Abstract
One crucial element for the evolution of cooperation may be the sensitivity to others' efforts and payoffs compared with one's own costs and gains. Inequity aversion is thought to be the driving force behind unselfish motivated punishment in humans constituting a powerful device for the enforcement of cooperation. Recent research indicates that non-human primates refuse to participate in cooperative problem-solving tasks if they witness a conspecific obtaining a more attractive reward for the same effort. However, little is known about non-primate species, although inequity aversion may also be expected in other cooperative species. Here, we investigated whether domestic dogs show sensitivity toward the inequity of rewards received for giving the paw to an experimenter on command in pairs of dogs. We found differences in dogs tested without food reward in the presence of a rewarded partner compared with both a baseline condition (both partners rewarded) and an asocial control situation (no reward, no partner), indicating that the presence of a rewarded partner matters. Furthermore, we showed that it was not the presence of the second dog but the fact that the partner received the food that was responsible for the change in the subjects' behavior. In contrast to primate studies, dogs did not react to differences in the quality of food or effort. Our results suggest that species other than primates show at least a primitive version of inequity aversion, which may be a precursor of a more sophisticated sensitivity to efforts and payoffs of joint interactions.
Keywords: cooperation, refusal of unequal pay, Canis familiaris
Recent studies investigating human cooperation suggest that aversion to inequity may account for much of the variation observed in the data (1). Inequity aversion is defined as partners resisting inequitable outcomes. In humans, it seems to be based on the simultaneous evaluation of their costs and gains compared with those of their partner. It has been suggested that comparing one's own payoff and effort during cooperation with those of others and reacting negatively to an unequal reward distribution in regard to the effort invested were crucial for the evolution of cooperation (2). If an individual responds to a disadvantageous reward distribution, it would likely increase its relative fitness compared with those who do not (3). A simple version of inequity aversion concerns dyadic relationships rather than third-party intervention, thus it does not imply an interest in inequity that exists among other people but is based solely on the subject's own efforts and material payoff relative to the investment and payoff of others.
Until recently, it has been thought that sensitivity toward unequal reward or effort distribution is a uniquely human asset. However, several experiments carried out with capuchin monkeys (Cebus apella) (4) and chimpanzees (Pan troglodytes) (5) suggest otherwise (but see refs. 6–8). In these experiments, scientists have attempted to model social situations in which they can test an animal's sensitivity to inequity without requiring cooperation. In the initial study, Brosnan and colleagues (4) used an experimental setup, whereby an animal had to exchange a token with the experimenter to obtain a food reward. They found that, if tested with a partner in visual contact, the monkeys responded negatively to unequal reward distributions, e.g., they refused participation if they witnessed a conspecific obtain a more attractive food reward for equal effort, an effect amplified if the partner received such a reward without any effort at all. The effort effect, however, could not be replicated as clear in a later study (9). Thus, although controversial, it seems that when tested in an exchange task, capuchin monkeys and chimpanzees seem to be sensitive at least to the unequal reward distribution (4, 5). These results receive further support by a recent experimental study on cottontop tamarins (Saguinus oedipus) that found behavioral differences caused by unequal reward distributions in a cooperative problem solving task (10).
However, primates are not the only animals known to engage in cooperative actions (for review see ref. 11). Canids, for example, are known to engage in cooperative hunting [e.g., wolves, Canis lupus (12); African wild dogs, Lycaon pictus (13)] and cooperative rearing of pups [e.g., wolves (14), African wild dogs (15, 16); mongoose, Suricata suricatta (17)]. Whereas dog–dog cooperation seems to be impaired by the domestication process (18, 19), dogs clearly show effective, complex, and elaborate cooperation with humans (e.g., gun dogs, assistant dogs) (20). The continuous change in initialization of actions found in guide dogs and blind persons has not been reported among wolves. Coworking between dogs and humans often includes more than 1 dog interacting with humans, e.g., in hunting. Because dogs show high sensitivity to elements of human behavior that are directed both toward them and to others (21, 22) and some understanding of human intentions (3, 23, 24), we predict that dogs may respond differently when owners distribute rewards unequally among their dogs, and this includes asking for different efforts from the dogs for the same reward. We expect that dogs will at least show some primitive version of inequity aversion such as reacting to the presence or absence of rewards (see also ref. 25). Paying close attention to other dogs and adjusting their behavior accordingly has already been demonstrated in several other studies (26, 27).
Results
The aim of this work was to investigate whether domestic dogs are influenced by the inequity of rewards that were received for the same action in pairs of familiar dogs when working with the same experimenter. In a manner similar to the primate studies (4, 9), the experimenter requested the dogs to perform a certain action (instead of having to exchange a token for food, the dogs were asked to give their paw) to gain a food reward. Giving the paw is a command often trained rather for fun than for obedience (e.g., like sit, come), which means that it is usually carried out in connection with a reward and in a relaxed situation. Thus, like exchanging a token in the primate studies, giving the paw is probably not very effortful for the dog in terms of energy invested. However, in pilot studies we found that most dogs would stop giving the paw after 15–20 times if not rewarded.
For giving the paw to the experimenter on command (e.g., the hand of the experimenter was held out to the respective dog, and the command “paw” was spoken; the experimenter avoided any other communication with the dogs), (i) the subject and the partner received the same low-value reward [baseline condition: Equity test (ET)], (ii) the subject received a lower-value reward than its partner [Quality Inequity test (QI)], (iii) the subject received no reward whereas the partner received the low-value reward [Reward Inequity test (RI)], or (iv) both dogs received the low-value reward, but the partner did not have to give the paw to receive this reward [Effort Control (EC)]. In addition, each dog was tested without a partner in an asocial test session consisting of an assessment condition followed by a no-reward (NR) control condition. In the assessment condition, the subject received a low-value reward for giving the paw. This condition tested whether the dogs would, in general, give the paw to an unfamiliar person 30 times. With the NR control condition, we tested how long the dogs would continue giving the paw without a reward (for a summary of the conditions, see Table 1).
Table 1.
Test conditions of experiments 1 and 2
| Social vs asocial conditions | Test conditions | Subject |
Partner |
||
|---|---|---|---|---|---|
| Task | Reward | Task | Reward | ||
| Experiment 1 | |||||
| Social conditions | Equity (ET) | + | Low | + | Low |
| Quality Inequity (QI) | + | Low | + | High | |
| Reward Inequity (RI) | + | − | + | Low | |
| Effort Control (EC) | + | Low | − | Low | |
| Asocial conditions* | Assessment | + | Low | No partner | |
| No Reward (NR) control | + | − | No partner | ||
| Experiment 2 | |||||
| Social conditions | Reward Inequity (RI) | + | − | + | Low |
| Social Control (SC) | + | − | + | − | |
*The two asocial conditions were run within a single session.
Both the high-value reward (sausage) and the low-value reward (dark bread) were present and clearly visible for each dog in all conditions including the assessment and control sessions. Each test session consisted of a series of 30 trials, in each of which the partner performed immediately before the subject (or until the subject refused to work). The asocial control was conducted according to Bräuer and colleagues (6) to control also for the movement of the food (see Experimental Procedures for details and Fig. 1 for a sketch of the general setup). The order of the 4 social conditions and the 2 asocial conditions deviated from a fully counterbalanced design in that we randomized the sequence of the conditions across subjects, but we never started with the RI test or the NR condition. This latter restriction was administered to avoid frustration by the subject, which is likely if an animal were put into a completely novel situation, commanded by an unfamiliar person and then not rewarded for the commanded action. Thus, we first established the testing situation with conditions where both animals were rewarded before testing any of the NR condition. The asocial control was conducted for half of the dogs before and for half of the dogs after the social conditions.
Fig. 1.
Photos of the experimental setup. The experimenter avoided eye contact with the dogs. The owner was standing behind the dogs.
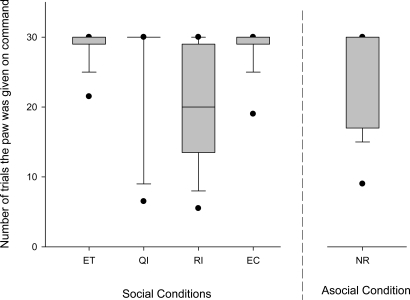
Overall, the 29 dogs (see Table S1 for breed and sex of dogs and sequence of test sessions in experiment 1) differed in the number of trials in which they continued to give the paw to the experimenter in the 4 test sessions (Friedman; Fr = 35.115; n = 29, P < 0.0001; corrected P < 0.01; Fig. 2). A priori planned comparisons revealed that subjects refused to give the paw to the experimenter earlier in the RI condition (RI) compared with the baseline condition (ET) (Dunn's multiple-comparisons test: P < 0.001; corrected P < 0.01). None of the other 2 test conditions differed significantly from the baseline condition (Dunn's multiple-comparisons test: ET–QI, P > 0.05; ET–EC, P > 0.05). Do dogs always refuse to give the paw when no reward is provided independently of the presence or absence of a rewarded partner? We found that partner presence makes a difference: subjects would stop significantly earlier to obey the command if a partner was present compared with the asocial control (RI–NR: Wilcoxon matched-pairs test: n = 25 (4 ties), T+ = 84, P = 0.034, corrected P < 0.05). The partner receiving a reward thus seemed a crucial factor responsible for refusing to give the paw in the reward inequity test (Fig. 2).
Fig. 2.
Box plots show the number of trials in which the subject gave the paw to the experimenter in the 4 different test conditions and the nonsocial control condition without reward. Shaded boxes represent the interquartile range, bars within shaded boxes are median values, and whiskers indicate the 5th and 95th percentiles. ET, equity test, where both animals receive a low-value reward; QI, quality inequity test, where the partner initially performed for a high-value reward followed by the subject asked to perform for a low-value reward; RI, reward inequity test, where the partner initially performed for a low-value reward followed by the subject performing but receiving no reward; EC, effort control, where the partner was initially handed a low-value reward without having to perform for it, after which the subject had to perform to receive the low-value reward; NR, asocial control, where the animal was tested alone and received no reward.
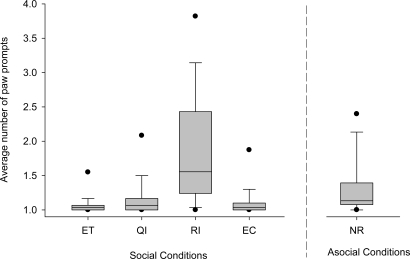
In addition to the number of trials during which the subject continued to obey the command, we analyzed the hesitation or willingness of the subject to do so. In our experiment, the experimenter would repeat the command up to 10 times. If the subject did not give the paw on command during this time then the session was terminated (for details, see SI Experimental Procedures). When we calculated the average number of times per trial that the experimenter had to prompt the subject to give the paw before it obeyed we found an overall significant difference among the 4 experimental conditions (Friedman; Fr = 38.011; n = 29, P < 0.0001, corrected P < 0.01; Fig. 3). Planned post hoc comparisons revealed the same difference as before with only RI differing significantly from the baseline condition (Dunn's multiple-comparisons test: ET–RI, P < 0.001, corrected P < 0.01; ET–QI, P > 0.05; ET–EC, P > 0.05). Moreover, like in our first measurement, the RI condition also differed significantly in the average number of times per trial the command had to be repeated compared with the asocial control condition (RI–NR, Wilcoxon matched-pairs test: n = 28 (1 tie); T+ = 362; P = 0.0001; corrected P < 0.01), suggesting that the subjects were less willing to give the paw to the experimenter when their partner was rewarded for the same action compared with not being rewarded when alone. The same results were found if all commands given (to sit up and to give the paw) were combined for the analyses.
Fig. 3.
Box plots show the average number of times per trial the experimenter had to ask the subject to give the paw in the 4 different test conditions and the nonsocial control condition without reward (NR). Shaded boxes represent the interquartile range, bars within shaded boxes are median values, and whiskers indicate the 5th and 95th percentiles. Abbreviations are as in the Fig. 2 legend.
If animals react to the inequity of the reward distribution, one would further expect that they check more often what the other animal gets. Accordingly, we analyzed whether subjects looked more often at their partner in one or the other condition. However, we found no significant difference among the 4 conditions (Friedman; Fr = 2.668; n = 28, P < 0.445). Finally, we analyzed whether subjects showed more signs of distress (defined as the average number of scratching, yawning, licking the mouth, avoiding the gaze of the partner per trial) in the inequity conditions compared with the control condition. We found an overall significant difference among the 4 experimental conditions (Friedman; Fr = 15.561; n = 29, P < 0.0014, corrected P < 0.01). Post hoc comparisons revealed a significant difference between the baseline and the RI condition (Dunn's multiple-comparisons test: ET–RI, P < 0.01, corrected P < 0.05), but no difference between the other 2 conditions and the baseline (Dunn's multiple-comparisons test: ET–QI, P > 0.05; ET–EC, P > 0.05). The subjects also were more stressed in the RI condition compared with the asocial control condition (Wilcoxon matched-pairs test: RI–NR, n = 29; T+ = 324.0, P < 0.021, corrected P < 0.05), suggesting that not getting a reward if the partner is rewarded is more stressful than not getting a reward in the absence of a rewarded dog.
So far, these results suggest that the dogs are sensitive to an unequal reward distribution rather than, or at least much more than, to a difference in quality or effort. However, it is possible that the animals reacted merely to the presence of the partner in the RI condition and not to the partner receiving food. Therefore, in a second experiment, we tested additional dogs in 2 further conditions, controlling for the presence of the partner. For giving the paw to the experimenter on command, (i) the subject and the partner both received no reward [baseline: Social Control (SC)] or (ii) the subject received no reward whereas the partner received the low value reward (RI test).
The general design of the experiment remained the same as the first experiment with (i) both the high-value reward (sausage) and the low-value reward (dark bread) present and clearly visible to each dog in both conditions; (ii) each test session consisting of a series of 30 trials (or until the subject refused to work), in each of which the partner performed immediately before the subject; and (iii) the incorporation of the movement of the food (for details, see Experimental Procedures). The social control was conducted for half of the dogs before and for half of the dogs after the RI test. For an overview of the sex and breed of the dogs and the sequence of sessions, see Table S2.
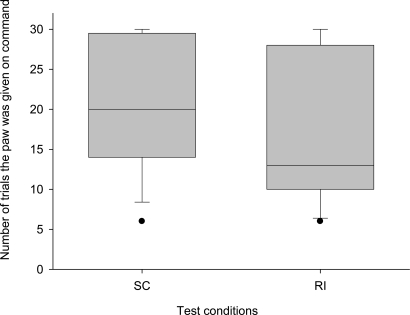
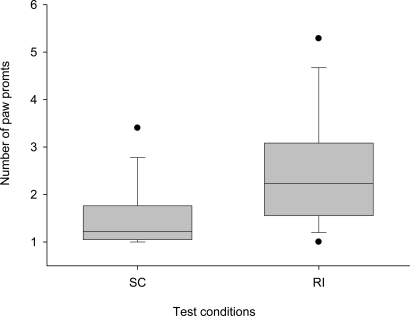
Overall, we found a trend toward a difference with the 14 tested dogs refusing to give the paw earlier in the RI condition compared with the SC condition (Wilcoxon matched-pairs test: n = 10 (4 ties), T+ = 10.0, P = 0.08, Fig. 4). Moreover, we found that the dogs hesitated significantly longer when obeying the command to give the paw in the RI condition compared with the SC condition (Wilcoxon matched-pairs test: n = 14, T+ = 99.0, P = 0.0017, Fig. 5). They also looked at the partner significantly more often and were more stressed in the RI condition compared with the SC condition (Wilcoxon matched-pairs test: looking at the partner, n = 14, T+ = 96.0, P = 0.004; stress signals, n = 14, T+ = 96.5, P = 0.0031), even though food was present and moved in both conditions. These results strongly suggest that the dogs react to the fact that the partner received the food and not just to the presence or absence of a partner dog.
Fig. 4.
Box plots show the number of trials the subject gave the paw to the experimenter in the 2 test conditions of experiment 2 (RI and SC). Shaded boxes represent the interquartile range, bars within shaded boxes are median values, and whiskers indicate the 5th and 95th percentiles.
Fig. 5.
Box plots show the average number of times per trial the experimenter had to ask the subject to give the paw in the 2 experimental conditions (RI and SC). Shaded boxes represent the interquartile range, bars within shaded boxes are median values, and whiskers indicate the 5th and 95th percentiles.
Discussion
Together, the results of experiments 1 and 2 provide evidence for the presence of sensitivity toward an unequal reward distribution in a non-primate species. The dogs refused earlier and hesitated longer to obey human commands and showed more stressed behavior in the social condition compared with the asocial control condition in the first experiment. In the second experiment, the dogs also showed a tendency to a higher refusal rate, a significantly longer hesitation, higher stress levels, and increased looking at the partner when the partner was rewarded and they themselves were not.
In regard to the original non-human primate studies on inequity aversion, several alternative explanations have been suggested to explain the behavior of the animals. First, it has been argued that the monkeys reacted toward the presence of the high-value food reward when receiving a low-value reward rather than the fact that the partner received the high-value reward (3, 8), even though this explanation was later ruled out by an experiment that controlled for the mere presence of high-value food (9). In our experiment, both the low- and the high-value reward were always present and clearly visible to both animals. Thus, it is unlikely that visibility of the high-value reward accounts for our results.
Another alternative hypothesis suggested that the monkeys were frustrated by receiving a low-value reward after having received a high-value reward in a previous session (7, 8). It had been shown that animals react differently, e.g., show frustration effects when they expect a certain outcome, so that the absence of the expected reward results in delays (28, 29) or reduced preference (28–31). This frustration effect (also called “contrast” effect) rather than aversion to inequity might explain the refusal of the less-preferred reward in studies where monkeys received a high-value reward before being tested with a low-value reward (7, 8), but a recent study using the original token exchange task confirmed previous results of inequity aversion even after controlling for the frustration effect (9). In both of our experiments, all NR control conditions were run after an assessment condition or warm-up trials so that all NR and all RI conditions were preceded by a condition where the subject received low-value rewards for giving the paw. Consequently, if the observed aversion to the unequal reward distribution could be explained by the frustration or contrast effect, we would not have expected any difference between the RI condition and both the asocial and social control conditions. In the first experiment, none of the subjects received any high-value rewards except once at the beginning of every test condition, thus a shift from high- to low-value reward cannot explain the results of the first experiment either.
Interestingly, our results differed from results of the primate studies in that we found no indication for sensitivity toward the quality of the food reward and the effort involved. Primates react to the quality of food, not just the presence/absence, and show more negative reactions than the dogs in this study (2, 9). The dogs' lack of sensitivity is also surprising in light of many studies demonstrating that a violation of expectancy of a certain food reward leads to a higher rejection rate in several species (28, 31). Although all owners whose dogs participated in this study confirmed that their dogs are more motivated to work if they receive sausage rather than bread as a reward, the dogs continued to give the paw for a low-value reward both in the social and the asocial assessment session, even with the high-value reward in front of them. There might, in fact, be several processes at work that could explain why we found no violation of expectancy effect in this study and why the dogs did not react to the QI condition: (i) All tested dogs were well trained used to work on a daily basis with their owners. This training effect might override the violation of expectancy effect, prompting the dogs to continue working as long as they receive a reward at all. Also the fact that they had to “work” for the reward might have enhanced the quality of the reward. (ii) In the social conditions, working next to a partner might have a facilitation effect, increasing the motivation of the subject to continue working even if only the partner is receiving the high-value reward and not they themselves. (iii) And possibly most importantly, the presence of the rewards themselves was such a strong motivator that they obscured the results of any QI assessment by the subjects. Further studies have to be conducted to reveal which of these explanations may be responsible for the lack of sensitivity for the quality of the reward that was observed in this study. Until we can answer these questions, it would be highly speculative to discuss whether this lack of sensitivity to the quality of the reward is a specific feature of domestic dogs or of canines in general. Of course, it would be tempting to assume that a domesticated species like the dog is less likely to react negatively than a nondomesticated species. However, attempting to answer this difficult question would require devising experiments in which the dogs may behave more like the primates that have been tested so far.
The strong motivating effect of the presence of rewards might explain also the lack of sensitivity toward differential effort invested by the subject and its partner. Alternatively, the insensitivity to the effort distribution and the food quality may be interesting in light of the evolution of cooperation. It has been argued that the psychological mechanism necessary for inequity aversion in regard to the invested effort and the reward requires animals to perceive a relation between relations, i.e., to compare the relation between its own effort and reward (1 token for 1 cucumber) with the relation between the partner's effort and reward (1 token for 1 grape, or no effort for 1 grape) (8). So far, it has only been shown that humans, chimpanzees, and, to a lesser extent, baboons are able to solve tasks that require the perception of relations between relations (32, 33). As a consequence, it is possible that dogs lack the cognitive abilities to show sensitivity to the outcome in relation to the effort invested. It is possible that sensitivity toward being rewarded or not may be the precursor to cognitively higher-level forms of inequity aversion.
The fact that the subjects refused earlier and hesitated longer to obey the command to give the paw to the experimenter when the partner received a reward but they themselves did not compared with the baseline condition as well as to the asocial and social control conditions suggests that dogs are sensitive to an unequal reward distribution. The lack of sensitivity toward the quality and the effort invested, however, also highlights the differences toward inequity aversion demonstrated in primates. Also, in contrast to the primates, the dogs never rejected food. Thus, the dogs were only responsive to disadvantageous inequity aversion (in contrast to advantageous inequity aversion characterizing humans) and were not willing to pay a cost by rejecting unfair offers (as is characteristic also of non-human primates), so there is a fundamental difference in the behavior of the primates and the dogs. The observed sensitivity toward the presence and absence of rewards may thus present a precursor of more sophisticated forms of inequity aversion. Further questions are raised concerning the evolutionary origin of at least a primitive form of inequity aversion: is it specific for primates and domesticated species working with humans, or is it more general feature present in other social species as well? In the latter case, did it evolve several times or does it have a very old origin and gradually developed into more and more sophisticated sensitivity to efforts and payoffs of joint interactions? Further studies thus need to determine (i) whether this ability is restricted to human-related species or to animals that cooperate with each other under natural conditions or rather a widely distributed phenomena in the animal kingdom and (ii) whether the lacking sensitivity toward the invested effort of the partner is specific to dogs either resulting from their evolutionary history of domestication or their developmental training by humans.
Experimental Procedures
A precondition for participation in the study was that the dogs had to know the command “to give the paw.” Details of the training and the experimental procedures are described in SI Experimental Procedures. Each subject was tested as subject and partner in all conditions. The ET was a baseline test in which both the subject and the partner performed for a low-value reward (dark bread). In the QI test, which determined their response to a high- and low-value reward distribution, the partner first performed for a high-value reward (sausage) followed by the subject asked to perform for a low-value reward (dark bread). In the RI test RI, which determined their response to an unequal presence of reward, the partner first performed for a low-value reward (dark bread) followed by the subject asked to perform but receiving no reward. In the EC test, the partner was initially handed a low-value reward without having to perform for it (dark bread as a gift), after which the subject had to perform to receive the low-value reward (dark bread).
To control that the dogs did react to the movement of the food in the social condition rather than to the partner being rewarded, as has been claimed for chimpanzees (6), the asocial control sessions included the movement of the food, i.e., the experimenter showed the food to the dog, moved the arm (food in the hand) toward the position where the other dog sat or would sit during the social conditions, opened the palm, closed the palm, moved the arm back and put the food back in the bowl in a way invisible to the subject before the subject was asked for the paw but did not receive a reward.
Each dog served as partner and subject in their respective dyad; the sequence was randomly chosen. The first subject was tested in all conditions before the roles were reversed. We carried out 2 test sessions per day with a 15-min break between them. The sequence of the 4 test sessions was counterbalanced between subjects with the only condition that it never started with the RI session. This condition assured that dogs in the asocial no reward condition and in the RI condition were down-shifted equally (e.g., received a low-value food reward in the session beforehand). The assessment and the asocial control sessions were either carried out before or after the test sessions in the same sequence.
In experiment 2, each of the 2 test sessions consisted of a series of 20 warmup trials (both subjects received the low-value reward for giving the paw on command) and 60 experimental trials, with trials alternating between the partner and the subject such that each individual received 40 trials per session (or until the subject refused to work), and the partner always performed immediately before the subject. In the SC condition, the subject and the partner were both asked to give the paw on command, but neither of them was rewarded. The RI condition was identical to the one in the first experiment. Again, to control for the movement of the food, the food was visibly lifted in front of the dogs but returned to the food bowl in the trials when they were not rewarded. The order of the 2 sessions was counterbalanced across subjects.
All tests were 2-tailed, and α was set at 0.05; trends are reported for 0.1 >α >0.05. When we analyzed subsets of data (comparing the RI and the NR conditions), the corresponding probabilities were corrected by using a sequential Bonferroni procedure (34). All results remained significant at the 5% level after the correction. Interobserver reliability for the dogs' behavior, based on coding 10 sessions of different dogs from video records, was calculated as Cohen κ values: giving the paw, 0.97; looking at the partner, 0.93; appeasement signals (yawning, scratching, licking the mouth, avoiding the gaze), 0.89.
Further details of the training and the experimental procedures are described in SI Experimental Procedures.
Supplementary Material
Acknowledgments.
We thank all our dogs and their owners for participating in our experiment. We are grateful to Karin Leitner for helping with the experiments and Frans B. M. de Waal, Anna Wilkinson, and 2 anonymous referees for discussions and comments on an earlier draft of this manuscript. This work was supported by the European Community Sixth Framework Program Contract NEST 012929, Royal Canin, Ltd., and Christian Palmers.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810957105/DCSupplemental.
References
- 1.Fehr E, Schmidt KM. A theory of fairness, competition, and cooperation. Q J Econ. 1999;114:817–868. [Google Scholar]
- 2.Fehr E, Fischbacher U. Social norms and human cooperation. Trends Cognit Sci. 2004;8:185–190. doi: 10.1016/j.tics.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Wynne CDL. Fair refusal by capuchin monkeys. Nature. 2004;428:140. doi: 10.1038/428140a. [DOI] [PubMed] [Google Scholar]
- 4.Brosnan SF, de Waal FBM. Monkeys reject unequal pay. Nature. 2003;425:297–299. doi: 10.1038/nature01963. [DOI] [PubMed] [Google Scholar]
- 5.Brosnan SF, Schiff HC, Waal FBM. Tolerance for inequity may increase with social closeness in chimpanzees. Proc R Soc London Ser B. 2004;1560:253–258. doi: 10.1098/rspb.2004.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bräuer J, Call J, Tomasello M. Are apes really inequity averse? Proc R Soc London Ser B. 2006;273:3123–3128. doi: 10.1098/rspb.2006.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roma PG, Silberberg A, Ruggiero AM, Suomi SJ. Capuchin monkeys, inequity aversion, and the frustration effect. J Comp Psychol. 2006;120:67–73. doi: 10.1037/0735-7036.120.1.67. [DOI] [PubMed] [Google Scholar]
- 8.Dubreuil D, Gentile MS, Visalberghi E. Are capuchin monkeys (Cebus apella) inequity averse? Proc R Soc London Ser B. 2006;273:1223–1228. doi: 10.1098/rspb.2005.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolkenten M.v., Brosnan SF, Waal FBM. Inequity responses of monkeys modified by effort. Proc Natl Acad Sci USA. 2007;104:18854–18859. doi: 10.1073/pnas.0707182104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cronin KA, Snowdon CT. The effects of unequal reward distributions on cooperative problem solving by cottontop tamarins, Saguinus oedipus. Anim Behav. 2008;75:245–257. doi: 10.1016/j.anbehav.2007.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clutton-Brock T. Breeding together: Kin selection and mutualism in cooperative vertebrates. Science. 2002;296:69–72. doi: 10.1126/science.296.5565.69. [DOI] [PubMed] [Google Scholar]
- 12.Mech D. The Wolf: The Ecology and Behavior of an Endangered Species. Garden City, NY: Natural History Press; 1970. [Google Scholar]
- 13.Creel S, Creel NM. Communal hunting and pack size in African wild dogs, Lycaon pictus. Anim Behav. 1995;50:1325–1339. [Google Scholar]
- 14.Harrington FH, Mech LD, Fritts SH. Pack size and wolf pup survival: Their relationship under varying ecological conditions. Behav Ecol Sociobiol. 1983;13:19–26. [Google Scholar]
- 15.Courchamp F, Macdonald DW. Crucial importance of pack size in the African wild dog Lycaon pictus. Anim Conserv. 2001;4:169–174. [Google Scholar]
- 16.Creel S, Creel NM, Mills MGL, Monfort SL. Rank and reproduction in cooperatively breeding African wild dogs: Behavioral and endocrine correlates. Behav Ecol. 1997;8:298–306. [Google Scholar]
- 17.Clutton-Brock TH, et al. Individual contributions to babysitting in a cooperative mongoose, Suricata suricatta. Proc R Soc London Ser B. 2000;267:301–305. doi: 10.1098/rspb.2000.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boitani L, Francisci F, Ciucci P. Population biology and ecology of feral dogs in central Italy. In: Serpell J, editor. The Domestic Dog: Its Evolution, Behaviour and Interactions with People. Cambridge, UK: Cambridge Univ Press; 1995. pp. 218–245. [Google Scholar]
- 19.Butler JRA, du Toit JT, Bingham J. Free-ranging domestic dogs (Canis familiaris) as predators and prey in rural Zimbabwe: Threats of competition and disease to large wild carnivores. Biol Conserv. 2004;115:369–378. [Google Scholar]
- 20.Naderi S, Miklósi Á, Dóka A, Csányi V. Cooperative interactions between blind persons and their dogs. Appl Anim Behav Sci. 2001;74:59–80. [Google Scholar]
- 21.Viranyi Z, Topal J, Gacsi M, Miklosi A, Csanyi V. Dogs respond appropriately to cues of humans' attentional focus. Behav Processes. 2004;66:161–172. doi: 10.1016/j.beproc.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Rooney NJ, Bradshaw JWS. Social cognition in the domestic dog: Behaviour of spectators towards participants in interspecific games. Anim Behav. 2006;72:343–352. [Google Scholar]
- 23.Soproni K, Miklosi A, Topal J, Csanyi V. Comprehension of human communicative signs in pet dogs (Canis familiaris) J Comp Psychol. 2001;115:122–126. doi: 10.1037/0735-7036.115.2.122. [DOI] [PubMed] [Google Scholar]
- 24.Riedel J, Buttelmann D, Call J, Tomasello M. Domestic dogs (Canis familiaris) use a physical marker to locate hidden food. Anim Cognit. 2006;9:27–35. doi: 10.1007/s10071-005-0256-0. [DOI] [PubMed] [Google Scholar]
- 25.Brosnan SF. Nonhuman species' reactions to inequity and their implications for fairness. J Soc Justice. 2006;19:153–185. [Google Scholar]
- 26.Range F, Viranyi Z, Huber L. Selective imitation in domestic dogs. Curr Biol. 2007;17:868–872. doi: 10.1016/j.cub.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 27.Virányi Z, Range F, Huber L. Attentiveness toward others and social learning in domestic dogs. In: Röska-Hardy L, Neumann-Held EM, editors. Learning from Animals? London: Psychology Press; 2008. [Google Scholar]
- 28.Amsel A. Frustration theory: Many years later. Psychol Bull. 1992;112:396–399. doi: 10.1037/0033-2909.112.3.396. [DOI] [PubMed] [Google Scholar]
- 29.Papini MR. Comparative psychology of surprising nonreward. Brain Behav Evol. 2003;62:83–95. doi: 10.1159/000072439. [DOI] [PubMed] [Google Scholar]
- 30.Tinklepaugh OL. An experimental study of representative factors in monkeys. J Comp Psych. 1982;8:197–236. [Google Scholar]
- 31.Papini MR, Dudley RT. Consequences of surprising reward omissions. Rev General Psychol. 1997;1:175–197. [Google Scholar]
- 32.Fagot J, Wasserman EA, Young ME. Discriminating the relation between relations: The role of entropy in abstract conceptualization by baboons (Papio papio) and humans (Homo sapiens) J Exp Psychol Anim Behav Process. 2001;27:316–328. [PubMed] [Google Scholar]
- 33.Tomasello M, Call J. Primate Cognition. Oxford, UK: Oxford Univ Press; 1997. [Google Scholar]
- 34.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.