Abstract
PKCη is expressed predominantly in the epithelial tissues; however, its role in the regulation of epithelial tight junctions (TJs) is unknown. We present evidence that PKCη phosphorylates occludin on threonine residues (T403 and T404) and plays a crucial role in the assembly and/or maintenance of TJs in Caco-2 and MDCK cell monolayers. Inhibition of PKCη by specific pseudo substrate inhibitor or knockdown of PKCη by specific shRNA disrupts the junctional distribution of occludin and ZO-1 and compromises the epithelial barrier function. Expression of dominant negative, PKCηK394R disrupts the TJ and barrier function, whereas wild-type PKCη and constitutively active PKCηA161E enhance the TJ integrity. Inhibition and knockdown of PKCη or expression of PKCηK394R induce dephosphorylation of occludin on threonine residues, whereas active PKCη elevates occludin phosphorylation. PKCη directly interacts with the C-terminal domain of occludin and phosphorylates it on highly conserved T403 and T404. T403/404A mutations result in the loss of occludin's ability to localize at the TJs, whereas T403/404D mutations attenuates the PKCη inhibitor-mediated redistribution of occludin from the intercellular junctions. These results reveal an important mechanism of epithelial TJ regulation by PKCη.
Keywords: differentiation, epithelium, protein kinase
The epithelial tight junctions (TJs) on one hand determine the cell polarity by forming a fence between the apical and basolateral membranes (1), and on the other hand, it prevents the diffusion of toxins, allergens, and pathogens from the lumen into the tissue (2). Additionally, TJs play essential roles in the regulation of cell–cell adhesion and the epithelium-to-mesenchymal transition (3). Dysfunctional TJs are associated with the pathogenesis of inflammatory diseases (2) and tumor metastasis (3). Therefore, understanding the molecular structure of TJs and the regulatory mechanisms that control the integrity of TJs is essential to advance our knowledge in epithelial homeostasis in health and disease.
The assembly of TJs involves at least 3 types of transmembrane proteins, occludin, claudins, and junctional adhesion molecule (4). The intracellular domains of occludin and claudins interact with the plaque proteins such as ZO-1, ZO-2, and ZO-3, which form the platforms for recruitment of scaffold proteins such as cingulin, Par-3, Par-6, etc.; this TJ protein complex is anchored into the perijunctional actomyosin ring. Although occludin knockout mice showed the formation of intact TJs in different epithelia (5), several studies indicated that occludin does play an important role in the regulation of TJ integrity (6, 7).
Protein kinases (8–10) and protein phosphatases (11) are either localized at the TJs or interact directly with the TJ proteins. Whereas atypical PKCs (PKCζ and PKCλ/ι) directly interact with the TJs (10), PKCε and PKCβI may indirectly regulate the integrity of TJs (12). PKCη, a novel PKC isoform, is predominantly expressed in epithelial tissues (13). The function of PKCη in the epithelial tissues and the targets for its kinase activity are unknown. Recent studies indicated that overexpression of PKCη induces differentiation of human keratinocytes (14) and inhibits mouse skin tumor promotion (15). PKCη knockout mice showed increased susceptibility to develop skin cancer (15) and impaired epithelial regeneration (16). The expression of PKCη was down-regulated in breast tumor tissues (17). Therefore, PKCη seems to play a crucial role in the differentiation of epithelial tissues, however, its role in the regulation of epithelial TJs is unknown.
In the present study, we provide evidence that PKCη plays a crucial role in the regulation of epithelial TJs and that PKCη phosphorylates occludin on T403 and T404, which appears to be required for the assembly of occludin into the TJs. The results of these studies reveal a unique mechanism of TJ regulation by PKCη.
Results and Discussion
Inhibition or Knockdown of PKCη Disrupts the Epithelial TJs.
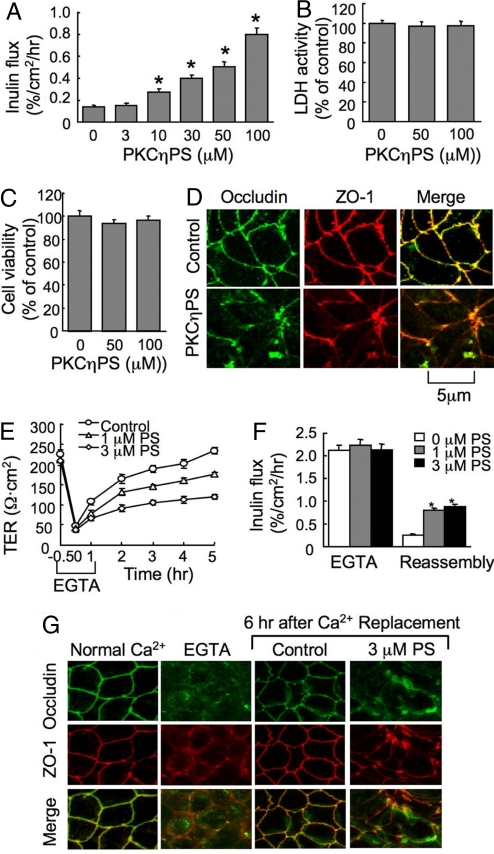
PKCη pseudo substrate (PKCηPS) dose-dependently increased the inulin permeability in Caco-2 cell monolayers (Fig. 1A) without affecting the LDH release (Fig. 1B) or cell viability (Fig. 1C). The control peptide with scrambled sequence did not affect inulin permeability [supporting information (SI) Fig. S1A]. PKCηPS induced a redistribution of occludin and ZO-1 from the intercellular junction into the intracellular compartment (Fig. 1D). Calcium-induced increase in TER (Fig. 2E), decrease in inulin permeability (Fig. 1F), and reorganization of occludin and ZO-1 at the intercellular junctions (Fig. 1G) were attenuated by PKCηPS, but not by the control peptide (Fig. S1B), indicating that PKCη plays an important role in TJ regulation. Similar to its effects in Caco-2 cells, PKCηPS induced disruption of TJs and barrier function and attenuated calcium-induced reassembly of TJs in MDCK cell monolayers (Fig. S2). PKCηPS however, did not affect the distribution of E-cadherin and β-catenin (Fig. S3), suggesting that PKCη may not regulate the adherens junctions.
Fig. 1.
PKCηPS disrupts TJs in Caco-2 cell monolayers. (A) Cell monolayers were incubated with varying concentrations of PKCηPS. Inulin permeability was measured at 1 hour. (B and C) Cell monolayers were incubated with 50 or 100 μM PKCηPS for 2 hours, and the incubation medium was assayed for LDH activity (B) and the cells analyzed for cytotoxicity by WST assay (C). (D) After 1-hour incubation with 50 μM PKCηPS, cell monolayers were fixed and double labeled for occludin and ZO-1. (E and F) Cell monolayers were incubated with EGTA to deplete calcium. TJ reassembly was induced by calcium replacement in the absence or presence of PKCηPS (PS). TJ assembly was evaluated by measuring TER (E) and inulin permeability (F). (G) At different stages of calcium switch-mediated TJ assembly with or without PKCηPS (PS), cell monolayers were double labeled for occludin and ZO-1 by immunofluorescence method. Values in A–C, E, and F are mean ± SEM (n = 6), and the asterisks indicate the values that are significantly different from corresponding values for cells incubated without PKCηPS.
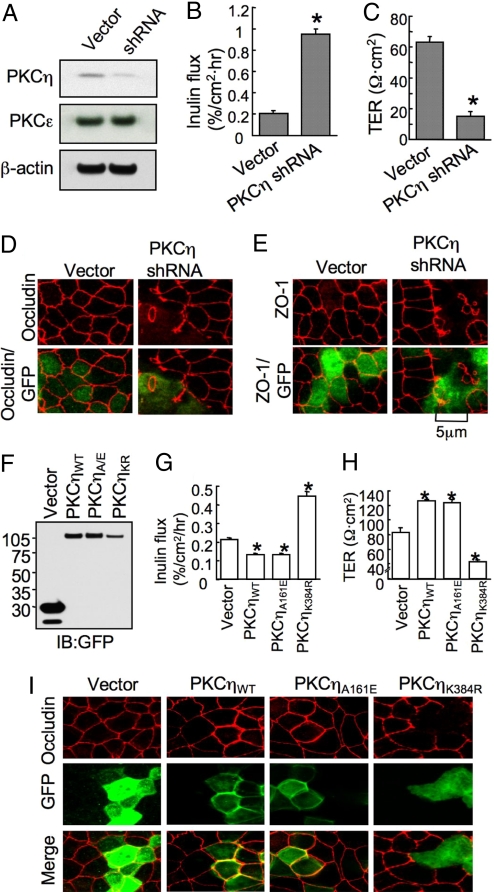
Fig. 2.
Reduced expression of PKCη attenuates the assembly of TJ in MDCK cells. Cells were transfected with PKCη-specific shRNA. (A) Protein extracts from vector or shRNA-transfected cells were immunoblotted for PKCη, PKCε, and β-actin. (B and C) Cells transiently transfected with the vector or shRNA were assessed for inulin permeability (B) and TER (C). (D and E) Cells transiently transfected with vector or shRNA were double stained for GFP and occludin (D) or ZO-1 (E). (F) Proteins from cells transfected with vector, PKCηWT-GFP, PKCηA161E-GFP or PKCηK384R-GFP were immunoblotted for GFP. (G and H) Inulin flux (G) and TER (H) were measured in cell monolayers transfected with vector or PKCη. (I) Cells transiently transfected with vector or PKCη constructs were double stained for GFP and occludin. Values in B, C, G, and H are mean ± SEM (n = 6). Asterisks indicate the values that are significantly (P < 0.05) different from corresponding value for vector-transfected cells.
Transfection of MDCK cells with shRNA in pRNAtinH1.2 vector (also express GFP) reduced the level of PKCη (Fig. 2A). Reduction of PKCη by shRNA was associated with a significant increase in inulin permeability (Fig. 2B) and a decrease in TER (Fig. 2C). Cells transiently transfected with vector or shRNA construct were double labeled for GFP and occludin or ZO-1 to compare the distribution of occludin and ZO-1 in GFP-positive and GFP-negative cells in the same monolayer. Junctional distribution of both occludin (Fig. 2D) and ZO-1 (Fig. 2E) was intact in GFP-negative cells, whereas it was disrupted in GFP-positive cells. Stable expression of shRNA also attenuated the organization of occludin and ZO-1 at the TJs and the development of barrier to inulin during the calcium-induced reassembly of TJs (Fig. S4). The effect of shRNA on TJs was reversed by cotransfection of a siRNA-resistant mutant PKCη (Fig. S5).
These results indicate that PKCη activity is required for the assembly and/or maintenance of the integrity of epithelial TJs. Similar effects of PKCηPS in Caco-2 and MDCK cells suggest that the role of PKCη in TJ regulation is a general phenomenon in epithelial tissues. The prevention of calcium-induced assembly of TJs by PKCηPS or PKCη-shRNA suggests that PKCη activity is required for the assembly of TJs. However, it is difficult to distinguish at this time whether PKCη is involved in the assembly of TJs or it is required only for the maintenance of assembled TJs. Occludin knockout mice showed no evidence of TJ disruption (16), which is possibly due to compensation by other PKC isoforms such as PKCζ and PKCλ.
Active PKCη Enhances and Dominant Negative PKCη Diminishes TJ Integrity.
PKCηWT-GFP (wild type), PKCηA161E-GFP (constitutively active) and PKCηK384R-GFP (dominant negative) were expressed in MDCK cells (Fig. 2F). The cells expressing PKCηWT-GFP or PKCηA161E-GFP exhibited significantly low inulin permeability (Fig. 2G) and high TER (Fig. 2H), whereas the expression of PKCηK384R-GFP significantly elevated inulin permeability (Fig. 2G) and reduced TER (Fig. 2H). Junctional distribution of occludin was unaffected by GFP expression in vector-transfected cells (Fig. 2I). Expression of PKCηWT-GFP or PKCηA161E-GFP enhanced the distribution of occludin at the intercellular junctions (particularly in GFP-positive cells), whereas PKCηK384R-GFP expression disrupted the junctional distribution of occludin (Fig. 2I). These results further validate the role of PKCη in the regulation of epithelial TJs. Elevated levels of junctional occludin in cells transfected with PKCηWT-GFP or PKCηA161E-GFP, particularly in GFP-positive cells compared with GFP-negative cells, indicate that active PKCη enhances the recruitment of occludin at the TJs.
PKCη Directly Interacts with Occludin.
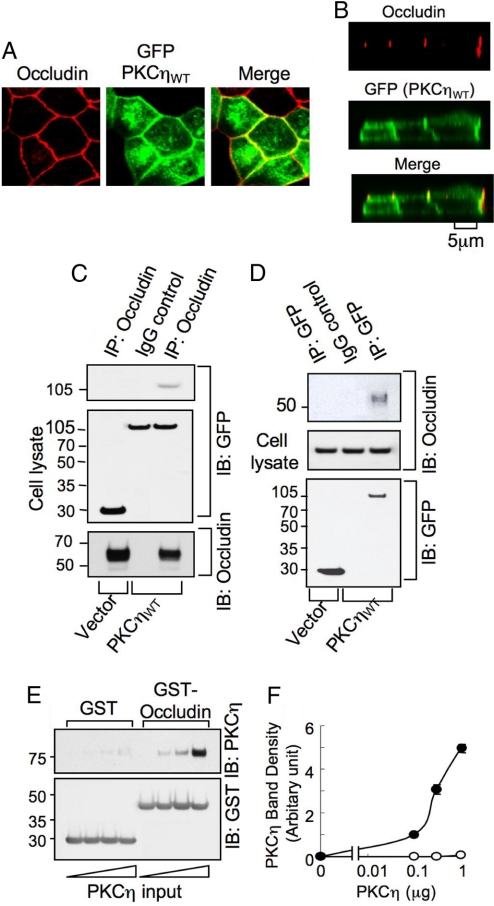
Studies were conducted to investigate the interaction of PKCη with the TJ protein complex in cells expressing PKCηWT-GFP. Immunofluorescence microscopy showed that a significant portion of cellular PKCηWT-GFP was localized at the intercellular junctions (Fig. 3A) and colocalized with occludin (Fig. 3B). Occludin was coimmunoprecipitated with anti-GFP immunocomplex prepared from cells transfected with GFP-PKCηWT, but not with anti-GFP immunoprecipitates prepared from vector-transfected cells (Fig. 3C). Similarly, anti-occludin immunocomplex showed the presence of PKCηWT-GFP (Fig. 3D). These results indicate that PKCη interacts with the TJ protein complex. To determine the direct interaction of PKCη with occludin we conducted a pair-wise binding assay using recombinant PKCη and GST-Occludin-C (the C-terminal region of human occludin). PKCη dose-dependently bound to GST-Occludin-C with only a trace amount of its binding to GST (Fig. 3 E and F). Colocalization and coimmunoprecipitation of PKCη with occludin suggest that PKCη may directly interact with the TJ proteins. The in vitro pair-wise binding studies demonstrated that PKCη binds directly to the intracellular C-terminal domain of occludin. Previous studies indicated that PKCζ and PKCλ may directly interact with TJ proteins (10, 11), whereas PKCβI and PKCε indirectly regulate TJ integrity (12). Our present study shows that similar to atypical PKC isoforms PKCη directly interacts with the TJs.
Fig. 3.
PKCη directly interacts with occludin. (A and B) MDCK cells expressing PKCηWT-GFP were fixed and double stained for occludin and GFP. The x–y (A) and x–z (B) fluorescence images were collected. (C and D) Anti-occludin immunocomplexes (nondenatured) from cells transfected with PKCηWT-GFP or vector were immunoblotted for occludin and GFP (C). Similarly, GFP was immunoprecipitated and immunoblotted for GFP and occludin (D). (E) Recombinant proteins GST or GST-Occludin-C were incubated with recombinant PKCη. GST and GST-Occludin-C were pulled down with GSH-agarose and immunoblotted for PKCη and GST. (F) Densitometric analysis of PKCη bands in experiment described in E. Values are mean ± SEM (n = 3).
PKCη Regulates Thr-Phosphorylation of Occludin.
We evaluated the effect of PKCηPS, PKCη-shRNA, and the expression of PKCη mutants on the Thr-phosphorylation of occludin. PKCηPS and expression of PKCη-shRNA reduced the Thr-phosphorylation of occludin in both Caco-2 and MDCK cell monolayers without altering the level of total occludin protein (Fig. 4 A and B); however, the Thr-phosphorylation of ZO-1 or Tyr-phosphorylation of occludin was unaffected. The expression of PKCηWT-GFP and PKCηA161E-GFP enhanced the Thr-phosphorylation of occludin, whereas it was reduced by PKCηK384R-GFP (Fig. 4C). Incubation of PKCη with GST-Occludin-C in vitro in the presence of ATP induced phosphorylation of GST-Occludin-C on Thr residues (Fig. 4 D and E); this phosphorylation was attenuated by PKCηPS (Fig. 4F). All these data point to the possibility that PKCη plays a crucial role in the Thr-phosphorylation of occludin in the cell. The in vitro phosphorylation studies indicated that PKCη directly phosphorylates Thr residues in the occludin C-terminal domain.
Fig. 4.
PKCη regulates Thr-phosphorylation of occludin. (A) Caco-2 and MDCK cell monolayers were incubated with or without 50 μM PKCηPS for 1 hour, or transfected with vector or PKCη-shRNA. p-Thr and p-Tyr were immunoprecipitated and immunoblotted for ZO-1 and occludin. (B) Occludin was immunoprecipitated from MDCK cells transfected with vector or shRNA and immunoblotted for p-Thr. (C) p-Thr immunoprecipitated from MDCK cells transfected with vector or PKCηWT-GFP, PKCηA161E-GFP, and PKCηK384R-GFP were immunoblotted for occludin. (D) GST-Occludin-C (10 μg) was incubated with varying amounts of PKCη in the absence or presence of ATP and immunoblotted for p-Thr and GST. (E) Densitometric analysis of p-Thr bands from experiments (+ATP) in D. Values are mean ± SEM from 3 independent experiments. (F) GST-Occludin-C was incubated with PKCη with or without ATP and in presence or absence of PKCηPS and immunoblotted for p-Thr and GST.
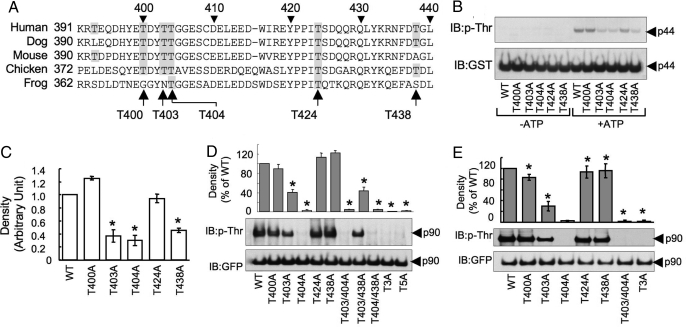
Sequence alignment of occludin from different species (Fig. 5A) indicated that T400, T403, T404, T424, and T438 of occludin are highly conserved through the evolution. To determine the phosphorylation of these residues by PKCη, we induced point mutations at these Thr residues in GST-Occludin-C. Mutation of T403, T404, and T438 to Ala partially reduced PKCη-induced phosphorylation (Fig. 5 B and C); multiple mutations of T403, T404, and T438 abolished the PKCη-mediated phosphorylation. The PKCη-induced phosphorylation of T403 and T404 was confirmed by mass spectrometric analysis (Fig. S6). To determine the Thr-phosphorylation sites in occludin in the cell, we mutated T400, T403, T404, T424, and T438 (single or multiple) to Ala in GFP-Occludin. Wild type and mutants of GFP-occludin were expressed in MDCK or Caco-2 cells. GFP from these cells was immunoprecipitated and immunoblotted for p-Thr. In both MDCK (Fig. 5D) and Caco-2 (Fig. 5E) cells, mutation of T403 resulted in partial loss of Thr-phosphorylation of occludin. However, mutation of T404 abolished the Thr-phosphorylation; similarly, multiple mutations that included T404A also abolished Thr-phosphorylation. Therefore, it is likely that PKCη phosphorylates occludin on T403 and T404 in vivo. A complete loss of Thr-phosphorylation by T404A mutation suggests that the phosphorylation of T404 may be a prerequisite step for the subsequent phosphorylation of T403.
Fig. 5.
PKCη phosphorylates occludin on specific Thr residues. (A) Alignment of amino acid sequence of the C-terminal region of occludin from different species. Conserved Thr residues are highlighted. The Thr residues in human occludin selected for mutation are identified by arrows. (B) GST-Occludin-C and its Thr-mutants (p44) were prepared and incubated with PKCη in the presence or absence of ATP. Reaction mixtures were immunoblotted to p-Thr and GST. (C) Densitometric analysis of p-Thr bands in experiments described in B. (D and E) GFP-Occludin (p90) and its Thr-mutants were expressed in MDCK (D) and Caco-2 (E) cells. Anti-GFP immunocomplexes were immunoblotted for p-Thr and GFP. Densitometric analysis was performed to evaluate the p-Thr densities. T3A refers to occludin in which T403, T404, and T438 were mutated and T5A to occludin with all 5 threonines mutated. In all experiments, values are mean ± SEM (n = 3). Asterisks indicate the values that are significantly different (P < 0.05) from corresponding value for vector-transfected cells.
Phosphorylation of T403 and T404 Is Required for the Assembly of Occludin at the TJs.
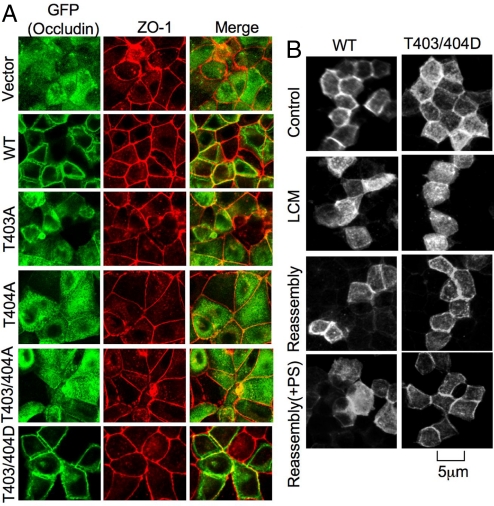
Subcellular localization of GFP-OccludinWT and its mutants in MDCK and Rat-1 (occludin null) cells was assessed by immunofluorescence visualization of GFP. In MDCK cells, GFP-OccludinWT appeared at the intercellular junctions at 1 hour after calcium-induced assembly of TJs, whereas T403/404A mutants were localized predominantly in the intracellular compartment (Fig. 6A). On the other hand, T403/404D mutants were organized at the intercellular junction (Fig. 6A). These results demonstrate that phosphorylation of T403 and T404 is necessary for the occludin to be organized at the plasma membranes and intercellular junctions. In Rat-1 fibroblasts, GFP-OccludinWT was localized at the plasma membrane forming new cell–cell contacts, which was colocalized with ZO-1 (Fig. S7). T403/404A mutants appeared almost exclusively in the intracellular compartment, whereas T403/404D mutants were localized predominantly in the plasma membrane. Interestingly, the expression of T403/404D mutants enhanced the junctional localization of ZO-1, whereas T403/404A mutant diminished junctional distribution of ZO-1. The inulin permeability (percentage of flux per hr/cm2) in MDCK cell monolayers that expressed T403/404D mutant (0.036 ± 0.006) was lower and in cell monolayers that expressed T403/404A mutant (0.20 ± 0.063) was higher than that in cell monolayers that expressed wild-type occludin (0.10 ± 0.026). Although occludin may not be required for the assembly of TJs, it may regulate the integrity of TJs, possibly by a phosphorylation-dependent mechanism.
Fig. 6.
Phosphorylation of occludin on T403 and T404 regulates its assembly into TJs. (A) MDCK cells were transfected with GFP-Occludin or its mutants. Transfected cells on transwells were preincubated with low calcium medium (LCM) followed by calcium restoration for 90 min before confocal imaging. Cells were double labeled for GFP and ZO-1. (B) MDCK cell monolayers transfected with GFP-OccludinWT or GFP-OccludinT403/404D were incubated with low calcium medium overnight, followed by replacement of calcium for 90 min in the presence or absence of PKCηPS (3 μM).
In MDCK cells, occludin mutants (T403/404A) were eventually organized at the intercellular junctions possibly because of dimerization with the endogenous occludin. Overall, these results indicate that phosphorylation of T403 or T404 is required for the assembly of occludin into the intercellular junctions. Administration of PKCηPS during calcium-induced assembly of TJs in MDCK cells attenuated the junctional organization of GFP-OccludinWT (Fig. 6B). However, PKCηPS failed to prevent the junctional organization of GFP-OccludinT403/404D. These results indicate that PKCη-induced phosphorylation of occludin on T403 and T404 is involved in calcium-induced assembly of occludin into the TJs. The precise role of Thr-phosphorylation is unclear, but likely to regulate occludin interactions with other components of TJs.
In summary, this study demonstrates that PKCη plays a critical role in the assembly and the maintenance of epithelial TJs by inducing Thr-phosphorylation of occludin on T403 and T404. Furthermore, this study demonstrates that phosphorylation of occludin on T403 and T404 is required for the assembly and/or maintenance of occludin in the TJs.
Methods
Reagents.
The PKCη pseudo substrate peptide, Myr-TRKRQRAMRRRVHQING (13) and a control peptide with scrambled sequence (Myr-RMINKARVRGRAQRHG-OH) were custom synthesized by GenScript. HRP-conjugated anti-GST and biotin-conjugated anti-p-Tyr antibodies were purchased from BD Biosciences. Rabbit polyclonal anti-occludin, anti-ZO-1, and anti-p-Thr and mouse monoclonal anti-occludin antibodies were purchased from Zymed. Alexa Fluor 488-conjugated anti-mouse IgG and anti-rabbit IgG antibodies were obtained from Molecular Probes. Cy3-conjugated anti-mouse IgG and anti-rabbit IgG, HRP-conjugated anti-mouse IgG and anti-rabbit IgG antibodies, and mouse monoclonal anti-β-actin antibodies were purchased from Sigma. Anti-mouse and anti-rabbit GFP antibodies were purchased from Clontech. Recombinant PKCη was purchased from Millipore.
Plasmids, shRNA, and Mutation.
For canine PKCη gene silencing, shRNA was designed by using the Dharmacon web site (siDesign Center, www.dharmacon.com/DesignCenter/) and cloning into pRNAtin-H1.2 vector (GenScript), which also contains cGFP gene. ShRNA-resistant PKCη was generated by mutation of 5 nucleotides at the siRNA target sequence without altering the amino acid sequence of resulting protein, and inserted into pmCherry vector. PKCηA161E and PKCηK384R in the pEFneo vector were kind gifts from Dr. Gottfried Baier (University of Innsbruck, Innsbruck, Austria). The PKCηA161E-GFP and PKCηK384R-GFP were subcloned into pAcGFP-N1 vector (Clontech). PKCηWT-GFP expression vector was generated from PKCηA161E-GFP by introducing E161A point mutation using QuickChange site-directed mutagenesis kit (Stratagene).
The cytoplasmic C-terminal region of human occludin encoding 378–522 (Occludin-C) was amplified from the pEGFP-occludin vector and was cloned into pGEX-2T vector (Amersham). Point mutations of T400, T403, T404, T424, and T438 to Ala or Asp (single or multiple mutations) were introduced in wild-type GFP-occludin or GST-Occludin-C nucleotide sequence as described above. The sequences of shRNA targeting region and the primers for PKCη and occludin are provided in Table S1. The GST-Occludin-C and its Thr mutants were expressed in Escherichia coli BL21 (DE3) and purified as described previously (11).
Cell Culture and Transfection.
Caco-2, MDCK II, and Rat-1 cells purchased from American Type Cell Culture were grown under standard cell culture conditions as described (11, 12). Cells were grown in Transwell inserts (6.5- to 24-mm diameter; Costar). PKCη shRNA, PKCη, and occludin expression vectors (0.3–1.0 μg of DNA) were transfected by using Lipofectamine 2000 (Invitrogen) in MDCK cells, Lipofectamine LTX and Plus reagent in Caco-2 cells, and Fugene HD (Roche Applied Science) in Rat-1 cells. Each empty vector was used as a negative control. Stably expressing PKCη shRNA and GFP-tagged occludin or PKCη proteins in MDCK cells were generated by G-418-mediated selection (0.6 mg/ml). Resistant cells were sorted in a Fluorescence Activated Cell Sorter (BD-LSR2).
TJ Assembly and Disruption.
TJ assembly was induced by the calcium switch method by EGTA treatment in Caco-2 cell monolayers (11) or low calcium medium in MDCK cells. Barrier function was evaluated by measuring TER and unidirectional flux of inulin (11). Cell viability was monitored by assay for lactate dehydrogenase release and mitochondrial dehydrogenase activity (cytotoxicity detection kit and cell proliferation reagent WST-1, Roche Applied Science).
Immunofluorescence Microscopy.
Cell monolayers were fixed in acetone/methanol (1:1) at 0 °C for 5 min, permeabilized in 0.2% Triton X-100, and incubated with primary antibodies (anti-ZO-1, anti-GFP, and anti-occludin) and secondary antibodies (goat Alexa Fluor 488-conjugated anti-mouse and anti-rabbit IgG antibodies and Cy3-conjugated anti-mouse and anti-rabbit IgG) in 3% nonfat milk as described (12). The fluorescence was visualized in a Zeiss LSM 5 laser scanning confocal microscope, and the images from Z-series sections (1-μm thickness) were collected by using Zeiss LSM 5 Pascal confocal microscopy software (release 3.2). Oil-immersion objective lens of 63× magnification with 1.4 numerical aperture was used to collect images. The z-series images were stacked by using the software, Image J (National Institutes of Health), and processed by Adobe Photoshop (Adobe Systems). Fluorescence at the intercellular junctions was quantitated by densitometry using Image J software.
Interaction Between Occludin and PKCη.
Interaction between occludin and PKCη was determined by coimmunoprecipitation studies as described previously for PP2A (11) and by colocalization by confocal microscopy. To determine the direct interaction between occludin and PKCη, pair-wise binding assay was conducted by using recombinant PKCη and GST-occludin-C as described (11).
Occludin Phosphorylation.
Occludin phosphorylation in vivo was determined by immunoprecipitation of p-Thr or p-Tyr from normal cells or those transfected with PKCη shRNA, GFP-tagged PKCη, GFP-tagged occludin, and their empty vectors followed by immunoblot analysis for occludin or GFP as described (11).
For in vitro phosphorylation, GST-occludin-C (20 μg) and its mutants were incubated with active PKCη (0.25–1.0 μg) in 90 μL of 20 mM Hepes buffer containing 0.5 mM ATP, 0.75 mM MgCl2, 0.2 mM CaCl2, 0.1 mg/ml phosphatidylserine, 10 μg/ml diacylglycerol, 0.03% Triton X-100, 0.25 mM glycerophosphate and 0.1 mM PMSF (pH 7.4) for 3 h at 30 °C on a rocker platform. After incubation, the reaction solution was immunoblotted for p-Thr.
Supplementary Material
Acknowledgments.
We thank Drs. Subramanian, Sarka Beranova-Giorgianni, and Li Chen for technical assistance. This work was supported by National Institutes of Health Grants R01-DK55532, R01-AA12307, DK061931, and 1S10-RR16679. T.S. is a recipient of Research Fellowships from the Japan Society for the Promotion of Science for Young Scientists.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802741106/DCSupplemental.
References
- 1.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 2.Turner JR. Molecular basis of epithelial barrier regulation: From basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z, et al. Raf 1 represses expression of the tight junction protein occludin via activation of the zinc-finger transcription factor slug. Oncogene. 2007;26:1222–1230. doi: 10.1038/sj.onc.1209902. [DOI] [PubMed] [Google Scholar]
- 4.Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: Leading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- 5.Saitou M, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medina R, Rahner C, Mitic LL, Anderson JM, Van Itallie CM. Occludin localization at the tight junction requires the second extracellular loop. J Membr Biol. 2000;178:235–247. doi: 10.1007/s002320010031. [DOI] [PubMed] [Google Scholar]
- 7.Van Itallie CM, Anderson JM. Occludin confers adhesiveness when expressed in fibroblasts. J Cell Sci. 1997;110(Pt 9):1113–1121. doi: 10.1242/jcs.110.9.1113. [DOI] [PubMed] [Google Scholar]
- 8.Stuart RO, Nigam SK. Regulated assembly of tight junctions by protein kinase C. Proc Natl Acad Sci USA. 1995;92:6072–6076. doi: 10.1073/pnas.92.13.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basuroy S, et al. Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J Biol Chem. 2003;278:11916–11924. doi: 10.1074/jbc.M211710200. [DOI] [PubMed] [Google Scholar]
- 10.Helfrich I, et al. Role of aPKC isoforms and their binding partners Par3 and Par6 in epidermal barrier formation. J Invest Dermatol. 2007;127:782–791. doi: 10.1038/sj.jid.5700621. [DOI] [PubMed] [Google Scholar]
- 11.Seth A, Sheth P, Elias BC, Rao R. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J Biol Chem. 2007;282:11487–11498. doi: 10.1074/jbc.M610597200. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki T, Seth A, Rao R. Role of phospholipase Cgamma-induced activation of protein kinase Cepsilon (PKCepsilon) and PKCbetaI in epidermal growth factor-mediated protection of tight junctions from acetaldehyde in Caco-2 cell monolayers. J Biol Chem. 2008;283:3574–3583. doi: 10.1074/jbc.M709141200. [DOI] [PubMed] [Google Scholar]
- 13.Osada S, et al. A phorbol ester receptor/protein kinase, nPKC eta, a new member of the protein kinase C family predominantly expressed in lung and skin. J Biol Chem. 1990;265:22434–22440. [PubMed] [Google Scholar]
- 14.Ohba M, et al. Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of the eta and delta isoforms of protein kinase C. Mol Cell Biol. 1998;18:5199–5207. doi: 10.1128/mcb.18.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chida K, Murakami A, Tagawa T, Ikuta T, Kuroki T. Cholesterol sulfate, a second messenger for the eta isoform of protein kinase C, inhibits promotional phase in mouse skin carcinogenesis. Cancer Res. 1995;55:4865–4869. [PubMed] [Google Scholar]
- 16.Chida K, et al. Disruption of protein kinase Ceta results in impairment of wound healing and enhancement of tumor formation in mouse skin carcinogenesis. Cancer Res. 2003;63:2404–2408. [PubMed] [Google Scholar]
- 17.Masso-Welch PA, et al. Altered expression and localization of PKC eta in human breast tumors. Breast Cancer Res Treat. 2001;68:211–223. doi: 10.1023/a:1012265703669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.