Abstract
Steroid receptor coactivator-1 (SRC-1) is a coactivator for nuclear hormone receptors such as estrogen and progesterone receptors and certain other transcription factors such as Ets-2 and PEA3. SRC-1 expression in breast cancer is associated with HER2 and c-Myc expression and with reduced disease-free survival. In this study, SRC-1−/− mice were backcrossed with FVB mice and then cross-bred with MMTV-polyoma middle T antigen (PyMT) mice to investigate the role of SRC-1 in breast cancer. Although mammary tumor initiation and growth were similar in SRC-1−/−/PyMT and wild-type (WT)/PyMT mice, genetic ablation of SRC-1 antagonized PyMT-induced restriction of mammary ductal differentiation and elongation. SRC-1−/−/PyMT mammary tumors were also more differentiated than WT/PyMT mammary tumors. The intravasation of mammary tumor cells and the frequency and extent of lung metastasis were drastically reduced in SRC-1−/−/PyMT mice compared with WT/PyMT mice. Metastatic analysis of transplanted WT/PyMT and SRC-1−/−/PyMT tumors in SRC-1−/− and WT recipient mice revealed that SRC-1 played an intrinsic role in tumor cell metastasis. Furthermore, SRC-1 was up-regulated during mammary tumor progression. Disruption of SRC-1 inhibited Ets-2-mediated HER2 expression and PyMT-stimulated Akt activation in the mammary tumors. Disruption of SRC-1 also suppressed colony-stimulating factor-1 (CSF-1) expression and reduced macrophage recruitment to the tumor site. These results suggest that SRC-1 specifically promotes metastasis without affecting primary tumor growth. SRC-1 may promote metastasis through mediating Ets-2-mediated HER2 expression and activating CSF-1 expression for macrophage recruitment. Therefore, functional interventions for coactivators like SRC-1 may provide unique approaches to control breast cancer progression and metastasis.
Virtually all transcription factors in mammals require coactivators to mediate their transcriptional activation functions (1). Through modulating gene expression regulated by hormones, growth factors, and cytokines, coactivators play crucial roles in many biological and pathological processes including cell proliferation, differentiation, carcinogenesis, and metastasis (1–3). The combinations, concentrations, and posttranslational modifications of these coactivators act to determine the specificity and efficiency of gene transcription (1, 3). The p160 steroid receptor coactivator (SRC) family contains 3 members: SRC-1 (NCOA1), SRC-2 (TIF2, GRIP1, or NCOA2), and SRC-3 (AIB1, ACTR, or NCOA3) (3). They share an overall similarity of 50–55% in their amino acid sequences, interact with nuclear hormone receptors and coactivate transcription through recruiting chromatin-remodeling and other transcriptional enzymes (3). In addition to nuclear receptors, members of the SRC family also interact with and coactivate other transcription factors such as Ets-2, PEA3, and E2F1 (4–11). Studies using mutant mouse models have shown that the members of the SRC family have both unique and partially redundant biological functions in development, somatic growth, steroid hormone response, metabolism, reproduction, cardiovascular system, and inflammatory response (3, 12–20).
In the SRC family, SRC-3 was first found to be amplified and overexpressed in breast cancer (21). Subsequent studies have shown that SRC-3 knockdown in breast cancer cells inhibits epidermal growth factor receptor (EGFR) activation, cyclin D1 expression, E2F1-mediated gene expression, and estrogen-induced cell proliferation (22–25). In mice with mammary carcinogenesis induced by oncoproteins or carcinogens, SRC-3 knockout suppresses IGF1 signaling pathway, Akt activation, cyclin D1 expression, mammary tumorigenesis, and metastasis (8, 26, 27). Moreover, SRC-3 overexpression in the mouse mammary epithelial cells caused spontaneous mammary tumors (28). These findings suggest that SRC-3 is a proto-oncoprotein of breast cancer.
To date, however, only a few studies have been conducted to investigate SRC-1 in breast cancer. Normal human mammary epithelial cells have minimal to no SRC-1 expression (8). However, SRC-1 expression increases in breast cancers. Increase of SRC-1 expression correlates with HER2 positivity, disease recurrence in HER2-positive breast cancers and resistance to endocrine therapy (8, 29). SRC-1 expression is inversely correlated with the expression of estrogen receptor β, a marker for better prognosis of disease-free survival in breast cancer (30). In addition, SRC-1 also interacts with Ets-2, both to enhance c-Myc expression in endocrine-resistant breast cancer cells (4, 5) and to promote ERα-mediated SDF-1 expression, facilitating cell proliferation and invasion (31). These findings suggest that patients with high expression of HER2 in combination with SRC-1 have a greater probability of recurrence compared with those who are HER2 positive but SRC-1 negative. However, the mechanistic role of SRC-1 in vivo during the entire process of breast cancer initiation and progression remains to be characterized.
In this study, we crossed SRC-1−/− mice with MMTV-PyMT mice to investigate the role of SRC-1 in mammary carcinogenesis. Although PyMT is not a human breast cancer oncogene, it activates c-Src/PI3K/Akt and Shc/ras/MAPK pathways, the same major protein kinase pathways as HER2 (32). Expression of the MMTV-PyMT transgene in mice causes rapid formation of mammary carcinomas with all identifiable stages similar to human breast cancer progression (33). Extensive lung metastasis also develops in all MMTV-PyMT mice (8, 33–35), which makes the animal model ideal for investigating the role of SRC-1 in breast cancer metastasis. Furthermore, biological markers expressed in PyMT-induced mammary tumors are consistent with those expressed in human breast cancers. For instance, the loss of ERα, PR, and integrin-β1 and the persistent expression of HER2 and cyclin D1 were observed in PyMT-induced tumors as they progressed to the malignant stage (35, 36). By inducing and characterizing the initiation and progression of mammary tumors in WT/PyMT and SRC-1−/−/PyMT mice, we have substantiated the role of SRC-1 in mammary cancer metastasis and uncovered molecular pathways responsible for SRC-1 to promote metastasis.
Results
Inactivation of SRC-1 Restores Mammary Ductal Differentiation but Does Not Inhibit Mammary Tumorigenesis in MMTV-PyMT mice.
In normal mouse mammary gland, relatively low levels of SRC-1 protein were detected in the luminal epithelial cells (LECs) and myoepithelial cells (MECs) by immunohistochemistry (IHC) (data not shown). To investigate the role of SRC-1 in breast cancer, we induced mammary carcinogenesis in WT and SRC-1−/− mice by using the MMTV-PyMT transgenic model. To match the FVB strain background of MMTV-PyMT mice, SRC-1−/− mice with a mixed 129SvEv/C57BL/6 strain background (13) were backcrossed with FVB mice for 6 generations. Mammary gland morphogenesis was similar in backcrossed FVB WT and SRC-1−/− mice. On the contrary, this phenotype differed from the slightly reduced mammary morphogenesis observed in SRC-1−/− mice with a mixed 129SvEv/C57BL/6 strain background (13). The FVB female mice have larger mammary glands with more ductal branches compared with C57BL/6 female mice. Subsequently, the backcrossed female FVB SRC-1+/− mice were bred with male MMTV-PyMT mice to produce female SRC-1+/−/WT and male SRC-1+/−/PyMT breeding pairs. From these breeding pairs, female WT/PyMT, SRC-1+/−/PyMT and SRC-1−/−/PyMT mice were generated for experiments. This breeding strategy guaranteed that all experimental mice had a uniform strain background and were heterozygous for the MMTV-PyMT transgene.
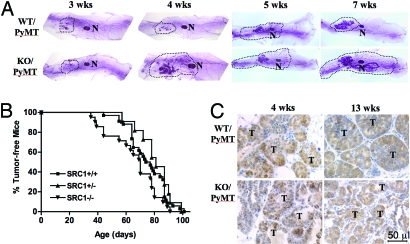
In WT/PyMT mice, the PyMT-induced LEC proliferation inhibited mammary epithelial differentiation and ductal elongation, resulting in a precocious phenotype of mammary gland development in most mice and tumorigenesis in all mice as reported (Fig. 1A) (33, 34). Interestingly, SRC-1 deficiency in SRC-1−/−/PyMT mice largely abrogated precocious mammary gland development. Although mammary glands of both WT/PyMT and SRC-1−/−/PyMT mice showed little outgrowth at 3 weeks of age because of low levels of ovarian steroids, the mammary glands of SRC-1−/−/PyMT mice exhibited more end buds, more ductal branches, and deeper ductal penetrance in the mammary fat pads compared with age-matched WT/PyMT mice (Fig. 1A). These results suggest that SRC-1 contributes to PyMT-induced inhibition of mammary ductal differentiation and elongation.
Fig. 1.
SRC-1 deficiency maintains mammary ductal branching and tumor cell differentiation but it does not affect mammary tumor formation. (A) Whole-mounted mammary glands from WT/PyMT and SRC-1−/− (KO)/PyMT mice with indicated ages. The fat pad areas with mammary ducts are outlined. N, lymph node. (B) Tumor-free curves of WT/PyMT (SRC1+/+, n = 34), SRC-1+/−/PyMT (SRC1±, n = 11) and SRC-1−/−/PyMT (SRC1−/−, n = 21) mice. There was no statistical difference between these curves (P > 0.05, log rank test). (C) Tissue sections of mammary tumors (T) isolated from WT/PyMT and SRC-1−/− (KO)/PyMT mice at ages of 4 and 13 weeks. The sections were immunostained with the SV40 T antibody (brown color) and counterstained with hematoxylin.
To detect mammary tumor formation, WT/PyMT, SRC-1+/−/PyMT and SRC-1−/−/PyMT mice were examined twice a week by palpation. Our examinations revealed that 50% of WT/PyMT, SRC-1+/−/PyMT and SRC-1−/−/PyMT mice developed mammary tumors at average ages of 75, 80, and 70 days, respectively. All SRC-1−/−/PyMT mice and all WT/PyMT and SRC-1+/−/PyMT mice developed mammary tumors within 90 and 100 days, respectively (Fig. 1B). Although the tumor-free curve of SRC-1−/−/PyMT mice slightly shifted to the left, which suggests a trend of earlier tumorigenesis, there were no significant differences in tumor latency among these 3 groups of mice (Fig. 1B). Next, we addressed whether SRC-1 deficiency affected PyMT tumor growth. The average time periods for mammary tumors to reach a diameter of 1.5 cm were, respectively, 22 ± 8 (n = 34), 21 ± 10 (n = 11), and 23 ± 8 (n = 21) days in WT/PyMT, SRC-1+/−/PyMT, and SRC-1−/−/PyMT mice after palpable tumors were detected, revealing no statistical differences (P > 0.05, one-way ANOVA). These results indicate that SRC-1 is not required for PyMT-induced mammary tumor initiation and growth.
Next, the histology of SRC-1−/−/PyMT and WT/PyMT mammary tumors was examined (Fig. 1C). In the mammary glands of WT/PyMT mice at 4 weeks, only some of the mammary ducts were lined with differentiated LECs and had a visible lumen, whereas many mammary ducts were filled with undifferentiated tumor cells. In the mammary glands of WT/PyMT mice at 13 weeks, many large solid tumors were observed and only a few acinar structures could be found in the tumor tissues (Fig. 1C). In contrast, in the mammary glands of SRC-1−/−/PyMT mice at both 4 and 13 weeks, LEC sheets and many ductal and acinar lumens were observed in the hyperplastic regions and tumor tissues (Fig. 1C). However, the MEC layer was disrupted at similar ages in both WT/PyMT and SRC-1−/−/PyMT mammary glands as assayed by IHC for smooth muscle α-actin (data not shown). The results suggest that SRC-1−/−/PyMT tumors are more differentiated than WT/PyMT tumors.
IHC using PyMT antibody demonstrated that PyMT levels were similar in LECs and mammary tumor cells of WT/PyMT and SRC-1−/−/PyMT mice (Fig. 1C). This was consistent with the similar latency and growth of mammary tumors in WT/PyMT and SRC-1−/−/PyMT mice. Therefore, the morphological differences of mammary gland development and mammary tumors between WT/PyMT and SRC-1−/−/PyMT mice are not attributed to any differential expression of the MMTV-PyMT transgene.
Disruption of SRC-1 Suppresses Mammary Tumor Metastasis to the Lung.
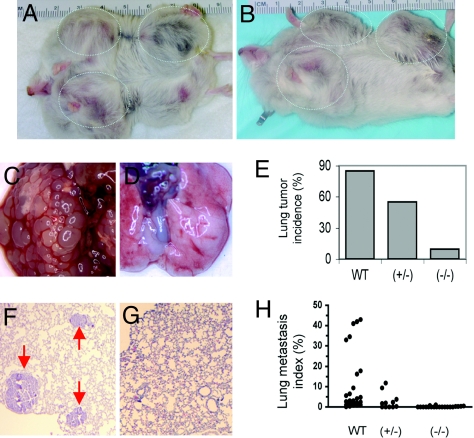
Considering the variations of mammary tumor formation among mice, the following standards were used to determine the time point for examining lung metastasis: mice were at least 100 days old; the palpable mammary tumor had been detected for at least 30 days; and the mammary tumor size was at least 1.5 cm in diameter. As soon as these 3 standards were satisfied, WT/PyMT, SRC-1+/−/PyMT and SRC-1−/−/PyMT mice usually had mammary tumors of comparable size (Fig. 2 A and B). These mice were killed and their lung samples were collected for examining focal metastatic tumors on the surface and inside of the lungs by using stereomicroscope and histological analysis. Macroscopic tumor foci on the lung surface were observed in 85% (28 of 33) of WT/PyMT mice, 55% (6 of 11) of SRC-1+/−/PyMT mice, and 9.5% (2 of 21) of SRC-1−/−/PyMT mice (Fig. 2 C–E). When nonadjacent sagittal lung sections were examined (Fig. 2 F and G), focal lung tumors were observed microscopically in 97% (32 of 33) of WT/PyMT mice, 64% (7 of 11) of SRC-1+/−/PyMT mice, and 29% (6 of 21) of SRC-1−/−/PyMT mice. Statistical calculation (χ2 test) revealed that the lung tumor frequency in SRC-1−/−/PyMT mice was significantly lower compared with the lung tumor frequencies in WT/PyMT (P < 0.0001) and SRC-1+/−/PyMT (P < 0.001) mice. The lung tumor frequency in SRC-1+/−/PyMT mice was also significantly lower than that in WT/PyMT mice (P < 0.001). To quantify the extent of metastasis, we measured the ratio of tumor area to lung area as described (8, 27). The average percentage of tumor area to lung area in SRC-1−/−/PyMT mice was reduced 98.6% and 94.3% when compared with that in WT/PyMT mice and SRC-1+/−/PyMT mice (Fig. 2H). In summary, our results clearly demonstrate that inactivation of SRC-1 drastically suppresses mammary tumor metastasis to the lung.
Fig. 2.
SRC-1 deficiency inhibits lung metastasis. (A and B) WT/PyMT (A) and SRC-1−/−/PyMT (B) mice developed mammary tumors in similar size. (C and D) Lung images of WT/PyMT (C) and SRC-1−/−/PyMT (D) mice. Note the numerous metastatic tumors in C. (E) Lung metastasis incidences as determined by observing lung surface tumors in WT/PyMT (WT, n = 33), SRC-1+/−/PyMT (+/−, n = 11) and SRC-1−/−/PyMT (−/−, n = 21) mice. (F and G) H&E-stained lung sections prepared from WT/PyMT (F) and SRC-1−/−/PyMT (G) mice. Arrows in F indicate lung tumors. (H) Lung metastasis index is calculated by (lung tumor area/lung area) × 100. The number of mice in each genotype group is the same as that in E.
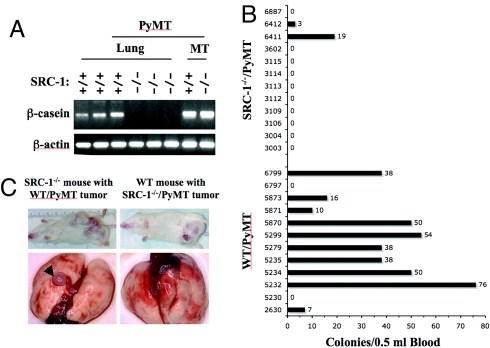
The mammary gland origin of these lung tumors was further confirmed by RT-PCR analysis of β-casein mRNA, which encodes a milk protein expressed specifically in the mammary epithelial cells (27). As expected, the β-casein mRNA was detected in both WT/PyMT and SRC-1−/−/PyMT mammary tumors. However, the β-casein mRNA was detected in the lung samples of WT/PyMT mice with lung tumors, but was undetectable in the lung samples of SRC-1−/−/PyMT mice without lung tumors (Fig. 3A). These results suggest that the low frequency of lung tumors in SRC-1−/−/PyMT mice is due to the suppression of metastasis from mammary tumor tissue.
Fig. 3.
SRC-1 has an intrinsic role to promote mammary tumor cells to enter the blood circulation. (A) Detection of β-casein mRNA by RT-PCR in the lungs of WT (SRC-1+/+)/PyMT mice and in the mammary tumors (MT) of WT/PyMT and SRC-1−/−/PyMT mice. The β-casein mRNA was undetectable in the lungs of SRC-1−/−/PyMT mice. The level of β-actin mRNA served as a loading control. (B) Colony formation assay of the mammary tumor cells in the blood. Mammary tumor cells in blood samples (0.5 ml each) collected from WT/PyMT (n = 12) and SRC-1−/−/PyMT (n = 12) mice were cultured to form colonies. Colonies were stained and counted. The numbers on the left and right sides of bars indicate the tag numbers of mice and the numbers of tumor cell colonies. (C) Mammary tumor transplantation assays. Transplanted WT/PyMT mammary tumors in SRC-1−/− mice and SRC-1−/−/PyMT mammary tumors in WT mice exhibited similar growth (i and ii). Lung tumors were observed in recipient mice with WT/PyMT tumors but not in recipient mice with SRC-1−/−/PyMT tumors (iii and iv).
The Intrinsic Role of SRC-1 in Mammary Tumor Metastasis.
To examine whether the suppression of metastasis in SRC-1−/−/PyMT mice was due to the loss of SRC-1 in the tumor cells or the host environment, we transplanted WT/PyMT and SRC-1−/−/PyMT tumor tissues to the mammary fat pads of SRC-1−/− and WT recipient mice, respectively. After transplantation, WT/PyMT tumors developed in 6 of 10 SRC-1−/− recipients. SRC-1−/−/PyMT tumors developed in 5 of 10 WT recipients. Again, tumor growth rates were comparable between 2 groups. When the tumor diameters reached 2.5 cm, lung surfaces and sections were examined for focal metastasis. Metastatic lung tumors were observed in all of the 6 SRC-1−/− recipients bearing WT/PyMT mammary tumors, indicating that the SRC-1−/− host environment does not suppress WT/PyMT tumor metastasis. In contrast, no lung tumors were observed in all of the 5 WT recipients bearing SRC-1−/−/PyMT mammary tumors (Fig. 3B). These results and the results from intact WT/PyMT and SRC-1−/−/PyMT mice support that SRC-1 has an intrinsic role in mammary tumor cells to promote lung metastasis.
SRC-1 Is Required for Intravasation of Mammary Tumor Cells.
To investigate the specific role of SRC-1 in the metastatic cascade, we quantified the number of mammary tumor cells in the blood circulation of WT/PyMT and SRC-1−/−/PyMT mice by collecting the blood from these mice at the experimental endpoint and counting the cancer cell colonies formed from the blood in culture. Cancer cell colony-forming units (CCCFUs) were found in 83% (10 of 12) of blood samples from WT/PyMT mice. An average of 31 colonies was identified in 0.5 ml of blood from each mouse. In contrast, CCCFUs were only found in 17% (2 of 12) of blood samples from SRC-1−/−/PyMT mice, and only 1.8 colonies were identified in 0.5 ml of blood from each mouse (Fig. 3C). Therefore, the appearance frequency and number of mammary tumor cells in the blood are significantly reduced in SRC-1−/−/PyMT mice compared with WT/PyMT mice (P < 0.001, unpaired t test). These results suggest that a major role of SRC-1 in metastasis is to promote mammary tumor cells to enter the circulation system.
SRC-1 Overexpression in PyMT Mammary Tumors Positively Correlates with HER2 Expression and Akt Activation.
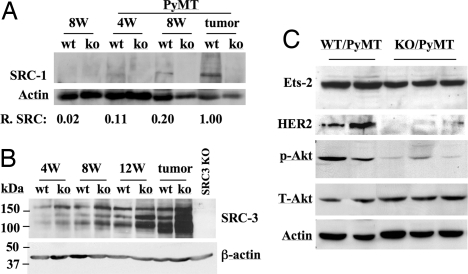
SRC-1 protein levels in WT mouse mammary glands were almost undetectable by Western blot analysis (Fig. 4A). However, clear SRC-1 protein bands were detected from the mammary gland samples of WT/PyMT mice at ages of 4 and 8 weeks. Furthermore, the SRC-1 levels were significantly increased in WT/PyMT tumors. As negative controls, SRC-1 was undetectable in the mammary gland samples of SRC-1−/− and SRC-1−/−/PyMT mice at all stages examined (Fig. 4A). In addition, the 160-kDa full-length SRC-3 protein and its shorter isoforms were also increased during mammary tumor progression in WT/PyMT and SRC-1−/−/PyMT mice (Fig. 4B). Interestingly, SRC-3 protein levels in SRC-1−/−/PyMT mammary glands and tumors were higher than that in WT/PyMT mammary glands and tumors at all stages. These results suggest that SRC-1 is up-regulated during the process of mammary gland tumorigenesis, and SRC-1 deficiency may result in an increase in SRC-3 expression during mammary gland tumorigenesis.
Fig. 4.
SRC-1 deficiency decreases the levels of Ets-2, HER2, and phosphorylated Akt (p-Akt). (A) Western blot analysis of SRC-1 in the mammary glands of WT and WT/PyMT mice at ages of 4 and 8 weeks and in the WT/PyMT mammary tumors. Mammary glands of SRC-1−/− (ko) and SRC-1−/− (ko)/PyMT mice and SRC-1−/− (ko)/PyMT mammary tumors served as negative controls. Relative SRC-1 (R. SRC) levels were determined by the ratio of SRC-1 band intensity to β-actin band intensity for each sample. The relative SRC-1 level in tumor was set to 1 unit. (B) Western blot analysis of SRC-3 in the mammary glands and solid mammary tumors of WT/PyMT (wt) and SRC-1−/−/PyMT (ko) mice at ages of 4, 8, and 12 weeks. A mammary tumor of SRC-3KO/PyMT mice was used as a negative control. The β-actin band for each sample served as a loading control. (C) Western blot analyses of Ets-2, HER2, p-Akt, and total Akt (T-Akt) in WT/PyMT and SRC-1−/− (KO)/PyMT mammary tumor samples. The β-actin served as a loading control.
PyMT activates both ERK (MAPK) and Akt pathways to induce mammary carcinogenesis (32). The total and active forms of ERK1 and ERK2 were similar in the mammary tumors of WT/PyMT and SRC-1−/−/PyMT mice (data not shown), suggesting that SRC-1 is not required for PyMT-induced activation of ERKs. Interestingly, the protein level of Ets-2, a transcription factor activated by MAPK and coactivated by SRC-1, was moderately reduced in SRC-1−/−/PyMT mammary tumors compared with WT/PyMT mammary tumors. Furthermore, the level of HER2, a target of Ets-2, was significantly reduced in SRC-1−/−/PyMT mammary tumors compared with WT/PyMT tumors (Fig. 4C). In addition, SRC-1 deficiency in the mammary tumors also resulted in a decrease in phosphorylated Akt without affecting the total Akt level (Fig. 4C). These results suggest that SRC-1 is required for Ets-2 up-regulation, Ets-2-mediated HER2 expression, and PyMT-stimulated Akt activation in the mammary tumors.
SRC-1 Deficiency Reduces Macrophage Recruitment to the Mammary Tumor Site.
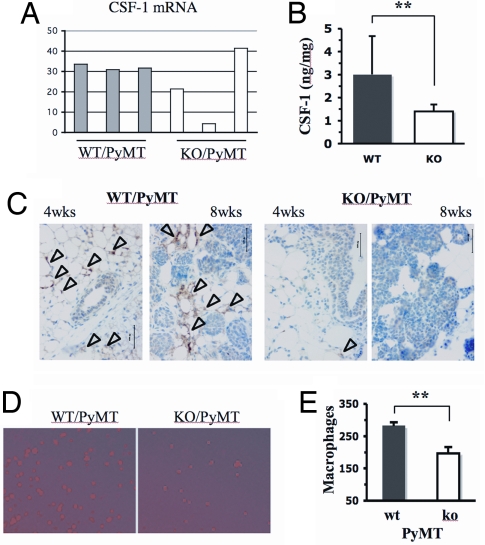
Malignant breast cancer cells usually secrete CSF-1 to attract macrophages to the tumor sites, which in turn produce EGF to stimulate breast cancer cell migration and invasion (37, 38). Real-time RT-PCR analysis revealed that CSF-1 expression was much lower in two of three SRC-1−/−/PyMT tumors compared with the three WT/PyMT tumors (Fig. 5A). To compare the secreted functional CSF-1 protein from these tumors, we isolated WT/PyMT and SRC-1−/−/PyMT mammary tumor cells from 3 mice for each group and measured their secreted CSF-1 in the medium. Our assays revealed that the CSF-1 secreted from WT/PyMT tumor cells was more than 2-fold higher compared with that secreted from SRC-1−/−/PyMT tumor cells (Fig. 5B). IHC for CD11b, a molecular marker of macrophage, detected many macrophages in the areas around the mammary ducts with hyperplasia at 4 weeks of age and in the mammary tumor areas at 8 weeks of age in WT/PyMT mice. In contrast, only a few macrophages could be detected in the mammary glands and tumors in SRC-1−/−/PyMT mice (Fig. 5C). Next, we compared the binding affinity of WT/PyMT and SRC-1−/−/PyMT mammary tumor cells to macrophages in culture. These tumor cells were isolated from primary mammary tumors and cultured to confluence. Fluorescent dye-labeled live macrophages were added to the monolayer of tumor cells. After incubation and washing away the unbound macrophages, the average number of macrophages associated with WT/PyMT mammary tumor cells was significantly higher than the number of macrophages associated with SRC-1−/−/PyMT cells (Fig. 5 D and E). These results demonstrate that SRC-1 is able to enhance CSF-1 expression in the mammary tumors to recruit macrophages to the tumor site, which may promote tumor cell migration, invasion and metastasis.
Fig. 5.
SRC-1 deficiency reduces CSF-1 expression and macrophage recruitment. (A) The relative levels of CSF-1 mRNA in WT/PyMT (n = 3) and SRC-1−/− (KO)/PyMT (n = 3) mammary tumors were measured by real-time RT-PCR and normalized to the 18S RNA. (B) The secreted CSF-1 protein in the culture medium of WT/PyMT (WT) and SRC-1−/−/PyMT (KO) tumor cells. Cells of 3 independent cell lines for each genotype group were maintained in serum-free medium for 48 h. CSF-1 (ng) in the medium was measured by ELISA and normalized to total amount of cell protein (mg). The measurements were performed in duplicate, and the experiments were repeated twice. **, P < 0.01 by unpaired t test. (C) Detection of macrophages by IHC in mammary glands and tumors of WT/PyMT and SRC-1−/− (KO)/PyMT mice at 4 and 8 weeks. Macrophages (brown color) were detected by using antibody against CD11b. (D) Mammary tumor cell-macrophage adhesion assay. WT/PyMT tumor cells retained more macrophages (red color) than SRC-1−/−/PyMT tumor cells did. (E) The average number of macrophages attached to WT/PyMT tumor cells is significantly larger than that attached to SRC-1−/− (ko)/PyMT tumor cells (P < 0.01, n = 5, t test).
Discussion
Although recent studies have suggested that increased SRC-1 expression in breast cancer may be detrimental (7, 29, 39), the precise in vivo role of SRC-1 in breast cancer initiation and progression remains to be defined. In this study, we have shown that SRC-1 is not required for PyMT-induced mammary tumor initiation and growth. Interestingly, SRC-1 deficiency antagonizes PyMT-induced precocious development of mammary gland, maintains a more differentiated morphology of mammary tumors, and significantly reduces mammary tumor cell intravasation and metastasis to the lung. Moreover, the role of SRC-1 in promotion of metastasis is intrinsic in the tumor cells as demonstrated by reciprocal transplantation of WT/PyMT and SRC-1−/−/PyMT tumors to SRC-1−/− and WT recipient mice. These results clearly indicate that SRC-1 plays a potent role in promoting mammary tumor metastasis to the lung, possibly through enhancing tumor cell intravasation.
The specific role of SRC-1 in promoting metastasis without affecting primary tumor initiation and growth is unique and different from the role of SRC-3 in mammary tumorigenesis. SRC-3 deficiency suppresses both the primary mammary tumor formation in MMTV-ras, MMTV-HER2 and carcinogen-treated mice and the lung metastasis in MMTV-ras and MMTV-PyMT mice (8, 26, 27, 40). Similar to SRC-3, several other proteins that enhance metastasis also promote primary tumor growth. These proteins include ras, HER2, c-myc, EGFR, hepatocyte growth factor, metalloproteinases, β1,6-N-acetylglucosaminyltransferase V, metastasis-associated gene-1, and osteopontin (41–43). Functional inhibition of these genes usually affects both primary tumor growth and metastasis.
Increased SRC-1 level during mammary tumor progression in MMTV-PyMT mice recapitulates SRC-1 overexpression in HER2-positive human breast cancers (7, 29). Since MMTV-PyMT-induced mammary tumors at advanced stages are usually ERα-negative and PR-negative, we focused our attention to addressing how overexpressed SRC-1 interacted with PyMT-activated oncogenic pathways, including the Shc/ras/MAPK and the c-Src/PI3K/Akt pathways (32). We found that, although SRC-1 deficiency did not directly affect PyMT-induced MAPK activation, it reduced the levels of Ets-2 and HER2. Because Ets-2 is a MAPK target and SRC-1 is a coactivator of Ets-2 that mediates HER2 expression (4, 5), SRC-1 may promote MAPK signaling through up-regulating the downstream components of the MAPK signaling pathway. We also found that SRC-1 deficiency suppressed Akt activation without changing total Akt level. Possibly, SRC-1 may be involved in Akt activation through increasing HER2 expression during mammary tumor progression. Because both the Akt and MAPK signaling pathways play critical roles in breast cancer cell migration, invasion, and metastasis, the down-regulation of these pathways by SRC-1 inactivation may be responsible, at least in part, for the suppression of lung metastasis observed in SRC-1−/−/PyMT mice.
The number of macrophages recruited to breast tumors has been found to be associated with invasiveness and metastasis in breast cancer (44). A paracrine loop has been proposed to explain the crucial role of macrophages in breast cancer metastasis (37). In this loop, the cancer cells express and secrete CSF-1 to recruit macrophages; in turn, macrophages express and secrete EGF to stimulate tumor cell migration and invasion (37). It also has been shown that CSF-1 deficiency suppresses PyMT-induced mammary tumor metastasis in mice (38). Our data demonstrate that SRC-1 deficiency inhibits CSF-1 expression in certain mammary tumors. We showed that fewer numbers of macrophages were recruited to the mammary tumor sites in SRC-1−/−/PyMT mice compared with WT/PyMT mice. In agreement with these in vivo data, SRC-1−/−/PyMT tumor cells in culture also secreted much less CSF-1 protein into the medium and showed lower affinity to interact with macrophages compared with WT/PyMT tumor cells. These findings suggest that SRC-1 may promote breast cancer metastasis through a direct or indirect pathway to enhance CSF-1 expression. Intriguingly, CSF-1 expression is not affected in some of the SRC-1−/−/PyMT mammary tumors. This is consistent with the fact that some of the SRC-1−/−/PyMT mice still develop lung metastasis, and individual differences may occur because of the relative expression levels of SRC-3 among tumors. Indeed, our data indicate that certain SRC-1−/−/PyMT mammary glands and tumors expressed higher SRC-3 compared with WT/PyMT mammary glands and tumors.
In conclusion, we have identified an important role for SRC-1 in promotion of mammary tumor metastasis. Our study suggests that SRC-1 specifically promotes metastasis without affecting primary tumor growth. SRC-1 may promote metastasis by facilitating Ets-2-mediated HER2 expression and activating CSF-1 expression to recruit macrophages to the mammary tumors. These findings may partially explain the poor prognosis of SRC-1-positive breast cancers. Further understanding of the molecular mechanisms and the gene networks regulated by SRC-1 in breast cancer metastasis may provide new additional strategies to inhibit breast cancer metastasis.
Methods
Mice and Mammary Tumorigenesis.
The previously described SRC-1−/− mice (13) were backcrossed with FVB mice for 6 generations. MMTV-PyMT mice with FVB background were obtained from the Jackson Laboratory (33). The backcrossed female SRC-1−/− mice were used to breed with male MMTV-PyMT mice to generate female WT/PyMT, SRC-1+/−/PyMT and SRC-1−/−/PyMT mice. Mammary tumorigenesis was examined by palpation, and tumor volume was measured as described in ref. 27. Whole-mounted mammary glands were also prepared and stained as described (27, 35).
Examination of Lung Metastasis.
Mice were killed at defined experimental endpoints as described in Results. Lung metastasis was analyzed by examining the visual tumors on the lung surface and by measuring the ratio of tumor area to total area on the lung sections as described (8, 27).
Tumor Transplantation.
The WT/PyMT and SRC-1−/−/PyMT mammary tumor tissues (≈1 mm3 in size) were sliced from primary tumors of different donor mice and reciprocally implanted into the mammary fat pads of 8- to 12-week-old female SRC-1−/− and WT recipient mice as described (8, 35). Tumor growth was monitored once a week. For analysis of lung metastasis, the recipient mice were killed when tumor size reached 2.5 cm in diameter.
Colony Formation Assay of Mammary Tumor Cells in Blood.
The procedure was modified from one described in refs. 8 and 45. At the experimental endpoint, blood samples (0.5 ml/mouse) were collected from the right atrium via heart puncture. Heparin was used as an anticoagulant. Each sample was mixed with 9 ml of DMEM with 10% FCS and cultured for 3 weeks. The formed tumor cell colonies were stained with crystal violet and then counted.
IHC.
IHC was performed on tissue sections of WT/PyMT and SRC-1−/−/PyMT mammary glands and tumors as described (8, 26, 27). The monoclonal antibodies against PyMT and CD11b were purchased from Oncogene Research and Abcam.
RT-PCR.
Total RNA samples were extracted from the lungs and mammary tumors of WT/PyMT and SRC-1−/−/PyMT mice as described in ref. 27. RT-PCR for analysis of β-casein and β-actin mRNAs was also described in ref. 27. Real-time RT-PCR with SYBR Green for analyses of CSF-1 mRNA and 18S RNA was performed as described (8, 46). The primers for detection of mouse CSF-1 mRNA were 5′-gaacactgtagccacatgattgg and 5′-tggcatgaagtctccatttgac. The relative level of CSF mRNA was normalized to the18S RNA level.
CSF-1 Measurement.
WT/PyMT and SRC-1−/−/PyMT mammary tumor cells were isolated from mammary tumors of WT/PyMT and SRC-1−/−/PyMT mice (n = 3) as described (8, 27). Cells were cultured to reach 90% confluence in 6-well plates in DMEM with 10% serum, 10 μg/ml insulin, and 10−4 M β-mecaptoethanol. Cells were washed and incubated in serum-free DMEM for 2 days. The CSF-1 concentration in the conditioned medium was measured by using the Quantikine Mouse M-CSF ELISA Kit (R&D Systems). The total amount of CSF-1 in the medium was normalized to the total protein extracted from the cells in the culture for each well.
Western Blot Analysis.
Western blot analyses of tissue lysates prepared from mammary glands and tumor tissues of WT, SRC-1−/−, WT/PyMT, and SRC-1−/−/PyMT mice were performed as described (11, 26, 27). The antibodies against SRC-1, Ets-2, and HER2 were from Santa Cruz Biotechnology. The antibodies against Akt, p-Akt, EGFR, and p-EGFR were from Cell Signaling. Western blot analysis for SRC-3 was described in ref. 8.
Macrophage Adhesion Assay.
WT/PyMT and SRC-1−/−/PyMT cells were isolated from mammary tumors of WT/PyMT and SRC-1−/−/PyMT mice as described (8, 27). The RAW264.7 murine macrophage cell line was obtained from American Type Culture Collection. For macrophage adhesion assay, WT/PyMT and SRC-1−/−/PyMT tumor cells were plated in 96-well plate at a density of 40,000 cells per well and cultured till confluence. Live RAW264.7 cells were labeled as described with CellTracker Orange CMTMR ((5-(and-6)-l((4-chloromethyl)benzoyl)amino)tetramethylrhodamine) from Invitrogen-Molecular Probes (47). Labeled live cells (20,000/well) were added to a 96-well plate with confluent tumor cells. After culturing at 37 °C for 1 h, cells were washed with PBS. The labeled macrophages attached to the tumor cells were examined under a fluorescent microscope. The macrophage numbers in 5 randomly chosen fields of view with 50× amplification were counted.
Acknowledgments.
We thank Suoling Zhou for technical assistance and Na Wu for critical reading of the manuscript. This work is supported in part by National Institutes of Health Grants CA112403, CA119689, DK58242, and DK59820 and American Cancer Society Award RSG-05-082-01-TBE.
Footnotes
The authors declare no conflict of interest.
References
- 1.O'Malley BW. Coregulators: From whence came these “master genes.”. Mol Endocrinol. 2007;21:1009–1013. doi: 10.1210/me.2007-0012. [DOI] [PubMed] [Google Scholar]
- 2.Lonard DM, Lanz RB, O'Malley BW. Nuclear receptor coregulators and human disease. Endocr Rev. 2007;28:575–587. doi: 10.1210/er.2007-0012. [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Li Q. Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol. 2003;17:1681–1692. doi: 10.1210/me.2003-0116. [DOI] [PubMed] [Google Scholar]
- 4.Al-azawi D, et al. Ets-2 and p160 proteins collaborate to regulate c-Myc in endocrine resistant breast cancer. Oncogene. 2008;27:3021–3031. doi: 10.1038/sj.onc.1210964. [DOI] [PubMed] [Google Scholar]
- 5.Myers E, et al. Associations and interactions between Ets-1 and Ets-2 and coregulatory proteins, SRC-1, AIB1, and NCoR in breast cancer. Clin Cancer Res. 2005;11:2111–2122. doi: 10.1158/1078-0432.CCR-04-1192. [DOI] [PubMed] [Google Scholar]
- 6.Goel A, Janknecht R. Concerted activation of ETS protein ER81 by p160 coactivators, the acetyltransferase p300 and the receptor tyrosine kinase HER2/Neu. J Biol Chem. 2004;279:14909–14916. doi: 10.1074/jbc.M400036200. [DOI] [PubMed] [Google Scholar]
- 7.Fleming FJ, et al. Expression of SRC-1, AIB1, and PEA3 in HER2 mediated endocrine resistant breast cancer; a predictive role for SRC-1. J Clin Pathol. 2004;57:1069–1074. doi: 10.1136/jcp.2004.016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin L, et al. The AIB1 oncogene promotes breast cancer metastasis by activation of PEA3-mediated matrix metalloproteinase 2 (MMP2) and MMP9 expression. Mol Cell Biol. 2008;28:5937–5950. doi: 10.1128/MCB.00579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louie MC, Revenko AS, Zou JX, Yao J, Chen HW. Direct control of cell cycle gene expression by proto-oncogene product ACTR, and its autoregulation underlies its transforming activity. Mol Cell Biol. 2006;26:3810–3823. doi: 10.1128/MCB.26.10.3810-3823.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mussi P, Yu C, O'Malley BW, Xu J. Stimulation of steroid receptor coactivator-3 (SRC-3) gene overexpression by a positive regulatory loop of E2F1 and SRC-3. Mol Endocrinol. 2006;20:3105–3119. doi: 10.1210/me.2005-0522. [DOI] [PubMed] [Google Scholar]
- 11.Yuan Y, et al. Genetic screening reveals an essential role of p27kip1 in restriction of breast cancer progression. Cancer Res. 2007;67:8032–8042. doi: 10.1158/0008-5472.CAN-07-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, et al. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci USA. 2000;97:6379–6384. doi: 10.1073/pnas.120166297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, et al. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 14.Gehin M, et al. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol Cell Biol. 2002;22:5923–5937. doi: 10.1128/MCB.22.16.5923-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mark M, et al. Partially redundant functions of SRC-1 and TIF2 in postnatal survival and male reproduction. Proc Natl Acad Sci USA. 2004;101:4453–4458. doi: 10.1073/pnas.0400234101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan Y, Liao L, Tulis DA, Xu J. Steroid receptor coactivator-3 is required for inhibition of neointima formation by estrogen. Circulation. 2002;105:2653–2659. doi: 10.1161/01.cir.0000018947.95555.65. [DOI] [PubMed] [Google Scholar]
- 17.Yuan Y, Xu J. Loss-of-function deletion of the steroid receptor coactivator-1 gene in mice reduces estrogen effect on the vascular injury response. Arterioscler Thromb Vasc Biol. 2007;27:1521–1527. doi: 10.1161/ATVBAHA.107.144477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishihara E, et al. SRC-1 null mice exhibit moderate motor dysfunction and delayed development of cerebellar Purkinje cells. J Neurosci. 2003;23:213–222. doi: 10.1523/JNEUROSCI.23-01-00213.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao L, Chen X, Wang S, Parlow AF, Xu J. Steroid receptor coactivator 3 maintains circulating insulin-like growth factor I (IGF-I) by controlling IGF-binding protein 3 expression. Mol Cell Biol. 2008;28:2460–2469. doi: 10.1128/MCB.01163-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu C, et al. An essential function of the SRC-3 coactivator in suppression of cytokine mRNA translation and inflammatory response. Mol Cell. 2007;25:765–778. doi: 10.1016/j.molcel.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anzick SL, et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 22.Louie MC, Zou JX, Rabinovich A, Chen HW. ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Mol Cell Biol. 2004;24:5157–5171. doi: 10.1128/MCB.24.12.5157-5171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh A, et al. The nuclear receptor coactivator AIB1 mediates insulin-like growth factor I-induced phenotypic changes in human breast cancer cells. Cancer Res. 2004;64:8299–8308. doi: 10.1158/0008-5472.CAN-04-0354. [DOI] [PubMed] [Google Scholar]
- 24.Planas-Silva MD, Shang Y, Donaher JL, Brown M, Weinberg RA. AIB1 enhances estrogen-dependent induction of cyclin D1 expression. Cancer Res. 2001;61:3858–3862. [PubMed] [Google Scholar]
- 25.Torres-Arzayus MI, et al. Targeting the AIB1 oncogene through mammalian target of rapamycin inhibition in the mammary gland. Cancer Res. 2006;66:11381–11388. doi: 10.1158/0008-5472.CAN-06-2316. [DOI] [PubMed] [Google Scholar]
- 26.Kuang SQ, et al. Mice lacking the amplified in breast cancer 1/steroid receptor coactivator-3 are resistant to chemical carcinogen-induced mammary tumorigenesis. Cancer Res. 2005;65:7993–8002. doi: 10.1158/0008-5472.CAN-05-1179. [DOI] [PubMed] [Google Scholar]
- 27.Kuang SQ, et al. AIB1/SRC-3 deficiency affects insulin-like growth factor I signaling pathway and suppresses v-Ha-ras-induced breast cancer initiation and progression in mice. Cancer Res. 2004;64:1875–1885. doi: 10.1158/0008-5472.can-03-3745. [DOI] [PubMed] [Google Scholar]
- 28.Torres-Arzayus MI, et al. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell. 2004;6:263–274. doi: 10.1016/j.ccr.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 29.Fleming FJ, Hill AD, McDermott EW, O'Higgins NJ, Young LS. Differential recruitment of coregulator proteins steroid receptor coactivator-1 and silencing mediator for retinoid and thyroid receptors to the estrogen receptor-estrogen response element by beta-estradiol and 4-hydroxytamoxifen in human breast cancer. J Clin Endocrinol Metab. 2004;89:375–383. doi: 10.1210/jc.2003-031048. [DOI] [PubMed] [Google Scholar]
- 30.Myers E, et al. Inverse relationship between ER-beta and SRC-1 predicts outcome in endocrine-resistant breast cancer. Br J Cancer. 2004;91:1687–1693. doi: 10.1038/sj.bjc.6602156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kishimoto H, et al. The p160 family coactivators regulate breast cancer cell proliferation and invasion through autocrine/paracrine activity of SDF-1alpha/CXCL12. Carcinogenesis. 2005;26:1706–1715. doi: 10.1093/carcin/bgi137. [DOI] [PubMed] [Google Scholar]
- 32.Dennis JW, Granovsky M, Warren CE. Glycoprotein glycosylation and cancer progression. Biochim Biophys Acta. 1999;1473:21–34. doi: 10.1016/s0304-4165(99)00167-1. [DOI] [PubMed] [Google Scholar]
- 33.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: Atransgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin EY, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Kuang SQ, Liao L, Zhou S, Xu J. Haploid inactivation of the amplified-in-breast cancer 3 coactivator reduces the inhibitory effect of peroxisome proliferator-activated receptor gamma and retinoid X receptor on cell proliferation and accelerates polyoma middle-T antigen-induced mammary tumorigenesis in mice. Cancer Res. 2004;64:7169–7177. doi: 10.1158/0008-5472.CAN-04-1176. [DOI] [PubMed] [Google Scholar]
- 36.Maglione JE, et al. Transgenic Polyoma middle-T mice model premalignant mammary disease. Cancer Res. 2001;61:8298–8305. [PubMed] [Google Scholar]
- 37.Wyckoff J, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 38.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agoulnik IU, et al. Role of SRC-1 in the promotion of prostate cancer cell growth and tumor progression. Cancer Res. 2005;65:7959–7967. doi: 10.1158/0008-5472.CAN-04-3541. [DOI] [PubMed] [Google Scholar]
- 40.Fereshteh MP, et al. The nuclear receptor coactivator amplified in breast cancer-1 is required for Neu (ErbB2/HER2) activation, signaling, and mammary tumorigenesis in mice. Cancer Res. 2008;68:3697–3706. doi: 10.1158/0008-5472.CAN-07-6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwirzke M, Schiemann S, Gnirke AU, Weidle UH. New genes potentially involved in breast cancer metastasis. Anticancer Res. 1999;19:1801–1814. [PubMed] [Google Scholar]
- 42.Nicolson GL. Breast cancer metastasis-associated genes: Role in tumour progression to the metastatic state. Biochem Soc Symp. 1998;63:231–243. [PubMed] [Google Scholar]
- 43.Granovsky M, et al. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat Med. 2000;6:306–312. doi: 10.1038/73163. [DOI] [PubMed] [Google Scholar]
- 44.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 45.Muraoka RS, et al. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest. 2002;109:1551–1559. doi: 10.1172/JCI15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Q, Chu MJ, Xu J. Tissue- and nuclear receptor-specific function of the C-terminal LXXLL motif of coactivator NCoA6/AIB3 in mice. Mol Cell Biol. 2007;27:8073–8086. doi: 10.1128/MCB.00451-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cumberledge S, Krasnow MA. Intercellular signalling in Drosophila segment formation reconstructed in vitro. Nature. 1993;363:549–552. doi: 10.1038/363549a0. [DOI] [PubMed] [Google Scholar]