Abstract
Most animals must travel to find food, incurring an unavoidable energy and time cost. Economic theory predicts, and experimental work confirms, that within species, increasing the distance traveled each day to find food has negative fitness consequences, decreasing the amount of energy invested in maintenance, repair, and reproduction. Here, we show that this relationship between daily distance traveled and reproductive success is fundamentally different between species and over evolutionary time in many lineages. Phylogenetically controlled analyses of 161 eutherian mammals indicate that, after controlling for body mass, evolutionary increases in the daily distance traveled are associated with corresponding increases in both total fertility (number of offspring per lifetime) and total offspring mass (grams of offspring per lifetime). This suggests that over evolutionary time, increasing travel distance is often part of a strategy for procuring more food energy and not necessarily a response to decreased food availability. These results have important implications for ecological comparisons among species, including assessments of habitat quality based on locomotor behavior.
Keywords: ecology, energetics, reproduction, life history, foraging economics
How far will an animal travel each day? A broad range of ecological pressures influence ranging decisions (1–7), making this seemingly straightforward question exceedingly difficult to answer definitively. In the simplest case, in which an animal requiring a net energy intake of Enet(J/day) acquires food energy at a constant rate B (J/m) and spends energy on travel at some rate C (J/m), it must travel sufficient distance, D (m/day), so that D (B–C) = Enet.
Perhaps surprisingly, despite considerable variation and complexity in foraging and ranging behaviors, this simple relationship between daily movement distance, food availability, and energy requirements is generally supported by behavioral observation in a broad range of species. While ecological constraints cannot always be linked to foraging behavior (7) and physiological constraints may limit ranging ability (8), large interspecific comparisons of foraging behavior indicate that daily movement distance, D, is primarily determined by the need to acquire sufficient food energy (2, 3, 6). For example, daily movement distance increases with body size and diet quality, reflecting both size-related increases energy requirements and the relative scarcity of high-quality, energy-dense foods on the landscape (2, 6). Further, decreases in an individual's rate of food acquisition, B, whether through experimental manipulation in the laboratory (9–14), increased foraging group size (1, 4, 6, 15) or through seasonal changes in food availability in the wild (16–19) typically leads to increases in the daily distance traveled.
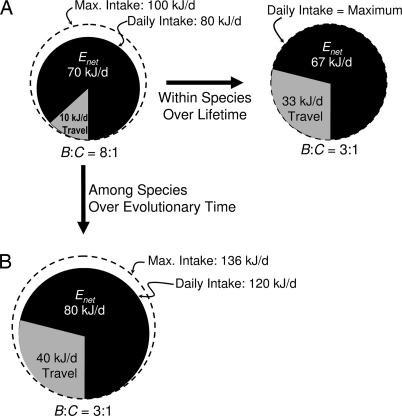
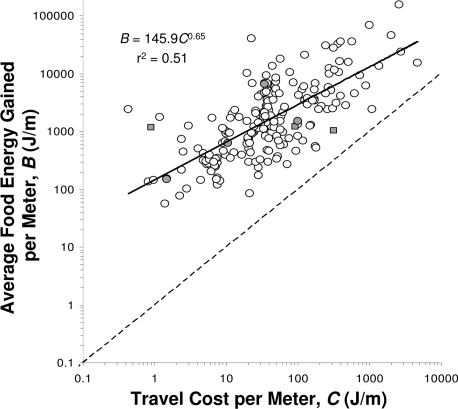
In principle, one might expect longer daily movement distances to be energetically beneficial, since greater D will lead to greater Enet as long as B > C (10, 20). In practice, however, while some species may increase foraging effort to maximize energy gain during food-rich periods (21, 22), intake and Enet are limited by an animal's maximum sustained (i.e., over several days) metabolic rate, typically 3–4× its resting metabolic rate (RMR) (maximum 6–7×; ref. 23). Since normal daily energy expenditure approaches this limit in wild populations, with field metabolic rates of 2–4× resting metabolism (24–26), a zero-sum system apparently prevails, in which increasing the energy spent on travel decreases the energy available for maintenance and reproduction (2) (Fig. 1A). Thus, rodents and birds challenged with decreased food per meter in experimental settings increase their ranging but lose weight and spend less energy on maintenance and reproduction (9–14), even when B > C and feeding is ad libitum. Similarly, daily movement distance increases (16–19) and body mass and condition decrease (27–30) during periods of food shortage for mammals in the wild, even though the average estimated food energy gained per meter for most free ranging mammals is over an order of magnitude greater than the travel cost per meter (Fig. 2).
Fig. 1.
Hypothetical energy budgets for a 100 g mammal (see ref. 24), showing proposed differences in travel costs versus net intake, Enet, over different time scales. (A) Within species, decreasing the ratio of food gained per meter, B, to travel cost per meter, C, from 8:1 to 3:1 increases daily travel distance and cost by 3.3× (right arrow), but limited maximum intake results in decreased Enet. (B) In contrast, with an evolutionary increase in maximum intake (down arrow) increasing daily travel 4× results in greater Enet even with the lower ratio B:C. Note that changes B:C do not dictate evolutionary changes in ranging and maximum intake in this model; see text for discussion.
Fig. 2.
Estimated food energy acquired per meter, B (J/m), versus the energy cost of travel per meter, C (J/m), for 161 mammal species. B is calculated by dividing estimated daily energy expenditure (kJ/day, equation 1 in ref. 24) by daily movement distance, D (km/day, Appendix 1). C was estimated from body mass using published allometric equations (156 terrestrial species, ref. 50; 5 aquatic species, ref. 51). Filled symbols: species for which direct measures of daily energy expenditure (circles) or both daily energy expenditure and cost of travel (squares) are available. As C increases with body size (42), the ratio of B:C changes with body size, from a mean of 65:1 for a 1 kg animal to 13:1 for a 800 kg animal. Dashed line indicates B = C. Note that C in this figure, also termed the “incremental cost of locomotion” (2) does not include the “postural cost” of travel (52).
While increased ranging and decreased Enet have been repeatedly demonstrated within numerous species, we propose that this relationship may be fundamentally different among species and over evolutionary time (Fig. 1B), at least in some lineages. Maximum daily metabolic rate may be constrained within a species (12) but is variable among species (23), indicating that this physiological constraint is evolutionarily labile. If so, longer daily movement distances and greater daily travel costs may be part of a strategy for expanding the daily energy budget and increasing net energy intake, thereby increasing the energy available for maintenance and reproduction, and not solely a strategy for compensating for decreased food availability (Fig. 1B). Under favorable ecological conditions, perhaps where food is predictably available and not easily depleted, species may be selected to increase ranging and daily intake to increase the energy available for maintenance and reproduction. Conversely, decreased daily travel may reflect an evolutionary shift toward smaller energy budgets and less net energy intake.
This model suggests that the reliability of food resources (e.g., lack of seasonality, resistance to depletion) may be more important than the ratio of B:C in encouraging evolutionary expansion or contraction of ranging and the daily energy budget. Energy budgets may expand when B:C increases and contract when B:C decreases, or vice versa (Fig. 1B), but changes (or stasis) in B:C do not dictate changes in the energy budget in our model, provided B > C. Instead, our model predicts expansion of ranging and the energy budget when increased ranging is profitable (e.g., food does not become depleted), and contraction when large energy budgets are a liability, perhaps in seasonal or stochastic environments prone to periods of extreme food shortage. In addition, factors unrelated to food availability, such as time constraints (31), limits to endurance (8), thermoregulatory constraints (32), or predation pressures (4), may limit ranging and consequently constrain the daily energy budget.
The implication that increased ranging may be an energy-maximizing response to favorable ecological conditions is consistent with recent work showing that both basal metabolic rate and activity level are positively correlated with food availability for rodents across different habitats (33) and seasons (34), as well as data suggesting that foraging effort and net intake may increase during food-rich periods in some species (21, 22). A positive interspecific relationship between ranging, food availability, and daily energy budget would also be consistent with the longstanding (26, 35), but contentious (36), hypothesis that RMR is positively correlated with food availability, since RMR is both a large component and strong correlate of field metabolic rate (24, 26; but see ref. 25). Again, while these examples suggest increased ranging is favored in habitats with increased food availability, our model suggests an increase in ranging and net energy intake can also occur with a decrease in the ratio of B:C (Fig. 1B), provided that maximum daily intake increases.
Here, we test the hypothesis that longer daily movement distances are often part of a strategy for maximizing net energy intake, using a large comparative dataset of 161 mammals to investigate whether evolutionary changes in daily movement distance are positively associated with reproductive investment. Given the diversity of ranging and reproductive strategies (31, 37), we do not expect increased ranging to be associated with greater reproductive investment in every case; larger ranging costs may well be associated with decreased reproductive investment in some cases, as suggested by intraspecific studies (9–13). Rather, we seek to compare ranging and reproduction to determine whether increased ranging is associated with increased reproduction in a significant proportion of lineages. Such results would shed new light on the diversity of foraging strategies seen in mammals, suggesting that evolutionary changes in ranging behavior may be correlated with expansion or contraction of daily energy budgets.
Results
Phylogenetically controlled multiple regression revealed a positive relationship between daily movement distance and both total fertility (offspring per lifetime; β = 0.265, P = 0.007, n = 109) and total offspring mass (grams offspring per lifetime, β = 0.155, P = 0.024, n = 108), once outliers were removed (Table 1). Other measures of reproductive and somatic investment, including litter mass (g/litter), litter mass per year (g/year), and lifespan (years), were not significantly related to travel distance (Table 1). Reducing the degrees of freedom due to polytomies in the phylogeny did not alter the results, nor did removing aquatic species (Table 1). Similarly, retaining outliers had negligible effects on results (Table 1).
Table 1.
Results of the phylogenetically controlled analysis
| Dependent Variable | Independent Variables | Degrees of Freedom | F | p | β |
|---|---|---|---|---|---|
| log Litter Mass | log Body Mass | 1 | 578.19 | <0.001 | 0.91 |
| log Daily Movement Distance | 1 | 0.30 | 0.588 | −0.02 | |
| Error | 143 | ||||
| log Offspring Mass per Year | log Body Mass | 1 | 155.05 | <0.001 | 0.75 |
| log Daily Movement Distance | 1 | 0.49 | 0.487 | 0.04 | |
| Error | 126 | ||||
| log Total Fertility | log Body Mass | 1 | 9.19 | 0.003 | −0.29 |
| log Daily Movement Distance | 1 | 7.69 | 0.007† | 0.27 | |
| Error | 107 | ||||
| log Total Offspring Mass | log Body Mass | 1 | 102.25 | <0.001 | 0.69 |
| log Daily Movement Distance | 1 | 5.25 | 0.024‡ | 0.16 | |
| Error | 106 | ||||
| log Maximum Lifespan | log Body Mass | 1 | 48.50 | <0.001 | 0.57 |
| log Daily Movement Distance | 1 | 0.29 | 0.591 | −0.04 | |
| Error | 124 |
†, P = 0.006 excluding aquatic spp., P = 0.012 including outliers.
‡, P = 0.057 excluding aquatic spp., P = 0.059 including outliers.
Conventional multiple regression analyses, using phylogenetic Order and species values of body mass and daily movement distance as independent variables and reproductive investment or maintenance measures as dependent variables, revealed significant positive relationships between daily movement distance and investment in maintenance and reproduction (Table 2). While effect sizes were generally small (partial η2 less than 0.1), daily movement distance was significantly positively correlated with litter mass (β = 0.113, P = 0.020, n = 150), offspring mass per year (β = 0.202, P = 0.002, n = 113), total fertility (β = 0.138, P = 0.016, n = 113), and total offspring mass (β = 0.213, P = 0.007, n = 111) (Table 2). As in the phylogenetic contrasts analysis, lifespan was not significantly associated with daily movement distance. Removing aquatic species did not significantly affect results.
Table 2.
Results of conventional species data multiple regression
| Dependent Variable | Independent Variables | Degrees of Freedom | F | p | η2 | β |
|---|---|---|---|---|---|---|
| log Litter Mass | log Body Mass | 1 | 515.27 | <0.001 | 0.788 | 0.681 |
| model adjusted r2 = 0.95 | log Daily Movement Distance | 1 | 5.58 | 0.020 | 0.039 | 0.113 |
| Phylogenetic Order | 8 | 9.55 | <0.001 | 0.355 | - | |
| log Offspring Mass per Year | log Body Mass | 1 | 126.81 | <0.001 | 0.514 | 0.451 |
| model adjusted r2 = 0.90 | log Daily Movement Distance | 1 | 10.46 | 0.002 | 0.080 | 0.202 |
| Phylogenetic Order | 8 | 14.00 | <0.001 | 0.483 | - | |
| log Total Fertility | log Body Mass | 1 | 19.94 | <0.001 | 0.164 | −0.153 |
| model adjusted r2 = 0.44 | log Daily Movement Distance | 1 | 5.98 | 0.016 | 0.055 | 0.138 |
| Phylogenetic Order | 8 | 6.09 | <0.001 | 0.323 | - | |
| log Total Offspring Mass | log Body Mass | 1 | 148.06 | <0.001 | 0.597 | 0.580 |
| model adjusted r2 = 0.91 | log Daily Movement Distance | 1 | 7.55 | 0.007 | 0.070 | 0.213 |
| Phylogenetic Order | 8 | 8.58 | <0.001 | 0.407 | - | |
| log Maximum Lifespan | log Body Mass | 1 | 63.70 | <0.001 | 0.349 | 0.155 |
| model adjusted r2 = 0.71 | log Daily Movement Distance | 1 | 0.27 | 0.606 | 0.002 | 0.016 |
| Phylogenetic Order | 9 | 14.49 | <0.001 | 0.523 | - |
Discussion
Three conclusions can be drawn from these results. First, the positive relationship between daily movement distance and lifetime reproductive output in both phylogenetically controlled and conventional multiple regression (Table 1 and 2) indicates that evolutionary increases in ranging are commonly associated with increased energy investment in reproduction. This supports our hypothesis that evolutionary increases in daily movement distance are often part of a strategy for increasing net energy intake, thereby making more energy available for maintenance and reproduction (Fig. 1B). Conversely, decreases in daily movement distance are often associated with decreased investment in maintenance and reproduction. Data on daily metabolic rates for these species in the wild (24) are necessary to determine whether these evolutionary changes in ranging are in fact associated with corresponding changes in the size of the daily energy budget, as suggested by our model.
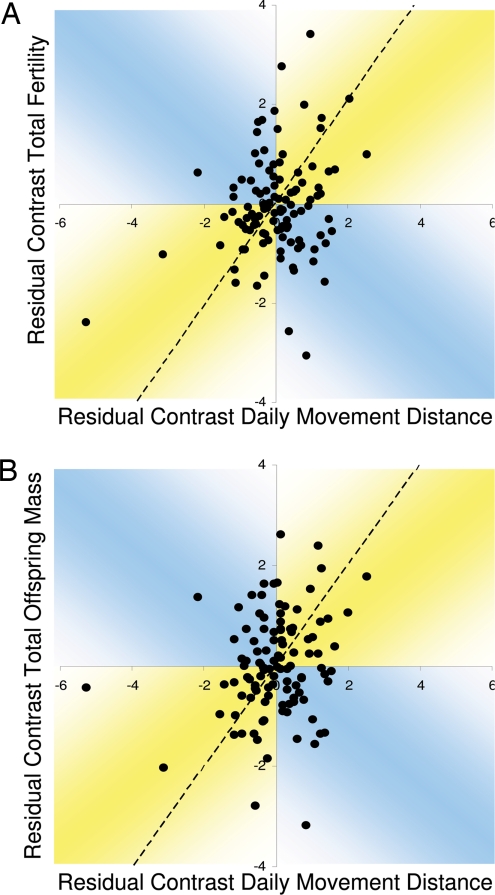
Second, the small effect size of daily movement distance, and the lack of a relationship between travel distance and reproductive output in some comparisons, demonstrates the considerable variability in the relationship between ranging and reproduction. Given the diversity of sources used to compile the ranging and life-history data (6, 38), a large portion of this apparent variability is likely due to differences in data quality and collection. Still, when the studentized residual contrasts, controlled for body mass, of ranging and reproductive output are plotted against each other (Fig. 3), it is apparent that evolutionary changes in daily movement distance are associated with a range of responses in reproductive output. For example, relatively large changes in daily movement distance (studentized residual > ±0.5) were negatively correlated with correspondingly large changes in total fertility and total offspring mass (studentized residual > ±0.5) in 34/90 and 33/85 cases, respectively (Fig. 3). This is similar to what is seen in most laboratory investigations of decreased food availability (9–13) and is consistent with the view of ranging as a cost that decreases the energy available for other activities (2). In contrast, in many cases, changes in daily movement distance have no significant effect on reproduction; relatively large changes in daily movement distance (studentized residual > ±0.5) were associated with little or no change in total fertility or total offspring mass (studentized residuals < ±0.5) in 14/90 and 11/85 cases, respectively (Fig. 3). This suggests that travel may be responding to food availability, with increased travel used to maintain net energy intake at some constant level in the face of decreased food availability. This is occasionally reported in laboratory experiments (14) and is commonly cited as the mechanism underlying increased ranging in food-poor seasons in wild populations (16–19). Finally, as suggested by our model, evolutionary increases in daily movement distance are often associated with increased investment in reproduction and maintenance. In the plurality of cases, relatively large changes in daily movement distance (studentized residuals > ±0.5) were positively correlated with large changes (studentized residuals > ±0.5) in total fertility (42/90 cases) and total offspring mass (41/85 cases) (Fig. 3).
Fig. 3.
Studentized residual phylogenetic contrasts, corrected for body mass, of daily movement distances versus (A) total fertility and (B) total offspring mass for 113 and 111 mammalian species, respectively. Dashed line indicates the Reduced Major Axis trendline. While some evolutionary changes in daily movement distance are negatively correlated with changes in reproduction (blue quadrants), in most instances, daily movement distance is positively associated with reproductive output (yellow quadrants). In contrast, within species, daily movement distance is negatively correlated with reproductive and somatic investment (see text).
Third, our data strongly support the hypothesis that the relationship between ranging and reproductive effort is fundamentally different over different timescales. Within species, over a lifetime, increased ranging is consistently associated with decreased net energy intake, reproductive effort, and maintenance, both in the wild (27–29) and in the lab (9–12). In contrast, among species, over evolutionary time, increased ranging is commonly associated with increased reproductive output (Tables 1 and 2; Fig. 3). We propose that this difference is due to the constraint of maximum daily metabolic rate (Fig. 1), which is relatively inflexible for an individual but may be labile over evolutionary time. This hypothesis could be tested with data on daily energy budgets for wild populations (24, 25, 33) or laboratory comparisons of maximum daily metabolic rate (12, 23).
The positive relationship between daily movement distance and investment in reproduction and maintenance may help explain some of the interspecific variation observed in daily movement distance. While the scaling of daily movement distance generally correspond to predicted energy requirements (3, 5, 6), considerable variation in ranging remains unexplained by body size, diet, and foraging group size (7). Our results suggest that species may increase daily movement distances as part of a strategy of increasing daily energy budgets: two species with similar body size, diet, and foraging group size may differ in daily movement distance if one adopts a larger daily energy budget. Including some measure of net energy intake, apart from body mass, may therefore improve predictive ranging models.
Our results suggest a new approach for interpreting interspecific differences in daily movement distance. Short daily movement distances are often viewed as a reflection of low diet quality (i.e., a diet focused on low energy-density foods) and/or relatively high food abundance (6) but results here suggest that short daily movement distances may also reflect the adoption of a smaller daily energy budget, with less energy invested in reproduction and maintenance. Conversely, while long daily movement distances are often viewed as diminishing the energy available for maintenance and reproduction (2), results here indicate that, in interspecific comparisons, longer daily movement distances may reflect an expansion of the energy budget, with greater investment in maintenance and reproduction. As suggested by previous work in ectotherms (39), contraction of the energy budget may be advantageous in seasonal or stochastic environments in which animals with large energy requirements are prone to starvation during food-poor periods, while expansion of the energy budget may be advantageous in more stable environments. Alternatively, decreased ranging and smaller energy budgets may be part of a risk-averse strategy focused on low-variance foods, while increased ranging and larger energy budgets are part of a risk-prone strategy of focusing on high-variance foods (40, 41). In linking expansion and contraction of the energy budget to daily movement distance, our model suggests that constraints on ranging, such as predation (4), endurance (8), or thermoregulation (32) may also constrain the energy budget.
These findings highlight the need for more direct measurements of energy budgets in wild populations. Laboratory and field studies have provided critical insight into foraging energetics and ecology within various species. Comparative efforts, using doubly labeled water (24, 25, 33) or similar techniques to measure foraging costs and benefits in a common currency of metabolic energy are necessary to understand how these species-specific strategies evolve.
Methods
We examined the relationship between mean daily movement distance with several life-history and reproduction traits for 161 mammalian species representing 7 Orders to determine whether, after controlling for phylogenetic relatedness and body mass, increased daily movement distance is associated with greater energy investment in reproduction and maintenance between species. Species means for body mass, daily movement distance, and several life-history variables were taken from the literature (ref. 6, 38,; see Table S1). Some life history traits were not available for all species (Table S1), and so sample sizes varied for each statistical test. Energy invested in reproduction was operationalized as litter mass (n = 150 species, Table S1; ref. 42) and as the mass of offspring produced per year calculated as the product of litter mass and litters per year (n = 113). Energy investment in maintenance was operationalized as expected maximum lifespan (n = 131). Combined investment in maintenance and reproduction was operationalized as total fertility (number of offspring per lifetime, n = 113) and total offspring mass (grams of offspring per lifetime, n = 111). For these lifetime investment variables we multiplied reproductive lifespan (i.e., maximum life span–age at first reproduction), by offspring per year to give total fertility or by mass of offspring per year to give total offspring mass.
Data were analyzed using phylogenetic independent contrasts (43). We used the tree topology and estimated divergence times from a recent mammal phylogeny (44) (Fig. S1). For these analyses, we transformed all variables using log10. This data transformation best met an important assumption of the test, the lack of relationship between the branch length and the absolute value of the standardized contrast (45, 46). We accounted for polytomies by calculating contrasts using zero branch lengths for all lineages originating from multifurcating nodes (47) and reduced the degrees of freedom accordingly. Body mass was entered as a second predictor variable in all multiple regressions to control for its effect on ranging, life-history, and reproduction. We considered outliers as data points with studentized residuals greater than ±3.0 and/or a Cook's distance greater than 1.0. If outliers were present, we reanalyzed the dataset with outliers removed. Independent contrasts were calculated using the PDAP module for Mesquite (48, 49). We used Statistica 6.0 to run the multiple regressions using independent contrasts, as well as to determine the possible presence of outliers.
Additionally, following previous workers (e.g., 6, 24, 26, 35), we analyzed the relationship between body mass, daily movement distance, and investment in reproduction and maintenance using conventional multiple regression of log10 transformed species means. Phylogenetic Order (Table S1) was entered as a fixed factor in a general linear model (SPSS 15.0) with body mass and daily movement distance entered as covariates. As in the phylogenetic contrasts analyses, we predicted that interspecific differences in daily movement distance would be positively correlated with reproductive output and maintenance (Fig. 1B), after controlling for the effects of body mass and phylogeny.
Supplementary Material
Acknowledgments.
Andrew Marshall, Andreas Koenig, Richard Wrangham, and three anonymous reviewers provided useful comments which substantially improved this manuscript. Support was provided by the Washington University Department of Anthropology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806105106/DCSupplemental.
References
- 1.Clutton-Brock TH, Harvey PH. Primate ecology and social organization. J Zoo Lond. 1977;183:1–39. [Google Scholar]
- 2.Garland T. Scaling the ecological cost of transport to body mass in terrestrial mammals. Am Nat. 1983;121:571–587. [Google Scholar]
- 3.Wrangham RW, Gittleman JL, Chapman CA. Constraints on group size in primates and carnivores, population density and day-range assays of exploitation competition. Behav Ecol Sociobiol. 1993;32:199–209. [Google Scholar]
- 4.Janson CH, Goldsmith ML. Predicting group size in primates, foraging costs and predation risks. Behav Ecol. 1995;6:326–336. [Google Scholar]
- 5.Chapman CA, Chapman LJ. Determinants of group size in primates: The importance of travel costs. In: Boinski S, Garber PA, editors. On the Move. Chicago: Univ of Chicago; 2000. pp. 24–42. [Google Scholar]
- 6.Carbone C, Cowlishaw G, Isaac NJB, Rowcliffe JM. How far do animals go? Determinants of day range in mammals. Am Nat. 2005;165:290–297. doi: 10.1086/426790. [DOI] [PubMed] [Google Scholar]
- 7.Snaith TV, Chapman CA. Primate group size and interpreting socioecological models: Do folivores really play by different rules? Evol Anth. 2007;16:94–106. [Google Scholar]
- 8.Garland T., Jr Laboratory endurance capacity predicts variation in field locomotor behaviour among lizard species. Animal Behav. 1999;58:77–83. doi: 10.1006/anbe.1999.1132. [DOI] [PubMed] [Google Scholar]
- 9.Perrigo G. Breeding and feeding strategies in deer mice and house mice when females are challenged to work for their food. Animal Behav. 1987;35:1298–1316. [Google Scholar]
- 10.Bautista LM, Tinbergen J, Wiersma P, Kacelnik A. Optimal foraging and beyond: How starlings cope with changes in food availability. Am Nat. 1998;152:543–561. doi: 10.1086/286189. [DOI] [PubMed] [Google Scholar]
- 11.Deerenberg C, Overkamp GJF, Visser GH, Daan S. Compensation in resting metabolism for experimentally increased activity. J Comp Physiol B. 1998;168:507–512. [Google Scholar]
- 12.Vaanholt LM, et al. Behavioural and physiological responses to increased foraging effort in male mice. J Exp Biol. 2007;210:2013–2024. doi: 10.1242/jeb.001974. [DOI] [PubMed] [Google Scholar]
- 13.Wiersma P, Verhulst S. Effects of intake rate on energy expenditure, somatic repair and reproduction of zebra finches. J Exp Biol. 2005;208:4091–4098. doi: 10.1242/jeb.01854. [DOI] [PubMed] [Google Scholar]
- 14.Wiersma P, Salomons HM, Verhulst S. Metabolic adjustments to increasing foraging costs of starlings in a closed economy. J Exp Biol. 2005;208:4099–4108. doi: 10.1242/jeb.01855. [DOI] [PubMed] [Google Scholar]
- 15.Pontzer H, Wrangham RW. The ontogeny of ranging in wild chimpanzees. Int J Primat. 2006;27:1–15. [Google Scholar]
- 16.Raemakers J. Causes of variation between months in the distance traveled daily by gibbons. Folia Primatol. 1980;34:46–60. doi: 10.1159/000155947. [DOI] [PubMed] [Google Scholar]
- 17.Ward RMP, Krebs CJ. Behavioural responses of lynx to declining snowshoe hare abundance. Can J Zool. 1985;63:2817–2824. [Google Scholar]
- 18.Anderson C. Subtrooping in a chacma baboon (Papio ursinus) population. Primates. 1981;23:445–458. [Google Scholar]
- 19.Gursky S. Effect of seasonality on the behavior of an insectivorous primate, Tarsius spectrum. Int J Primat. 2000;21:477–495. [Google Scholar]
- 20.Ydenberg RC, Welham CVJ, Schimd-Hempel R, Schimd-Hempel P, Beauschamp G. Time and energy constraints and the relationships between currencies in foraging theory. Behav Ecol. 1994;5:28–34. [Google Scholar]
- 21.Knott CD. Changes in orangutan caloric intake, energy balance, and ketones in response to fluctuating fruit availability. Int J Primat. 1998;19:1061–1079. [Google Scholar]
- 22.Doran D. Influence of seasonality on activity patterns, feeding behavior, ranging, and grouping patterns in Tai chimpanzees. Int J Primat. 1997;18:183–206. [Google Scholar]
- 23.Hammond KA, Diamond J. Maximal sustained energy budgets in humans and animals. Nature. 1997;386:457–462. doi: 10.1038/386457a0. [DOI] [PubMed] [Google Scholar]
- 24.Nagy KA, Girard IA, Brown TK. Energetics of free-ranging mammals, reptiles, and birds. Ann Rev Nutr. 1999;19:247–277. doi: 10.1146/annurev.nutr.19.1.247. [DOI] [PubMed] [Google Scholar]
- 25.Speakman JR, et al. Resting and daily energy expenditures of free-living field voles are positively correlated but reflect extrinsic rather than intrinsic effects. Proc Natl Acad Sci USA. 2003;100:14057–14062. doi: 10.1073/pnas.2235671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNab BK. The Physiological Ecology of Vertebrates: A View from Energetics. Ithaca, NY: Cornell Univ Press; 2002. [Google Scholar]
- 27.Koenig A, Borries C, Chalise MK, Winkler P. Ecology, nutrition, and timing of reproductive events in an Asian primate, the Hanuman langur (Presbytis entellus) J Zool. 1997;243:215–235. [Google Scholar]
- 28.Parker KL, Gillingham MP, Hanley TA, Robbins CT. Energy and protein balance of free-ranging black-tailed deer in a natural forest environment. Wildlife Monog. 1999;143:3–48. [Google Scholar]
- 29.Kuntz R, Kubalek C, Ruf T, Tataruch F, Arnold W. Seasonal adjustment of energy budget in a large wild mammal, the Przewalski horse (Equus ferus przewalskii) I. Energy intake. J Exp Biol. 2006;209:4557–4565. doi: 10.1242/jeb.02535. [DOI] [PubMed] [Google Scholar]
- 30.Pusey AE, Oehlert GW, Williams JM, Goodall J. Influence of ecological and social factors on body mass of wild chimpanzees. Int J Primatol. 2005;26:3–31. [Google Scholar]
- 31.Schoener TW. Theory of feeding strategies. Ann Rev Ecol Syst. 1971;2:369–404. [Google Scholar]
- 32.Schwaibald U, Pillay N. Behavioral strategies of the African ice rat Otomys sloggetti robertsi in the cold. Physiol Behav. 2006;88:567–574. doi: 10.1016/j.physbeh.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Mueller P, Diamond J. Metabolic rate and environmental productivity: well-provisioned animals evolved to run and idle fast. Proc Natl Acad Sci USA. 2001;98:12550–12554. doi: 10.1073/pnas.221456698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bozinovic F, Muñoz JL, Cruz-Neto AP. Intraspecific variability in the basal metabolic rate: testing the food habits hypothesis. Physiol Biochem Zool. 2007;80:452–460. doi: 10.1086/518376. [DOI] [PubMed] [Google Scholar]
- 35.McNab BK. The influence of food habits on the energetics of eutherian mammals. Ecol Monogr. 1986;56:1–19. [Google Scholar]
- 36.Harvey PH, Pagel MD, Rees JA. Mammalian metabolism and life histories. Am Nat. 1991;137:556–566. [Google Scholar]
- 37.Pianka ER. Natural selection of optimal reproductive tactics. Am Zool. 1976;16:775–784. [Google Scholar]
- 38.Ernest SKM. Life history characteristics of placental nonvolant mammals. Ecology. 2003;84:3402. [Google Scholar]
- 39.Pough FH. The advantages of ectothermy for tetrapods. Am Nat. 1980;115:92–112. [Google Scholar]
- 40.Caraco T. On foraging time allocation in a stochastic environment. Ecology. 1980;61:119–128. [Google Scholar]
- 41.Houston AI, McNamara JM. Risk-sensitive foraging and temperature. TREE. 1990;5:131–132. [Google Scholar]
- 42.Charnov EL, Ernest SKM. The offspring-size/clutch-size trade-off in mammals. Am Nat. 2006;167:578–582. doi: 10.1086/501141. [DOI] [PubMed] [Google Scholar]
- 43.Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- 44.Bininda-Emonds ORP, et al. The delayed rise of present-day mammals. Nature. 2007;446:507–512. doi: 10.1038/nature05634. [DOI] [PubMed] [Google Scholar]
- 45.Garland T, Jr, Harvey PH, Ives AR. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst Biol. 1992;41:18–32. [Google Scholar]
- 46.Garland T, Jr, Bennett AF, Rezende EL. Phylogenetic approaches in comparative physiology. J Exp Biol. 2005;208:3015–3035. doi: 10.1242/jeb.01745. [DOI] [PubMed] [Google Scholar]
- 47.Garland T, Jr, Diaz-Uriarte R. Polytomies and phylogenetically independent contrasts: Examination of the bounded degrees of freedom approach. Syst Biol. 1999;48:547–558. doi: 10.1080/106351599260139. [DOI] [PubMed] [Google Scholar]
- 48.Maddison WP, Maddison DR. Mesquite: A modular system for evolutionary analysis. [Accessed September 1, 2008];2007 Version 2.0. Available at http://mesquiteproject.org. [Google Scholar]
- 49.Midford PE, Garland T, Maddison WP. PDAP: PDTREE package for Mesquite. [Accessed September 1, 2008];2007 version 1.1. Available at http://mesquiteproject.org/pdap_mesquite/ [Google Scholar]
- 50.Taylor CR, Heglund NC, Maloiy GMO. Energetics and mechanics of terrestrial locomotion: I. Metabolic energy consumption as a function of speed and body size in birds and mammals. J Exp Biol. 1982;97:1–21. doi: 10.1242/jeb.97.1.1. [DOI] [PubMed] [Google Scholar]
- 51.Baudinette RV. The energetics and cardiorespiratory correlates of mammalian terrestrial locomotion. J Exp Biol. 1991;160:209–231. doi: 10.1242/jeb.160.1.209. [DOI] [PubMed] [Google Scholar]
- 52.Taylor CR. The energetics of terrestrial locomotion and body size in vertebrates. In: Pedley TJ, editor. Scale Effects in Animal Locomotion. New York: Academic; 1977. pp. 127–141. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.