blocks of stimuli (2 semantic, 2 mixed, 4 scrambled baseline), alternating between conditions (e.g., semantic, baseline, mixed, baseline, semantic, baseline, etc.). Half of the subjects completed 6 runs of the semantic blocking paradigm (semantic blocks and their mixed controls) followed by 6 runs of the phonological blocking paradigm (phonological blocks and their mixed controls); the other half of the subjects performed the phonological paradigm followed by the semantic paradigm.
In each paradigm, pictures within a set were presented an equal number of times. Each set of 6 pictures was repeated 4 consecutive times (cycles) in a different order, forming a block of 24 pictures. Thus, each participant saw 6 pictures in each of 12 semantic/phonological and 12 mixed sets, cycled 4 times for a total of 576 trials in each paradigm. In the baseline task, 12 scrambled pictures were presented in each of 48 blocks for a total of 576 trials. Each trial contained a fixation (500 ms), a blank screen (200 ms), stimulus presentation (650 ms), and a blank response period (1,150 ms). Throughout the experiment, participants were instructed to name pictures as quickly as possible without sacrificing accuracy. A session lasted ≈2 h.
Image acquisition.
Participants were scanned at the University of Pennsylvania by using a 3-Tesla Siemens Trio scanner with a standard 8-channel head coil. For each participant, T1-weighted anatomical images were obtained at the beginning of the session by using a 3D MPRAGE pulse sequence [repetition time (TR) = 1,620 ms, echo time (TE) = 3 ms, interval time (TI) 950 ms, voxel size 0.9766 × 0.9766 × 1 mm, matrix size 192 × 256 × 160] before T2*-weighted functional images were acquired. We acquired 152 sets of 16 interleaved, axial gradient echo, echoplanar images with TR = 2.5, TE = 30, 64 × 64 pixels in a 19.2-cm field of view, voxel size 3 mm × 3 mm × 5 mm for each run. The image acquisition period was 1,000 ms with a 1,500-ms gap (TR = 2,500 ms) to minimize movement artifacts (28) and allow the experimenter to hear overt responses. The gap was positioned (300-ms poststimulus onset) so that no participant produced a response during image acquisition. Online prospective motion correction was performed with a prospective motion correction (PACE) sequence.
Image processing and analysis.
Functional images were sinc-interpolated in time to correct for the fMRI acquisition sequence and were spatially smoothed with an 8-mm FWHM Gaussian kernel. We used the general linear model as implemented in VoxBo (www.voxbo.org) to analyze data. Analysis included an empirically-derived 1/f noise model, filters removing low temporal frequencies (< 0.0048 Hz), and regressors to model global signal variations and between-scan differences (29). Covariates of no interest were created for each of the 4 cycles of each condition of the picture naming task (e.g., semantic blocked 1, 2, etc.), each lasting 15 s. Each stimulus condition, modeled as a boxcar function, was convolved with a canonical hemodynamic response function.
Exp. 2.
Subjects.
We identified 12 individuals with chronic aphasia secondary to left hemisphere stroke (from a group initially reported in ref. 18) based on their ability and willingness to undergo neuroimaging procedures. All participants were right-handed, native speakers of English with an average age of 53 years (range 35–68), average education of 14 years (range 10–19), and average time from stroke of 63 months (range 10–175). All patients gave informed consent in accordance with the IRB of Albert Einstein Medical Center (behavioral experiment) and the University of Pennsylvania (neuroimaging study). See Table S2 for individuals' ages, months postonset, and aphasia classification.
Materials and procedure.
The stimuli were identical to those described above for the semantic blocking paradigm (patients did not perform the phonological blocking paradigm). Unlike subjects in the fMRI study, patients completed the task at their own pace, with a 5-s response deadline. We varied the response-stimulus interval within subjects (1 or 5 s). The first complete response before the 5-s deadline was taken as the picture-naming response. The target name, correctly pronounced, was scored as correct. Anything else was scored as an error (see ref. 18 for a complete account of the behavioral methods).
The behavioral measure of interest was the growth of interference across naming cycles. For each subject, we calculated the error rate for each picture when in a semantic block and when in a mixed block, by cycle, and then we computed linear contrasts of blocking effect across cycles. For each participant, this process resulted in a linear contrast f value that described the growth of interference over cycles (independent of overall magnitude). Individual behavioral performance was based on 1,150 trials.
Lesion analyses.
For 6 participants, T1-weighted MRI volumes were collected on a 3-Tesla Siemens scanner. Each volume consisted of 160 contiguous axial slices covering the entire brain (matrix size 192 × 256; 1-mm voxels). For 6 participants who were unable or unwilling to undergo an MRI scan, we obtained computed tomography (CT) data in 16-slice scanners. CT scan volumes consisted of at least 44 contiguous axial slices covering the entire brain (matrix size 512 × 512; 2.5- to 3-mm slice thickness; in 1 case, 32 slices of 9-mm slice thickness and in another case 33 slices of 5-mm slice thickness).
We identified lesion boundaries on a standard Montreal Neurological Institute (MNI) template by using MRIcro (30) after reslicing the MNI template to match the angle of acquisition for each participant's scan. We matched each slice of the template to a slice in the participant's scan and manually drew the lesion contour onto the corresponding template on axial slices. With MRI data, this produced a contiguous 3D lesion volume. As CT scans provide fewer slices than the template, we manually interpolated between CT slices to render the lesion on each slice of the template. All lesion reconstructions were performed by the same experimenter; however, for 5 patients, a second experimenter repeated the procedure to allow for a reliability assessment. The degree of reliability (mean percentage volume difference = 23 ± 11; and mean percentage discrepant voxels = 6 ± 5) (discrepant is defined as >2 voxels from the other manually drawn lesion volume), was comparable to the same measures of interrater reliability reported elsewhere (31).
Each participant's lesion volume was then resliced at the angle of the MNI template and transferred to a higher-resolution version of the template (1 × 1 × 1 mm; available as ch2.img in MRIcro) using MRIcro's tri-linear interpolation function. This procedure was necessary to use the Automated Anatomical Labeling (AAL) map in MRIcro (aal.img). Using the AAL map, we defined 2 anatomical ROIs: the LIFG (inferior orbitalis, triangularis, and opercularis), and a temporal lobe area (superior and middle temporal gyri; cf. ref. 17).
Across the 12 patients, the range of overall damage and in each ROI was: overall lesion volume, 41–231 cc; LIFG, 0–77%; left temporal cortex, 0–44%. Table S2 includes for each patient overall lesion volume, the proportion of lesion damage in the LIFG, left temporal cortex and their corresponding anatomical subdivisions in MRICRO (LIFG: triangularis, opercularis, and orbitalis; left temporal cortex: superior and middle temporal gyri; based on ref. 32).
To further assess the specificity of the relation between LIFG damage and interference during word production, we performed 2 whole-brain analyses. First, we compared the distribution of brain lesions in an overlay subtraction analysis, which revealed anatomical lesions unique to the high-growth interference group compared with the low-growth interference group (33, 34). The advantage of this method is that lesions common to both groups are not revealed, only lesions different between groups are shown. The disadvantage of this analysis is that participants must be divided into dichotomous groups, neglecting the reality that behavior is on a continuum (35). To address this concern, and more importantly to give a statistical evaluation of the relationship between behavior and brain damage across the whole brain at every voxel (cf. ref. 36), we used a nonparametric test, a permutation analysis (12, 26) (see Fig. 2D).
Supplementary Material
Acknowledgments.
We thank Esther Lee and Adelyn Brecher for contributions to the data collection and analysis and Gary Dell for helpful discussions. This research was supported by National Institutes of Health Grants HD007425 (to T.T.S.), R01 DC000191 (to M.F.S.), MH60414 (to S.L.T.-S.), and MH067008 (to S.L.T.-S.).
Footnotes
An analysis of this study was presented at the Academy of Aphasia, October 23–25, 2005, Amsterdam, The Netherlands (37).
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805874106/DCSupplemental.
References
- 1.Dell GS. A spreading-activation theory of retrieval in sentence production. Psychol Rev. 1986;93:283–321. [PubMed] [Google Scholar]
- 2.Levelt WJM, Roelofs A, Meyer AS. A theory of lexical access in speech production. Behav Brain Sci. 1999;22:1–75. doi: 10.1017/s0140525x99001776. [DOI] [PubMed] [Google Scholar]
- 3.Roelofs A. A spreading-activation theory of lemma retrieval in speaking. Cognition. 1992;42:107–142. doi: 10.1016/0010-0277(92)90041-f. [DOI] [PubMed] [Google Scholar]
- 4.Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Grodzinsky Y, Santi A. The battle for Broca's region. Trends Cognit Sci. 2008;12:474–480. doi: 10.1016/j.tics.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Hagoort P. On Broca, brain, and binding: A new framework. Trends Cognit Sci. 2005;9:416–423. doi: 10.1016/j.tics.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Martin RC. Language processing: Functional organization and neuroanatomical basis. Annu Rev Psychol. 2003;54:55–89. doi: 10.1146/annurev.psych.54.101601.145201. [DOI] [PubMed] [Google Scholar]
- 9.Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc Natl Acad Sci USA. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson-Schill SL, et al. Verb generation in patients with focal frontal lesions: A neuropsychological test of neuroimaging findings. Proc Natl Acad Sci USA. 1998;95:15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarter M, Berntson GG, Cacioppo JT. Brain imaging and cognitive neuroscience: Toward strong inference in attributing function to structure. Am Psychol. 1996;51:13–21. doi: 10.1037//0003-066x.51.1.13. [DOI] [PubMed] [Google Scholar]
- 12.Kimberg DY, Coslett HB, Schwartz MF. Power in voxel-based lesion-symptom mapping. J Cognit Neurosci. 2007;19:1067–1080. doi: 10.1162/jocn.2007.19.7.1067. [DOI] [PubMed] [Google Scholar]
- 13.Belke E, Meyer AS, Damian MF. Refractory effects in picture naming as assessed in a semantic blocking paradigm. Q J Exp Psychol A. 2005;58:667–692. doi: 10.1080/02724980443000142. [DOI] [PubMed] [Google Scholar]
- 14.Damian MF. Locus of semantic interference in picture–word interference tasks. Psychol Bull Rev. 2003;10:111–117. doi: 10.3758/bf03196474. [DOI] [PubMed] [Google Scholar]
- 15.Damian MF, Als LC. Long-lasting semantic context effects in the spoken production of object names. J Exp Psychol Learn Mem Cognit. 2005;31:1372–1384. doi: 10.1037/0278-7393.31.6.1372. [DOI] [PubMed] [Google Scholar]
- 16.Damian MF, Vigliocco G, Levelt WJM. Effects of semantic context in the naming of pictures and words. Cognition. 2001;81:B77–B86. doi: 10.1016/s0010-0277(01)00135-4. [DOI] [PubMed] [Google Scholar]
- 17.Maess B, Friederici AD, Damian M, Meyer AS, Levelt WJM. Semantic category interference in overt picture naming: Sharpening current density localization by PCA. J Cognit Neurosci. 2002;14:455–462. doi: 10.1162/089892902317361967. [DOI] [PubMed] [Google Scholar]
- 18.Schnur TT, Schwartz MF, Brecher A, Hodgson C. Semantic interference during blocked-cyclic naming: Evidence from aphasia. J Mem Lang. 2006;54:199–227. [Google Scholar]
- 19.Vigliocco G, Vinson DP, Damian MF, Levelt WJM. Semantic distance effects on object and action naming. Cognition. 2002;85:B61–B69. doi: 10.1016/s0010-0277(02)00107-5. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy RA, Kartsounis LD. Wobbly words: Refractory anomia with preserved semantics. Neurocase. 2000;6:487–497. [Google Scholar]
- 21.Wilshire CE, McCarthy RA. Evidence for a context-sensitive word retrieval disorder in a case of nonfluent aphasia. Cognit Neuropsychol. 2002;19:165–186. doi: 10.1080/02643290143000169. [DOI] [PubMed] [Google Scholar]
- 22.Roelofs A. Phonological segments and features as planning units in speech production. Cognition. 1999;14:173–200. [Google Scholar]
- 23.Buckner RL, et al. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- 24.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 25.Vitkovitch M, Humphreys GW. Perseverant responding in speeded naming of pictures: It's in the links. J Exp Psychol Learn Mem Cogn. 1991;17:664–680. [Google Scholar]
- 26.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol. 2005;15:219–224. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Huang J, Carr TH, Cao Y. Comparing cortical activations for silent and overt speech using event-related fMRI. Hum Brain Mapp. 2002;15:39–53. doi: 10.1002/hbm.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aguirre GK, Zarahn E, D'Esposito M. Empirical analyses of BOLD fMRI statistics. 2. Spatially smoothed data collected under null-hypothesis and experimental conditions. NeuroImage. 1997;5:199–212. doi: 10.1006/nimg.1997.0264. [DOI] [PubMed] [Google Scholar]
- 30.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- 31.Fiez JA, Damasio H, Grabowski TJ. Lesion segmentation and manual warping to a reference brain: Intra- and interobserver reliability. Hum Brain Mapp. 2000;9:192–211. doi: 10.1002/(SICI)1097-0193(200004)9:4<192::AID-HBM2>3.0.CO;2-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 33.Berti A, et al. Shared cortical anatomy for motor, awareness, and motor control. Science. 2005;309:488–491. doi: 10.1126/science.1110625. [DOI] [PubMed] [Google Scholar]
- 34.Karnath HO, Berger MF, Kuker W, Rorden C. The anatomy of spatial neglect based on voxelwise statistical analysis: A study of 140 patients. Cereb Cortex. 2004;14:1164–1172. doi: 10.1093/cercor/bhh076. [DOI] [PubMed] [Google Scholar]
- 35.Wu DH, Waller S, Chatterjee A. The functional neuroanatomy of thematic role and locative relational knowledge. J Cognit Neurosci. 2007;19:1542–1555. doi: 10.1162/jocn.2007.19.9.1542. [DOI] [PubMed] [Google Scholar]
- 36.Bates E, et al. Voxel based lesion-symptom mapping. Nat Neurosci. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- 37.Schnur TT, Lee E, Coslett HB, Schwartz MF, Thompson-Schill SL. Brain Lang. 2005;95:12–13. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.