Abstract
The colonic epithelial lining undergoes constant replacement, driven by epithelial stem cells in crypts of Lieberkühn. Stem cells lost because of damage or disease can be replaced by adjacent crypts that undergo fission. The close proximity of an extraordinary number of luminal microbes creates a challenge for this repair process; infection must be prevented while immune system activation and epithelial stem cell genetic damage must be minimized. To understand the factors that modulate crypt/stem cell replacement in the mouse colon, we developed an in vivo acute injury system analogous to punch biopsy of the skin. In contrast to epidermal stem cells, colonic epithelial progenitors did not migrate over the wound bed. Instead, their proliferative expansion was confined to crypts adjacent to wound beds and was delayed to the latter phase of healing. This increased epithelial proliferation was coincident with the infiltration of Trem2 expressing macrophages and increased expression of IL-4 and IL-13 in the wound bed. Interestingly, Trem2−/− mice displayed slow and incomplete wound healing of colonic mucosal injuries. We found the latter phase of healing in Trem2−/− mice showed a diminished burst of epithelial proliferation, increased expression of IFN-γ and TNF-α, diminished expression of IL-4 and IL-13, and increased markers of classical macrophage activation. Ablation of these cytokines in injured WT and Trem2−/− mice demonstrated that their expression ultimately determined the rate and nature of wound healing. These studies show that Trem2 signaling is an important pathway to promote healing of wounds in the colon where stem cell replacement is necessary.
Keywords: biopsy injury, epithelium, macrophage, stem cell, wound healing
The colonic epithelium forms the physical barrier between the host and the colon's luminal contents. It is primarily composed of a single layer of terminally differentiated absorptive enterocytes with contributions from two smaller populations: mucous-secreting goblet cells and enteroendocrine cells. This epithelium undergoes constant replacement driven by tripotent epithelial stem cells located at the base of numerous epithelial invaginations (approximately 400/mm2) called crypts of Lieberkuhn (1). This absorptive barrier encloses a vast population of microbes (upwards of 1012 organisms/ml) that normally promotes numerous beneficial physiologic processes in the host (2, 3). Despite the symbiotic nature of this interaction, breaches of the colonic epithelium, particularly injuries involving focal loss of epithelial stem cells, put the host at risk for localized and systemic infection.
A number of experimental systems have been developed to study the regulatory mechanisms of colonic epithelial homeostasis in response to injury including drugs (e.g., dextran sodium sulfate and indomethacin) and activating mutations within the immune system (e.g., IL-10−/− mice) (4, 5). Previous studies using these systems have suggested that the proliferative response of the colonic epithelium to chronic injury stimuli requires detection of these microbes through Myd88-mediated Toll-like receptor signaling (6–8).
Although these systems produce severe colonic injury, including focal epithelial stem cell loss, they are less conducive to determining the exact mechanism of subsequent wound healing because of the imprecise timing and location of the injury foci. Therefore, we developed a simpler injury system analogous to a widely used punch biopsy system in skin (9) to facilitate our understanding of the process of colonic wound repair. Using this system, we found that the cellular process of healing differs from skin and that Trem2 is an important regulator of inflammatory cytokines that greatly influences the efficiency of epithelial stem cell/barrier replacement.
Results and Discussion
Enhanced Epithelial Proliferation Is Confined to Wound-Adjacent Crypts and Is Delayed in Biopsy Injured WT Mice.
Under the visual guidance of a straight-type rigid miniature endoscope (10, 11), we used 3 French biopsy forceps to create 3–5 discrete, oval-shaped wounds in the mucosal lining of the distal colon of each mouse (Fig. 1A). We selected lesions for further analysis that produced an obvious depression in the mucosa measuring 1.75 ± 0.25 mm along the major axis and 0.25 ± 0.05 mm along the minor axis at the time of the initial biopsy (day 0). We analyzed images of individual lesions that were obtained during repeat endoscopy every two days to quantify changes in the surface area of the depressed region of each lesion. In WT, adult C57BL/6 mice, we observed a reproducible reduction in the average lesion surface area over time: Two days postinjury the wound area was reduced by 45.4% (SD = 9.5%; day 0 = baseline), by day 4 it was reduced by 70.7% (SD = 8.5%), and by day 6 by 98.7% (SD = 1.0%; Fig. 1 A and B).
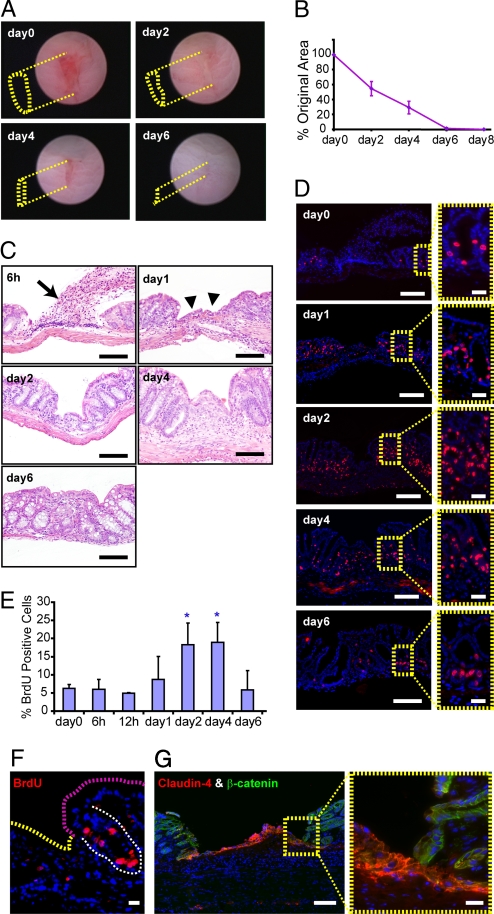
Fig. 1.
Mucosal regeneration of WT mice after colonic biopsy injury. (A) Serial endoscopic views of a biopsy injury site in a WT mouse colon. The yellow dotted lines depict the depressed area margin of the lesion at each time point. No depressed area was observed at day 6. The length of the major axis = 1.75 mm (day 0), = 1.5 mm (day 2), = 1.0 mm (day 4) and = 0.6 mm (day 6). (B) Plot of the average surface area of lesions ± SD relative to the original size of the wound (n = 20 lesions from 5 mice). (C) H+E-stained sections of WT mouse colons at various times post biopsy injury. At 6 h, fibrin, platelets, neutrophils, and red blood cells were extruded and attached to surface of the injured area (arrow). By day 1, clotted material was no longer present and in its place an epithelial cell monolayer had formed over the wound bed (arrowheads). Between days 2 and 4, the cell census of adjacent crypts was expanded. By day 6, crypts repopulated the wound bed. Bars = 100 μm. (D) Serial sections from C stained with BrdU to label cells in S-phase. Insets (yellow dashed boxes) show crypts adjacent to wound. Bars = 100 μm (Insets = 20 μm). (E) Quantification of epithelial cell proliferation (percentage of BrdU positive cells) in the three crypts adjacent to the wound (n = 12 lesions/group from 3 mice per group). At days 2 and 4 postinjury, epithelial cell proliferation was significantly increased as compared with day 0 (P < 0.01; blue asterisks). (F) BrdU stained sections of a WT mouse one day post biopsy injury showing two populations of BrdU negative surface epithelial cells emanating from a crypt (BrdU positive cells). (Scale bar: 20 μm.) (G) Section of WT injury stained with antisera directed against claudin-4 (red) and β-catenin (green). Only the WAE cells are claudin-4 positive (Inset). (Scale bars: 100 μm for main panel and 20 μm for the Inset.)
Analysis of histology and epithelial proliferation showed two distinct phases of healing. Several processes occurred during the first phase. At 6 h postinjury, fibrin, platelets, neutrophils, and red blood cells attached to surface of the wound bed (Fig. 1C). By day 1–2 this cellular material was replaced by a monolayer of epithelial cells (Fig. 1C). Reduction of wound bed surface areas during this initial phase appeared to depend on contracture of the surface epithelium around the wound as adjacent crypts slanted inwards toward the wound bed. Epithelial proliferation did not increase in these crypts during the first day after injury as determined by bromodeoxyuridine (BrdU) incorporation (Fig. 1D).
Two features defined the second phase of mucosal regeneration. First, a statistically significant increase in epithelial proliferation occurred that was confined in space (to the 2–4 crypts adjacent to the wound bed) and time (days 2 and 4 postinjury; P < 0.01, Fig. 1 D and E). Second, an increase in the number of stromal cells was observed in the wound bed beginning at day 2 postinjury. By day 6, recovery of the injured area was nearly complete as epithelial proliferation and stromal cell census had returned to preinjury levels (Fig. 1C).
The epithelial cells that initially covered the wound during the first phase (wound associated epithelial [WAE] cells) did not proliferate from the time of injury to day 4 postinjury. During this time frame, we never observed WAE cells that incorporated BrdU after a one hour pulse label (Fig. 1F for day 1 and data not shown for day 2 and day 4). In addition to being postmitotic, WAE cells expressed high levels of claudin-4 (Fig. 1G), were shorter, and expressed decreased levels of villin relative to surface epithelial cells located distant from the wound bed (supporting information (SI) Fig. S1A). Interestingly, claudin-4 is known to be expressed on follicular associated epithelial cells that overlay mucosal lymphoid aggregates (12, 13). We found that the WAE cells contained the tight junction protein occludin, but not ZO-1, in their cytoplasm (Fig. S1 B and C). Specific mislocalization of occludin in cultured epithelial cells has been associated with leaky tight junctions (14). The source of the WAE cells appeared to be the crypts that were adjacent to the wound bed. To test this idea, we performed a pulse–chase experiment using BrdU incorporation to label crypt epithelial progenitors at the time of biopsy injury (Fig. S1 D and E). After a two day chase period, we readily found BrdU-positive WAE cells (Fig. S1 F and H). After a four day chase period, BrdU-positive WAE cells were no longer present, indicating rapid migration and turnover of these cells (Fig. S1G).
Overall, the process of epithelial wound repair differs when comparing colon to skin. In colon, epithelial proliferation is delayed whereas epidermal stem cells increase proliferation at the time of injury (9). In the colon, postmitiotic WAE cells initially cover the wound bed, whereas in the skin, proliferative epidermal stem cells migrate underneath a persistent clot and later regenerate the epidermis (9).
Efficient Wound Healing Did Not Require Neutrophils or TLR-Signaling.
Recent studies have suggested that colonic epithelial stem cells are strongly influenced by their local stromal microenvironment and Myd88-dependent Toll-like receptor signaling during mucosal injury (7, 15). We quantified the major stromal cell types in both the wound bed and adjacent crypt areas of WT and Myd88−/− mice. Two cell types showed significant increases in both strains: neutrophils peaked during the first phase of healing (6 h postbiopsy) and macrophages peaked during the second phase (days 2–4 postbiopsy; Fig. S2 A and B). In contrast, the number and location of B and T lymphocytes, dendritic cells, myofibroblasts, and endothelial cells were not obviously altered within wound sites in either strain (Fig. S2A).
The increase in stromal neutrophils and macrophages at defined times suggested accompanying alterations in cytokine production. Therefore, we performed qRT-PCR analysis using total RNA extracted from biopsy injury sites, including a 0.5-mm margin of the adjacent colonic mucosa. Interestingly, mRNAs indicative of Th1 cytokine stimulation (TNF-α and IFN-γ) were enriched in the wounds of WT mice before the emergence of the WAE cells (6 h; Fig. S2C). As expected, Myd88−/− mice did not show an elevation of Th1 cytokine RNAs. In contrast, mRNAs encoding Th2 cytokines were elevated (IL-4 and IL-13) in wounds of WT and Myd88−/− mice during the second phase of wound healing (day 2–4; Fig. S2C).
We hypothesized that the major cell types and cytokines elevated at specific times during wound healing might be essential for mucosal regeneration. We treated biopsy-injured WT mice with antibodies directed against (i) GR-1 to ablate neutrophils by >95% (similar to previous injury studies; ref. 7, 16) and (ii) TNF-α and IFN-γ (17–19) to ablate Th1 cytokines. We found that in both of these experiments, the process of wound healing was indistinguishable from isotype control-treated mice (data not shown). These results indicate that decreasing either neutrophils or Th1 cytokines that are normally elevated during the early phase of healing did not influence that overall rate of wound healing.
We previously showed that the TLR signal adaptor, Myd88 was required in bone marrow derived cells to stimulate epithelial proliferation in crypts adjacent to mucosal ulcers that formed during DSS injury (7). Therefore, we tested whether Myd88 was necessary to increase epithelial proliferation during the second phase of healing in our biopsy injury system. Interestingly, our analysis of biopsy injuries in Myd88−/− mice showed a time course of healing and epithelial cell proliferation were indistinguishable from similarly biopsy injured WT mice. (Fig. S3). Thus, efficient wound healing appears to be Myd88-independent for acute colonic mucosal injuries. One possible explanation for the difference of the two systems is that DSS inhibits epithelial proliferation that requires both TLR and prostaglandins to rescue it in WT mice (15). This finding is similar to skin, where Myd88 signaling was required for proper wound healing only when punch biopsies were inoculated with pathogenic microbes (20).
Efficient Wound Healing Requires Trem2 Signaling.
We next evaluated other macrophage-expressed genes that can modulate Th1/Th2 cytokine production. We considered Trem2 for two major reasons. First, Trem2 is a cell surface receptor (with as yet undetermined ligand) that is specifically induced in macrophages by IL-4/IL-13. Second, Trem2 signaling is important in injury responses (21–26). In situations where low levels of TLR ligands are present, Trem2 suppresses TLR signaling (23, 24). Greater levels of TLR ligands suppress the expression of Trem2, thereby abrogating its function. The osteoclast (a close relation to the macrophage) is another cell type that requires Trem2 function (22). We found that in WT and Myd88−/− mice, Trem2 mRNA was induced to its maximum levels at day 2 postinjury, concurrent with peak macrophage accumulation. Trem2 mRNA levels returned to baseline at day 6, when wound repair was complete (Fig. 2A). Immunofluorescence analysis showed that Trem2 protein expression was detectable only in a subpopulation of macrophages within the wound bed of WT and Myd88−/− mice (Fig. 2 and Fig. S4A).
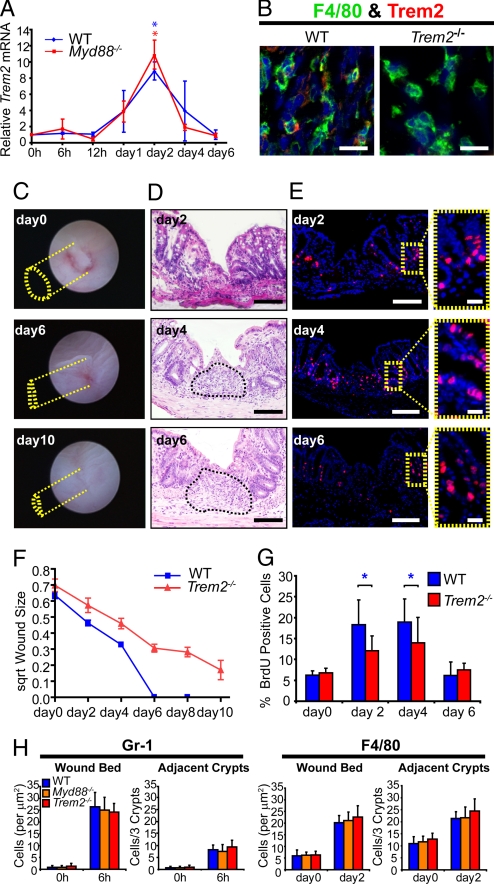
Fig. 2.
Trem2 is required for proper colon mucosal healing. (A) Plot of Trem2 mRNA expression (mean ± SD) at times postinjury. WT and Myd88−/− mice showed similar patterns of Trem2 mRNA up-regulation that peaked two days after biopsy (n = 12 lesions/group from 4 mice per group, P < 0.01; asterisks indicate statistically significant differences for WT in blue and Myd88 in red versus their own 0 h baselines). (B) Double-label immunohistochemistry from WT and Trem2−/− mice showing expression of Trem2 protein (red) and F4/80-positive macrophages (green) located in the wound bed two days after biopsy. (C) Serial endoscopic views of a biopsy injury site from an adult Trem2−/− mouse colon. The yellow dotted lines depict the margin of the lesion at each time point. A depressed area remained at day 6 and persisted through day 10. The length of the major axis = 1.5 mm (day 0), = 1.0 mm (day 6), and = 0.8 mm (day 10). (D) H+E-stained sections of Trem2−/− mouse colons at times postbiopsy injury. The surface of the wound bed was covered by epithelial cells by day 2 in Trem2−/− mice. Stromal cells persisted in the wound bed (asterisk) at day 6. (E) Serial sections from (C) stained with BrdU (Insets of crypts adjacent to the wound bed in yellow dashed boxes). (F) Plot of square root (to generate linear graphs) of the mean (± SD) surface area of lesions in Trem2−/− mice (orange) and WT (blue) mice showed healing was delayed in the absence of Trem2. These healing curves are statistically significantly different (see Table S1 for description). (G) Quantification of percentage BrdU incorporation in three crypts adjacent to injured areas (WT and Trem2−/− are compared at each time point, n = 12 lesions/group from 3 mice per group, blue asterisk indicates P < 0.01 by student's t test). (H) Plots of Gr-1 positive neutrophils at 6 h postinjury (Left) and F4/80-positive macrophages at day 2 postbiopsy injury (Right) in WT, Myd88−/−, and Trem2−/− mice (n = 16 lesions/group from 4 mice per group). (Scale bars: 10 μm in B; 100 μm in D and E; and 20 μm in E Insets.
Colonic biopsy injury of Trem2−/ mice resulted in significantly slowed and incomplete mucosal healing (out to day 10 postinjury; Fig. 2 C–F). Histological analysis of biopsy injury sites of Trem2−/− mice showed WAE cells formed normally over the wound bed by day 2 postinjury. However, in contrast to WT injuries, the wound beds of Trem2−/− mice exhibited a sustained increase in stromal cells at day 6 and beyond (Fig. 2D). Interestingly, the crypts adjacent to biopsy injuries in Trem2−/− mice displayed decreased epithelial cell proliferation (and, as a surrogate, crypt height) at day 2 and day 4 postinjury as compared with WT mice (Fig. 2G and Fig. S4B), despite the fact that neutrophil and macrophage elevation in the wound bed showed a similar timing and peak magnitude between these groups of mice (Fig. 2H).
Trem2 Signaling Influences the Cytokine Balance in Colonic Wounds That Effects Macrophage Activation State and Healing.
Trem2 expression was an important factor in the control of the balance of Th1 and Th2 cytokines during the second phase of mucosal healing. In contrast to WT mice, TNF-α and IFN-γ mRNA expression in Trem2−/− mice was significantly elevated during the second phase of wound healing (Fig. 3A). Interestingly, expression of IL-4 and IL-13 were decreased in the second phase of wound healing in Trem2−/− mice as compared with WT (Fig. 3A).
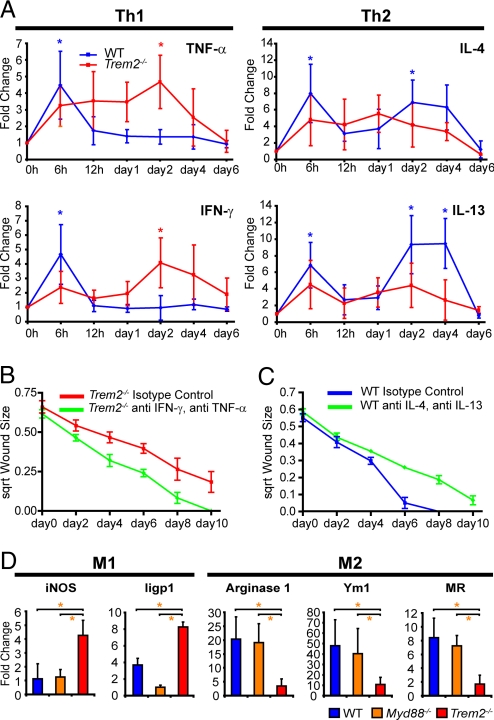
Fig. 3.
IL-4 and Il-13 are required for proper biopsy wound healing. (A) Plots of mRNA expression (mean ± SD) for Th1 and Th2 cytokines in biopsy injured WT and Trem2−/− mice. For each mouse genotype, comparisons between individual time points post injury were compared with uninjured areas (0 h). Statistically significant differences (P < 0.01) are denoted by either blue asterisks (WT) or red asterisks (Trem2−/−). (B and C) Administration of neutralizing antibodies for TNF-α and IFN-γ accelerated the healing in Trem2−/− mice. Administration of neutralizing antibodies for IL-4 and IL-13 delayed wound healing in WT mice. For both experiments, the healing curves were statistically different (P < 0.001) as determined by generalized estimating equations, n = 12 lesions from 3 mice per group (see Table S1 for description and p values). (D) Plots of mean (± SD) fold-change of relative mRNA expression as determined by qRT-PCR analysis of wound beds procured at day 2 post injury (baseline of expression is 0h for each genotype) for WT, Myd88−/−, Trem2−/− mice (n = 12 lesions/group from 3 mice per group). Statistically significant differences (P < 0.01) as determined by Student's t tests between groups are indicated by an orange asterisk.
We performed two experiments to test the role of these cytokines in wound healing. First, we ablated TNF-α and IFN-γ in biopsy-injured Trem2−/− mice using neutralizing antibodies, which significantly (but not completely) rescued the defects in healing and epithelial census of crypts adjacent to the wound by day 4 postinjury (Fig. 3B and Fig. S5A). In a second group of experiments, we treated biopsy-injured WT mice with neutralizing antibodies directed against IL-4 and IL-13 that resulted in wounds that did not completely heal by day 10 (similar to Trem2−/− mice; Fig. 3C). We also evaluated Stat6−/− mice as Stat6 is a downstream mediator of IL-4/IL-13. These mice also showed abnormal wound repair and displayed decreased epithelial census in crypts adjacent to the wound (Fig. S5 B–E). Thus, control of the expression of these cytokines in the wound bed, in part through the influence of Trem2, is important for efficient and complete wound repair.
Recent studies have suggested that Th2 cytokines, especially IL-4 and IL-13, can stimulate an “alternative” (M2) activation of macrophages (27–28) as opposed to “classical” (M1) activation of macrophages by Th1 cytokines that is stimulated by IFN-γ. We therefore examined mRNA expression of markers of M1 and M2 activated macrophages in the wound bed. We found that inducible nitric oxide synthase and Iigp (M1 markers) were significantly increased in Trem2−/− mice as compared with WT and Myd88−/− mice (Fig. 3D). At the same time points, arginase 1, Ym1 and mannose receptor (M2 markers) mRNA expression were significantly decreased in Trem2−/− mice (Fig. 3D).
Taken together, these data suggest that Trem2 signaling not only inhibits expression of M1 markers of activation but also promotes expression of markers of M2 activation of wound bed macrophages by terminating proinflammatory cytokine production. Because Trem2 is detected in wound bed macrophages, we infer that alteration in M1/M2 marker expression occurs in these cells. Trem2 activity in macrophages then terminates proinflammatory cytokine production either in macrophages themselves or another as yet unidentified cell type in the wound bed. Trem2 also is required to maintain high levels of the expression of Th2 cytokines that facilitate wound healing. We found that one source for IL-4 appears to be a subset of T cells in the wound bed (Fig. S6) as was previously demonstrated in the spleens of mice with a parasitic infection (29). Future studies will be required to further delineate the functional cellular source(s) of these cytokines.
These findings can be generalized to other inflammatory systems of the colon. We found that diminished wound healing of biopsy injuries also occurred in WT mice treated with dextran sodium sulfate (DSS)(Fig. S5F). DSS creates an injury with an M1 signature in the mesenchyme (7) further supporting the idea that this type of macrophage activation inhibits colonic wound healing.
Prospectus.
Using a novel in vivo model of acute mouse colonic mucosal regeneration, we found that Trem2 promotes efficient wound healing by inhibiting cytokines that can enhance M1 macrophage activation and promoting cytokines that can promote M2 macrophage activation in the wound bed (Fig. 4). An important question is the cellular source(s) of IL-4 and/or IL-13. One aspect of wound healing that is correlated with the presence of M2 macrophages is an increase in epithelial census and proliferation in wound-adjacent crypts. One possible target that requires additional study is arginase 1 produced from M2 macrophages. This molecule can shift l-arginine utilization to production of polyamines, which might contribute to adjacent epithelial proliferation through c-myc and p21 (27, 30, 31). The resulting elongated crypts may then undergo fission (32) and thus participate in wound healing.
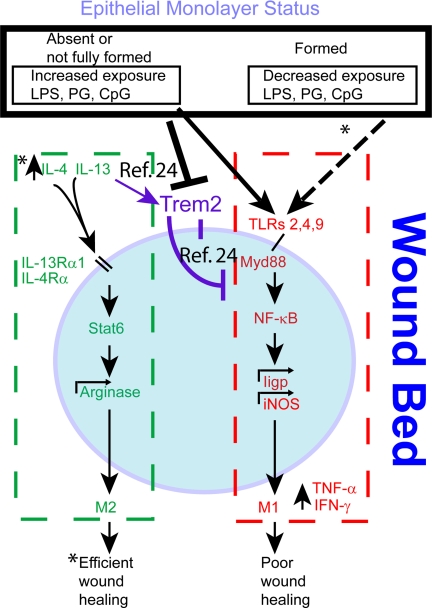
Fig. 4.
Trem2 sits at a focal point for the promotion of wound repair. Trem2 expression peaks in wound associated macrophages (blue circle) at day 2 post injury at a time when the WAE cell layer is formed and IL-4 and IL-13 also increase. IL-4 and IL-13 are known to increase Trem2 expression on macrophages (24). Our hypothesis is that the WAE cells form a barrier that limits exposure to bacterial products (either none or low levels). At low levels of TLR antigens, Trem2 is known to inhibit Myd88-mediated TLR signaling (23, 24). At higher levels of TLR antigens, Trem2 expression is inhibited (24), thus relieving the inhibition of TLR signaling that we hypothesize leads to an increase in proinflammatory cytokines in macrophages and/or other wound bed cells. Key unanswered questions are the nature of the interaction of the WAE cells and macrophage activation, the precise functional sources of IL-4 and IL-13 in this system and the role of the activated macrophage products on colonic epithelial progenitor proliferation and thus wound healing (all indicated by asterisks in diagram).
We also propose that further understanding the role of WAE cells in colonic wound repair will be important as the timing of their appearance correlates with Trem2 expression. We predict that circumstances where this barrier (and thus WAE cells) is chronically damaged will decrease Trem2 expression in recruited macrophages and lead to inefficient healing as macrophages will be directly exposed to high levels of bacterial TLR antigens. With infection by bacterial pathogens, this course of action may be temporarily preferable so that M1-activated macrophages can clear the wound bed of microbes. We predict this system will provide a framework to test the role and mechanism of beneficial indigenous microbes (given as “probiotics”) in wound healing. Under conditions of colonic damage, specific microbial components either stimulate Th1 type cytokines or Th2 type cytokines (33). These cytokines may modulate the type of macrophage activation state to influence the nature of wound healing that occurs. In addition, these findings suggest that patients with defects that effect barrier function or immune cell activation in the intestine will promote Th1 cytokines during injury that will overwhelm the ability of Trem2 to inhibit this process and cause impaired healing.
Materials and Methods
Mice.
All animal studies were performed according to protocols approved by the Washington University School of Medicine Animal Studies Committee. All strains were maintained on a C57BL/6J (B6) genetic background and were maintained free of specified pathogens in a barrier facility under a strict 12-h light cycle. Adult (8- to 16-week-old) male mice were used for all of the experiments.
Endoscopic Procedures.
To create discrete mucosal injuries in the mouse colon and to monitor their regeneration, we used a high-resolution miniaturized colonoscope system (10, 11). This system consisted of a miniature rigid endoscope (1.9-mm outer diameter), a xenon light source, a triple chip high resolution CCD camera, and an operating sheath with 3 Fr. instrument channel and water injection bulb to regulate inflation of the mouse colon (all from Karl Storz). Endoscopic procedures were viewed with high resolution (1024 × 768 pixels) images on a flat panel color monitor.
Procedure for Biopsy Injury.
The night before the initial biopsy injury, food was removed from the mouse cages. The following morning, mice were anesthetized by using ketamine and xylazine. The endoscope with outer operating sheath was inserted to the mid-descending colon and the mucosa was surveyed to the ano-rectal junction. Then, we inserted flexible biopsy forceps with a diameter of 3 French, and removed single full thickness areas of the entire mucosa and submucosa. We took particular care to avoid penetration of the muscularis propria. Each mouse was biopsy injured at 3–5 sites along the dorsal side of the colon (spacing was >5 mm).
Detailed descriptions of methods for quantification of wound regeneration, antibody neutralization studies, immunohistochemistry, and RNA gene expression analysis can be found in the SI Supplemental Materials and Methods.
Supplementary Material
Acknowledgments.
We thank S. Bloom, W. Stenson, E. Unanue, and H. Virgin for comments on the manuscript. This work was funded by National Institutes of Health Grant DK071619, the Pew Scholars Foundation, and the Washington University Digestive Disease Research Core (National Institutes of Health Grant P30-DK52574).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803343106/DCSupplemental.
References
- 1.Chang WW, Leblond CP. Renewal of the epithelium in the descending colon of the mouse. I. Presence of three cell populations: Vacuolated-columnar, mucous and argentaffin. Am J Anat. 1971;131:73–99. doi: 10.1002/aja.1001310105. [DOI] [PubMed] [Google Scholar]
- 2.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 3.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 4.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 5.Elson CO, et al. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 6.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukata M, et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055–G1065. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 9.Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 10.Becker C, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Becker C, et al. In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut. 2005;54:950–954. doi: 10.1136/gut.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamagawa H, et al. Characteristics of claudin expression in follicle-associated epithelium of Peyer's patches: Preferential localization of claudin-4 at the apex of the dome region. Lab Invest. 2003;83:1045–1053. doi: 10.1097/01.lab.0000078741.55670.6e. [DOI] [PubMed] [Google Scholar]
- 13.Owen RL. Uptake and transport of intestinal macromolecules and microorganisms by M cells in Peyer's patches a personal and historical perspective. Semin Immunol. 1999;11:157–163. doi: 10.1006/smim.1999.0171. [DOI] [PubMed] [Google Scholar]
- 14.Clayburgh DR, Shen L, Turner JR. A porous defense: The leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282–291. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]
- 15.Brown SL, et al. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest. 2007;117:258–269. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001;167:1601–1608. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 17.Schreiber RD, Hicks LJ, Celada A, Buchmeier NA, Gray PW. Monoclonal antibodies to murine gamma-interferon which differentially modulate macrophage activation and antiviral activity. J Immunol. 1985;134:1609–1618. [PubMed] [Google Scholar]
- 18.Sheehan KC, Ruddle NH, Schreiber RD. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989;142:3884–3893. [PubMed] [Google Scholar]
- 19.Mandik-Nayak L, Huang G, Sheehan KC, Erikson J, Chaplin DD. Signaling through TNF receptor p55 in TNF-alpha-deficient mice alters the CXCL13/CCL19/CCL21 ratio in the spleen and induces maturation and migration of anergic B cells into the B cell follicle. J Immunol. 2001;167:1920–1928. doi: 10.4049/jimmunol.167.4.1920. [DOI] [PubMed] [Google Scholar]
- 20.Miller LS, et al. Myd88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cella M, et al. Impaired differentiation of osteoclasts in TREM-2 deficient individuals. J Exp Med. 2003;198:645–651. doi: 10.1084/jem.20022220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamerman JA, et al. Cutting edge: Inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Immunol. 2006;177:2051–2055. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- 24.Turnbull IR, et al. Cutting edge: TREM2 attenuates macrophage activation. J Immunol. 2006;177:3520–3524. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. 2007;4:675–689. doi: 10.1371/journal.pmed.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 27.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 28.Mantovani A, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Anthony RM, et al. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray RM, Zimmerman BJ, McCormack SA, Patel TB, Johnson LR. Polyamine depletion arrests growth of IEC-6 and Caco-2 cells by different mechanisms. Am J Physiol Gastrointest Liver Physiol. 2001;281:G37–G43. doi: 10.1152/ajpgi.2001.281.1.G37. [DOI] [PubMed] [Google Scholar]
- 31.Liu L, et al. Polyamine-modulated expression of c-myc plays a critical role in stimulation of normal intestinal epithelial cell proliferation. Am J Physiol Cell Physiol. 2005;288:C89–C99. doi: 10.1152/ajpcell.00326.2004. [DOI] [PubMed] [Google Scholar]
- 32.Greaves DL, et al. Mitochondrial DNA mutations are established in human colonic stem cells, and mutated clones expand by crypt fission. Proc Natl Acad Sci USA. 2006;103:714–719. doi: 10.1073/pnas.0505903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pena JA, et al. Genotypic and phenotypic studies of murine intestinal lactobacilli: Species differences in mice with and without colitis. Appl Environ Microbiol. 2004;70:558–568. doi: 10.1128/AEM.70.1.558-568.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.