Abstract
Fisheries can have a large impact on marine ecosystems, because the effects of removing large predatory fish may cascade down the food web. The implications of these cascading processes on system functioning and resilience remain a source of intense scientific debate. By using field data covering a 30-year period, we show for the Baltic Sea that the underlying mechanisms of trophic cascades produced a shift in ecosystem functioning after the collapse of the top predator cod. We identified an ecological threshold, corresponding to a planktivore abundance of ≈17 × 1010 individuals, that separates 2 ecosystem configurations in which zooplankton dynamics are driven by either hydroclimatic forces or predation pressure. Abundances of the planktivore sprat above the threshold decouple zooplankton dynamics from hydrological circumstances. The current strong regulation by sprat of the feeding resources for larval cod may hinder cod recovery and the return of the ecosystem to a prior state. This calls for the inclusion of a food web perspective in management decisions.
Keywords: alternative dynamics, ecological thresholds, ecosystem resilience, Baltic Sea, climate versus top-down control
Quantifying to what extent the synergetic interplay of top-down and bottom-up (including climate) processes controls structure and functioning of ecosystems has long been a running subject of scientific debate (1–4). In marine systems, particularly, understanding the combined effect of fishing and climate on ecosystem dynamics is of central importance for the management of exploited resources (5). Trophic cascades, defined by top-down control and the propagation of indirect effects between nonadjacent trophic levels, have been demonstrated in terrestrial and aquatic systems (6, 7). In pelagic marine ecosystems, however, empirical evidence of multilevel trophic cascades, from top predators to primary producers, has seldom been presented (8–10). On the other hand, with respect to global warming, it has been shown that climate changes may affect the whole pelagic food web by either changing system productivity (11, 12) or shifting the timing of ecological events and disrupting trophic links (13). Top-down and bottom-up (including climate) forces can also operate in concert, and their relative strength may vary in response to ecosystem alterations (14, 15). In open marine systems, it has also been suggested that environmental forcing may change system functioning by altering the strength and direction of the trophic control (i.e., in the North Pacific, summarized in ref. 16). However, empirical evidence showing temporal shifts between the 2 opposite processes of trophic control are extremely rare in open marine ecosystems, and they typically have been related to the direct effect of contrasting climate regimes (17, 18).
Sudden changes in ecosystem functioning may eventually result in promoting alternative stable states, as shown by both theoretical and experimental investigations (19) and supported by observational studies (9, 20). In fact, the resulting biotic and/or physical feedbacks that have arisen after the shift may stabilize the system in a state difficult to reverse (19). In this order, the dynamical systems theory may help explain the lack of recovery of some previously overharvested fish species despite robust management controls of the fishery (20). Consequently, identifying how and under which circumstances ecosystems respond to anthropogenic and climate forces bears vast management implications (5).
Here, we use information collected during three decades (1974–2005) to show evidence for a reorganization of the central Baltic Sea ecosystem caused by cascading effects of the top predator collapse. We provide quantitative evidence that the underlying mechanisms driving the trophic cascade allowed the establishment of 2 alternative ecosystem configurations that are separated by an ecological threshold (i.e., a certain abundance of zooplanktivorous fish) and characterized by different system structure, functioning, and stability. Our study provides an important contribution to the ongoing intense debate on the consequences of top predator declines in marine systems (21).
Results and Discussion
Ecological Threshold and Shifts in Ecosystem Functioning.
During the past 3 decades, the central Baltic Sea ecosystem has been characterized by an overall community-wide trophic cascade in summer that involves 4 levels of the food web: top piscivore fish (the gadoid cod), zooplanktivorous fish (the clupeid sprat), zooplankton, and phytoplankton (10). The trophic cascade was triggered by the remarkable drop in cod biomass (10), which was related to the effect of high fishing pressure intertwined with unfavorable recruitment conditions; that is, the lack of salt- and oxygen-rich water inflows from the North Sea (22). Since the late 1980s, the cod stock has been low, and it has not shown any tendency to recover (22). Our results show that after the cod collapse, the consequent dramatic increase in the sprat stock affected quantitatively and qualitatively the structure of the zooplankton community, as suggested by correlation analyses. In fact, during the observed period, not only did the total zooplankton biomass decrease (see also ref. 10), but the proportion of cladocerans in the zooplankton community also declined (Table 1). This pattern can be explained by the selectivity of clupeids on cladocerans because of their low escape response and the conspicuousness of egg-carrying individuals (24). No statistical relationship was found between zooplankton trends and the hydrological variability [supporting information (SI) Table S1]. The other main zooplanktivorous fish species of the Baltic Sea, the herring, do not significantly affect the open sea zooplankton, likely because of the different spatial and ontogenetic patterns in its feeding habits compared with sprat (10).
Table 1.
Correlations between sprat abundance and zooplankton parameters in the whole time series and in the 2 alternative configurations
| Correlated variables | Whole time series, |
Cod-dominated, |
Sprat-dominated |
||||||
|---|---|---|---|---|---|---|---|---|---|
| r | P | n | r | P | n | r | P | n | |
| Sprat abundance–zooplankton biomass | −0.45 | 0.01 | 29 | −0.12 | 0.65 | 16 | −0.60 | 0.03 | 13 |
| Sprat abundance–% cladocerans | −0.51 | <0.01 | 29 | 0.12 | 0.66 | 16 | −0.56 | 0.04 | 13 |
| Sprat abundance–% large copepod stages | −0.06 | 0.77 | 29 | 0.05 | 0.85 | 16 | −0.47 | 0.11 | 13 |
| Sprat abundance–% large copepod stages 0–50 m proxy for vertical distribution | −0.30 | 0.14 | 25 | 0.12 | 0.67 | 15 | −0.69 | 0.03 | 10 |
| Sprat abundance–PC1 zooplankton | −0.45* | 0.01* | 29 | 0.008 | 0.98 | 16 | −0.67 | 0.01 | 13 |
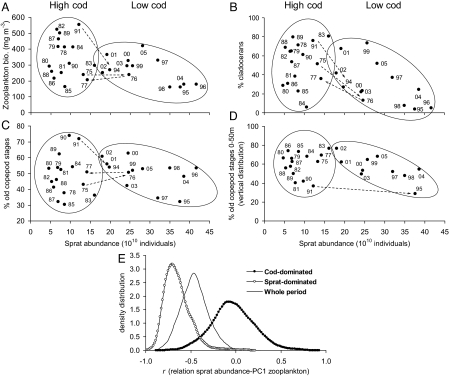
The alternative configurations were separated by piecewise regression and TGAM analyses (Fig. S1, Table S2, and Table S3). The rationale in considering sprat abundance rather than biomass in the sprat–zooplankton interactions is that sprat show strong density-dependent growth due to feeding competition in the Baltic Sea (10, 23). Correlation coefficients (r) and significance values (P) are indicated; n, number of observations. In bold are the significant correlations, at α = 0.05.
*After correcting for autocorrelation: r = −0.37, P < 0.05.
In addition to the overall top-down regulation of zooplankton, we also found quantitative evidence of a shift in the functioning of the central Baltic Sea ecosystem during the last 3 decades. The shift appears to be linked to an ecological threshold (piecewise regression and threshold generalized additive model (TGAM) analyses; Fig. S1, Table S2, and Table S3) that separates 2 alternative ecosystem configurations characterized by different strengths of the trophic interactions. The ecological threshold corresponds to a total sprat abundance of ≈17 × 1010 individuals and allows identifying one cod-dominated configuration characterized by low sprat abundance and a marked independence between zooplankton and sprat variations, and one sprat-dominated configuration in which cod biomass is low and zooplankton become strongly controlled by sprat predation (correlation analyses in Table 1; Fig. 1). In the latter configuration, the sprat control on both zooplankton biomass and species composition is substantially higher compared with the whole period investigated (Fig. 1 A and B and Table 1), suggesting a shift in the strength of sprat predation pressure on zooplankton. In the sprat-dominated configuration, the effect of sprat abundance on the stage (age/size) composition of copepods and on their vertical distribution also became noticeable. In fact, with increasing sprat abundance, the relative biomass of older copepod stages declined, and a higher proportion of their biomass occurred in the deeper layers of the water column in daytime (Fig. 1 C and D and Table 1). These patterns are explained by the fact that clupeids actively select large stages of copepods (24). The shift in the strength of the relationships between sprat and zooplankton is also illustrated by the change in the density distribution of correlation coefficients obtained through bootstrap resampling (Fig. 1E). The observed planktivore regulation of quantitative and qualitative characteristics of the zooplankton dynamics, as well as of its vertical distribution, are in line with the response observed in experimentally manipulated lakes (25), but so far has never been shown for marine ecosystems.
Fig. 1.
Alternative configurations of the central Baltic Sea ecosystem. The 2 configurations are illustrated as the relation between sprat abundance and (A) zooplankton biomass; (B) proportion (%) of cladocerans in the zooplankton community; (C) proportion (%) of large copepod stages in the copepod group; and (D) proportion (%) of large copepod stages occurring in the upper 50-m depth, proxy for vertical distribution. The 2 configurations correspond to the situations of high cod/low sprat (left ellipses) and of low cod/high sprat (right ellipses), respectively, and were separated by piecewise regression and TGAM. Numbers associated with each point indicate observation year. Ellipses were drawn by eyes to assemble the points belonging to either configuration. The dashed lines show the transit from one configuration to the other. See Table 1 for the statistics of the correlations between sprat abundance and zooplankton parameters, in the whole study period and in the 2 configurations. (E) Density distribution of the correlation coefficients between sprat abundance and PC1 of zooplankton parameters, which was obtained by bootstrap resampling (10,000 times) in the whole study period and in the 2 configurations. See Table S4 for statistical comparisons among the distributions.
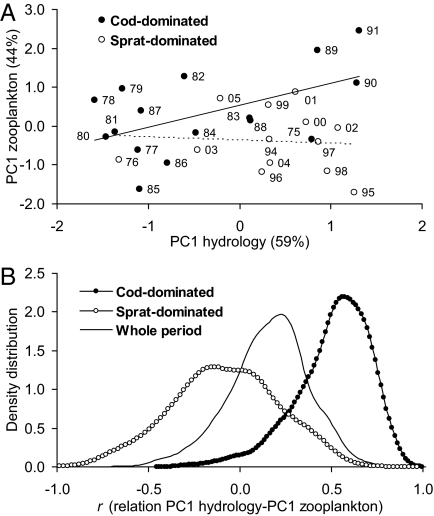
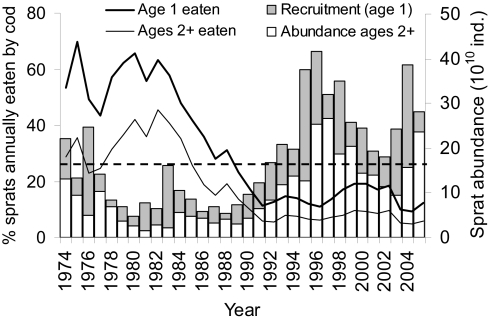
In the cod-dominated configuration, on the other hand, sprat and zooplankton are clearly uncoupled (Fig. 1 and Table 1), likely because sprat abundance is not high enough to regulate the zooplankton resource. Being unconstrained by sprat predation pressure, in the cod-dominated configuration zooplankton dynamics are driven mainly by hydrological conditions, as evidenced by TGAM (Fig. S1 and Table S3) and supported by correlation analyses (Fig. 2A) and the density distribution of correlation coefficients (Fig. 2B and Table S4). Based on our results, we suggest that in the cod-dominated configuration cod acts as an ecological attractor, being able to control sprat abundance and buffer high-sprat recruitment events. This is illustrated clearly during the period 1975–1977, when a rapid increase in sprat abundance above the threshold (due to temperature-driven unusually high sprat recruitment; ref. 26) shifted the system from one configuration to the other and back again within 1 year. We argue that these sudden and temporary shifts in zooplankton regulation mechanisms arise when the cod stock is large enough to depress strong sprat year classes in a very short time (1 year in this case) through predation mortality (Fig. 3). Following the collapse of the cod population, the system was no longer able to depress the high-sprat recruitment events, which could thus translate into a large and long-lasting sprat population. As highlighted by the discontinuous link between the temporal patterns of zooplankton and hydrological factors, our results suggest that high-sprat abundances decouple zooplankton dynamics from hydrology (Fig. 2) and become the main forcing of zooplankton variations. Overall, this emphasizes that changes in ecosystem functioning can be a result of variations at the higher trophic levels directly affected by human exploitation, and not merely the consequence of climate change (17, 18). Specifically, and in contrast to what has been shown in other systems (27), in the Baltic Sea the changes in the dynamic properties of zooplankton were not directly related to climate-driven hydrological variations, but rather to an alteration of the interaction strength between highly harvested species (piscivore cod and planktivore sprat).
Fig. 2.
Dual relationship between zooplankton and hydrological factors in the 2 alternative configurations. (A) Relationships between the PC1 of hydrological factors (temperature and salinity 0–100 m in spring and summer) and PC1 of zooplankton parameters in the 2 configurations. Cod-dominated: r = 0.53, P = 0.03; sprat-dominated: r = 0.074, P = 0.81. Factor loadings show association of zooplankton PC1 with total zooplankton biomass, whereas PC1 of hydrology is related mostly to salinity in summer. Numbers associated with each point indicate observation year. (B) Density distribution of the correlation coefficients between the PC1 of hydrological factors and PC1 of zooplankton parameters, obtained by bootstrap resampling (10,000 times) in the whole study period and in the 2 configurations. See Table S4 for statistical comparisons among the distributions. In bold are the significant correlations, at α = 0.05.
Fig. 3.
Trends in annual sprat predation mortality and sprat abundance in relation to the ecological threshold. The columns represent sprat total abundance divided into recruits (age 1) and older individuals (ages 2+). The lines show the trends in the proportion of sprats that are eaten annually by cod (proportion of age t sprats that die from age t to age t + 1 because of cod predation). The horizontal dashed line indicates the sprat abundance threshold that separates the 2 ecosystem configurations (see Fig. 1).
Hints for Alternative Stable States and Final Remarks.
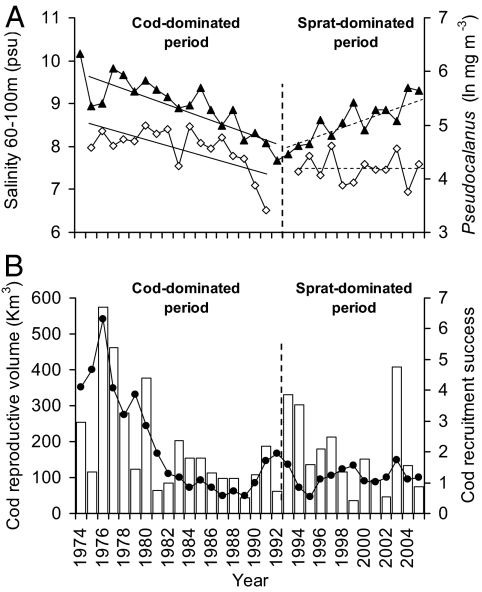
The shift in the main driver of zooplankton dynamics shown here may help explain the failure of cod recruitment during the past decade, despite improved hydrological conditions for egg and larval survival (see below and SI Text). Aside from the importance of parental stock size and age structure (28), cod recruitment strength in the Baltic Sea is mainly linked to hydrological circumstances [i.e., the reproductive volume (RV); ref. 29]. Higher salinity of the mid-deep waters enhances the buoyancy of cod eggs, preventing them from sinking into hypoxic water layers (29), but also favors the development of one of the key zooplankton prey for cod larvae; that is, the copepod Pseudocalanus spp. (30). Our results indicate that the direct link between zooplankton and hydrological conditions disappears when the population of the major planktivore rises above a certain threshold (Fig. 2). Specifically, the increase in deep water salinity after the sprat outburst was not translated into the expected increase in the Pseudocalanus spp. (Fig. 4A), despite the high productivity of the system (10). Likewise, after the early 1990s, cod recruitment success (RS) was decoupled from its RV (Fig. 4B). Particularly, the peaks in cod RV that occurred after the sprat outburst were not followed by the expected improvement in cod RS (Fig. 4B and SI Text), indicating a shift also in the main drivers of cod recruitment. These dual relationships between biological factors and hydrological circumstances in periods of high and low sprat abundance can be indicative of system hysteresis and alternative stable states in the central Baltic Sea ecosystem (19). Our study suggests that under conditions of high sprat abundance, cod recruitment may be jeopardized by the top-down regulation of sprat on the food resources for cod larvae. Although a conceptual framework of this cultivation/depensation effect (31) has been proposed before for the Baltic Sea (30), our study adds to it a critical aspect, showing the discontinuous behavior of the mechanisms involved and identifying the putative sprat abundance threshold responsible for the discontinuity. However, other prey-to-predator feedback loops could operate to delay cod recovery, such as sprat predation on cod eggs (32) and changes in the size structure of cod prey (33).
Fig. 4.
Potential ecological mechanisms hindering the success of cod recruitment. (A) Trends in salinity (▲) and Pseudocalanus spp. biomass (average spring-summer) (◇) in the central Baltic Sea. Pseudocalanus spp. is one of the main prey for sprat and larval/postlarval cod (30). Salinity between 60 and 100 m of depth was considered here because this plankter occurs mainly in deeper water layers, where it encounters favorable salinity conditions for reproduction (30). Salinity and Pseudocalanus spp. are positively correlated in the cod-dominated period (r = 0.67, P < 0.01), whereas in the sprat-dominated period the correlation disappears (r = −0.51, P = 0.09). Overall relation: r = 0.44, P = 0.02 (r* = 0.17, P* = 0.36). (B) Trends in cod reproductive volume (columns) and cod recruitment success (●). Relation between cod recruitment volume and recruitment success in the cod-dominated period (r = 0.71, P < 0.001) and in the sprat-dominated period (r = 0.27, P = 0.34). Overall relation: r = 0.59, P = 0.001. The vertical dashed lines indicate the time when sprat rose above the abundance threshold without any further reversal (see also Fig. 3). In bold are the significant correlations, at α = 0.05.
Empirical food web data can provide relevant information for disentangling the synergetic effects of human-induced disturbances (e.g., overfishing) and climate change on marine ecosystems. Harvested species may be seen as part of a large, dynamic, trophic network, with a high probability of being susceptible to top-down control, generating cascading effects through the food web (34). Our study highlights the role that human perturbations may have in promoting shifts in ecosystem functioning that are potentially difficult to reverse. Examples of failure of top predator recovery after release from extensive exploitation have been reported in several areas (20), emphasizing the crucial importance of linking food web dynamics, resilience, biodiversity, anthropogenic disturbances, and climate change across ecosystems (35). In particular, our study suggests that the restraint of sprat population below the critical abundance threshold may be favorable for Baltic cod recruitment and can contribute to reducing the magnitude of the summer algal blooms, which have been very intense in the Baltic Sea during the last decade (10). We claim that fisheries management, apart from achieving the necessary restriction in cod fishing pressure, should develop a framework for implementing an ecosystem approach that takes into consideration food web dynamics and the synergetic interplay of human and climatic drivers (5). This will effectively help maintain a healthy predator–prey relationship in marine ecosystems.
Materials and Methods
Time series of cod biomass (age 2+) and sprat abundance (age 1+) at the start of the year in the Baltic Sea were retrieved from official stock assessment reports (22). Fish population data were calculated by Extended Survivors Analysis, which is a standard methodology used in the International Council for the Exploration of the Sea stock assessment framework. Predation mortality rates were extracted from the multispecies assessment report (36).
The Latvian Fish Resources Agency provided raw data of summer abundance per 1 m3 of the major zooplankton species in the Gotland Basin (central Baltic Sea); that is, the copepods Pseudocalanus spp., Temora longicornis, and Acartia spp. (divided in the copepodite stages CI–CV and adults), and the cladocerans Bosmina coregoni maritima, Evadne nordmanni, and Podon spp. These species represent the main prey for sprat in the study area (37) and were sampled in daytime at several depth intervals from the surface down to a maximum depth of 100 m (or to sea bottom for shallow stations). Further details on sampling procedure and plankton identification can be found in the literature (37). Because sprat feed primarily in the open sea during the main feeding period [that is, summer (38, 39)], we focused our investigation on the open-sea stations (≥100-m depth). This avoided also the potential confounding effect of different sampling depths in the construction of zooplankton time series. Zooplankton biomasses per 1 m3 were calculated from abundances by using standard wet weights (40). For each copepod species, we pooled the different development stages into younger (CI–CIV) and older (CV to adult) stages. To investigate the zooplankton daytime depth distribution, we used the relative biomass of zooplankton occurring in the 0- to 50-m depth (37). Basic time series of cod, sprat, and summer zooplankton are presented in Fig. S2.
Hydrographic parameters (temperature and salinity), collected monthly in the central Baltic Sea, were provided by the Swedish Meteorological and Hydrological Institute (www.smhi.se). Time series of water temperature (°C) and salinity [practical salinity units (psu)] were averaged over the 0- to 100-m depth strata (samples at surface and at depth intervals of 10 m).
Principal component analysis was used to extract the main time trends from the zooplankton and hydrological time series. In the construction of the principal components, missing values (4 points in the vertical distribution of the old copepod stages) were predicted from linear trend regression. In all of the other analyses, we did not fill missing points with estimated values.
Pearson's product moment correlations were used to relate the different variables considered in the study. We checked the time series for autocorrelation by using the autocorrelation function. When present, autocorrelation can bias the statistical inference in correlation analyses, increasing the type I error rate (41). However, autocorrelation was absent in one or both of the time series in almost every correlation analysis, and thus corrections were not necessary (26). Only in 2 correlation analyses did both time series present autocorrelation. In these cases, we adjusted the time series of the dependent variable by using the “first-differencing” method to remove its autocorrelation (41), and we showed the results by using both untransformed and transformed data (the latter indicated by *).
Piecewise regression analysis (42) was used to detect discontinuities in the correlations between sprat abundance and zooplankton parameters. The model estimates the point of discontinuity in the relationship between 2 variables and the parameters of the 2 linear regressions identified. In our study this analysis was used to individuate the threshold in the sprat–zooplankton relationship.
To verify the point of phase transition (18) highlighted by the piecewise regression analysis, we applied a TGAM to the PC1 values of the zooplankton parameters time series. TGAMs are an extension of nonparametric regression techniques (43) and were chosen here for their ability to represent an abrupt change in the relationships between dependent and independent variables (i.e., a phase transition) at a specific threshold value t (18). PC1 of zooplankton parameters was analyzed in relation to PC1 of hydrological factors and to sprat abundance time series. Sprat abundance was used as the threshold variable. The threshold value was selected minimizing the generalized cross validation score (GCV) of the whole model (18). The searching algorithm runs the model for 100 possible threshold values between the 0.1 lower and the 0.9 upper quantiles.
The strength of the link between sprat abundance (as well as hydrology) and zooplankton through the whole period investigated and in the 2 configurations identified by piecewise regression and TGAM analyses was also assessed by quantifying the probability density distribution of correlation coefficients obtained by bootstrap resampling (27). This analysis involved a random pairwise sampling with replacement where each time series was resampled 10,000 times. The number of elements in each bootstrap sample equals the number of elements in the original data set. The probability density distribution of the corresponding correlation coefficients was then computed using nonparametric Kernel smoothing. Kolmogorov–Smirnov tests were used to compare the estimated density distributions of the correlation coefficients.
Cod reproductive volume in the central Baltic Sea was calculated by using a contouring program that estimates the volume of water whose hydrographic conditions are considered suitable for the development of cod eggs; that is, with a salinity >11 psu and an oxygen concentration >2 ml·L−1 (29). These criteria are based on studies that have established the relation between Baltic cod egg survival and cod egg buoyancy, vertical distribution, and oxygen concentration (29). Cod recruitment success is defined here as the number of recruits at age 0 (thousand individuals)/spawning biomass (tonnes) (1 tonne = 1,000 kg) (data from refs. 22 and 36).
The significance level was set at 5% for all of the statistical tests used in the analyses. Statistical analyses were performed by using Matlab 7 (The MathWorks, Inc.), Statistica 6 (StatSoft, Inc.), and R (R Foundation for Statistical Computing).
Supplementary Material
Acknowledgments.
We thank L. Ciannelli, who made the R script of the TGAM available, and M. Plikshs for cod reproductive volume time series. We are indebted to J. Bascompte, A. De Roos, S. Hansson, and G. Sugihara for valuable comments on earlier versions of this manuscript, and to M. Clarke for English editing. The comments of 3 anonymous reviewers significantly improved the manuscript. The Latvian Fish Resources Agency furnished zooplankton data. We are grateful to the Swedish Meteorological and Hydrological Institute (SMHI), SHARK database, which provided hydrological profiles. M. Casini was partially funded by Oscar och Lili Lamms Minne Stiftelsen. The research of J.-C.M. is a contribution to the priority program AQUASHIFT (the impact of climate variability on aquatic ecosystems, Leibniz Institute of Marine Sciences, IFM-GEOMAR).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806649105/DCSupplemental.
References
- 1.Ecology. Special feature: Top-down and bottom-up forces. Ecology. 1992;73:723–765. [Google Scholar]
- 2.Menge BA, et al. Benthic-pelagic links and rocky intertidal communities: bottom-up effects on top-down control? Proc Natl Acad Sci USA. 1997;94:14530–14535. doi: 10.1073/pnas.94.26.14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winder M, Schindler DE. Climate change uncouples trophic interactions in an aquatic ecosystem. Ecology. 2004;85:2100–2106. [Google Scholar]
- 4.Vinueza LR, Branch GM, Branch ML, Bustamante RH. Top-down herbivory and bottom-up El Niño effects on Galápagos rocky-shore communities. Ecol Monog. 2006;76:111–131. [Google Scholar]
- 5.Brander KM. Global fish production and climate change. Proc Natl Acad Sci USA. 2007;104:19709–19714. doi: 10.1073/pnas.0702059104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pace ML, Cole JJ, Carpenter SR, Kitchell JF. Trophic cascades revealed in diverse ecosystems. Trends Ecol Evol. 1999;14:483–488. doi: 10.1016/s0169-5347(99)01723-1. [DOI] [PubMed] [Google Scholar]
- 7.Shurin JB, et al. A cross-ecosystem comparison of the strength of trophic cascades. Ecol Lett. 2002;5:785–791. [Google Scholar]
- 8.Frank KT, Petrie B, Choi JS, Legget WC. Trophic cascades in a formerly cod-dominated ecosystem. Science. 2005;308:1621–1623. doi: 10.1126/science.1113075. [DOI] [PubMed] [Google Scholar]
- 9.Daskalov GM, Grishin AN, Rodionov S, Mihneva V. Trophic cascades triggered by overfishing reveal possible mechanisms of ecosystem regime shifts. Proc Natl Acad Sci USA. 2007;104:10518–10523. doi: 10.1073/pnas.0701100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casini M, et al. Multi-level trophic cascades in a heavily exploited open marine ecosystem. Proc R Soc London B Biol Sci. 2008;275:1793–1801. doi: 10.1098/rspb.2007.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ware DM, Thomson RE. Bottom-up ecosystem trophic dynamics determine fish production in the Northeast Pacific. Science. 2005;308:1280–1284. doi: 10.1126/science.1109049. [DOI] [PubMed] [Google Scholar]
- 12.Frederiksen M, Edwards M, Richardson AJ, Halliday NC, Wanless S. From plankton to top predators: Bottom-up control of a marine food web across four trophic levels. J Anim Ecol. 2006;75:1259–1268. doi: 10.1111/j.1365-2656.2006.01148.x. [DOI] [PubMed] [Google Scholar]
- 13.Edwards M, Richardson AJ. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature. 2004;430:881–884. doi: 10.1038/nature02808. [DOI] [PubMed] [Google Scholar]
- 14.Wilmers CC, Post E, Peterson RO, Vucetich JA. Predator disease out-break modulates top-down, bottom-up and climatic effects on herbivore population dynamics. Eco Lett. 2006;9:383–389. doi: 10.1111/j.1461-0248.2006.00890.x. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz OJ, Kalies EL, Booth MG. Alternative dynamic regimes and trophic control of plant succession. Ecosystems. 2007;9:659–672. [Google Scholar]
- 16.Progress in Oceanography. Special issue on marine ecosystem structure and dynamics. Prog Oceanogr. 2006;68:115–342. [Google Scholar]
- 17.Hunt GL, Jr, et al. Climate change and control of the southeastern Bering Sea pelagic ecosystem. Deep Sea Res Part II Top Stud Oceanogr. 2002;49:5821–5853. [Google Scholar]
- 18.Litzow MA, Ciannelli L. Oscillating trophic control induces community reorganization in a marine ecosystem. Ecol Lett. 2007;10:1124–1134. doi: 10.1111/j.1461-0248.2007.01111.x. [DOI] [PubMed] [Google Scholar]
- 19.Scheffer M, Carpenter SR. Catastrophic regime shifts in ecosystems: linking theory to observation. Trends Ecol Evol. 2003;18:648–656. [Google Scholar]
- 20.Bakun A, Weeks SJ. Adverse feedback sequences in exploited marine systems: Are deliberate interruptive actions warranted? Fish Fish. 2006;7:316–333. [Google Scholar]
- 21.Heithaus MR, Frid A, Wirsing AJ, Worm B. Predicting ecological consequences of marine top predator declines. Trends Ecol Evol. 2008;23:202–210. doi: 10.1016/j.tree.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 22.International Council for the Exploration of the Sea. Report of the Baltic Fisheries Assessment Working Group. Copenhagen: International Council for the Exploration of the Sea; 2006. p. 24. ICES CM 2006/ACFM. [Google Scholar]
- 23.Casini M, Cardinale M, Hjelm J. Inter-annual variation in herring (Clupea harengus) and sprat (Sprattus sprattus) condition in the central Baltic Sea: What gives the tune? Oikos. 2006;112:638–650. [Google Scholar]
- 24.Viitasalo M, Flinkman J, Viherluoto M. Zooplanktivory in the Baltic Sea: A comparison of prey selectivity by Clupea harengus and Mysis mixta, with reference to prey escape reactions. Mar Ecol Prog Ser. 2001;216:191–200. [Google Scholar]
- 25.Carpenter SR, Kitchell JF, editors. The Trophic Cascade in Lakes. Cambridge, UK: Cambridge Univ Press; 1993. [Google Scholar]
- 26.MacKenzie BR, Köster FW. Fish production and climate: Sprat in the Baltic Sea. Ecology. 2004;85:784–794. [Google Scholar]
- 27.Molinero JC, et al. Climate control on the long-term anomalous changes of zooplankton communities in the Northwestern Mediterranean. Global Change Biol. 2008;14:11–26. [Google Scholar]
- 28.Cardinale M, Arrhenius F. The influence of stock structure and environmental conditions on the recruitment process of Baltic cod estimated using a generalized additive model. Can J Fish Aquat Sci. 2000;57:2402–2409. [Google Scholar]
- 29.MacKenzie BR, Hinrichsen HH, Plikshs M, Wieland K, Zezera AS. Quantifying environmental heterogeneity: Habitat size necessary for successful development of cod Gadus morhua eggs in the Baltic Sea. Mar Ecol Prog Ser. 2000;193:143–156. [Google Scholar]
- 30.Möllmann C, Müller-Karulis B, Kornilovs G, John AM., St. Effects of climate and overfishing on zooplankton dynamics and ecosystem structure: Regime shifts, trophic cascades, and feedback-loops in a simple ecosystem. ICES J Mar Sci. 2008;65:302–310. [Google Scholar]
- 31.Walters C, Kitchell JF. Cultivation/depensation effects on juvenile survival and recruitment: Implication for the theory of fishing. Can J Fish Aquat Sci. 2001;58:39–50. [Google Scholar]
- 32.Jarre-Teichmann A, et al. Stock-recruitment relationship for cod (Gadus morhua) in the central Baltic Sea incorporating environmental variability. Arch Fish Mar Res. 2002;48:97–123. [Google Scholar]
- 33.Van Leeuwen A, De Roos AM, Persson L. How cod shapes its world. J Sea Res. 2008;60:89–104. [Google Scholar]
- 34.Bascompte J, Melian CJ, Sala E. Interaction strength combinations and the overfishing of a marine food web. Proc Natl Acad Sci USA. 2005;102:5443–5447. doi: 10.1073/pnas.0501562102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folke C, et al. Regime shifts, resilience, and biodiversity in ecosystem management. Annu Rev Ecol Evol Syst. 2004;35:557–581. [Google Scholar]
- 36.International Council for the Exploration of the Sea. Report of the Study Group on Multispecies Assessment in the Baltic. Copenhagen: International Council for the Exploration of the Sea; 2006. ICES CM 2006/BCC:07. [Google Scholar]
- 37.Kornilovs G, Sidrevics L, Dippner JW. Fish and zooplankton interaction in the Central Baltic Sea. ICES J Mar Sci. 2001;58:579–588. [Google Scholar]
- 38.Aro E. A review of fish migration patterns in the Baltic. Rapp P-v Réun Cons int Explor Mer. 1989;190:72–96. [Google Scholar]
- 39.Arrhenius F, Hansson S. Food consumption of larval, young and adult herring and sprat in the Baltic Sea. Mar Ecol Prog Ser. 1993;96:125–137. [Google Scholar]
- 40.Hernroth L, editor. Recommendations on methods for marine biological studies in the Baltic Sea. Mesozooplankton biomass assessment. Baltic Mar Biol. 1985;10:1–32. [Google Scholar]
- 41.Pyper BJ, Peterman RM. Comparison of methods to account for autocorrelation in correlation analyses of fish data. Can J Fish Aquat Sci. 1998;55:2127–2140. [Google Scholar]
- 42.Toms JD, Lesperance ML. Piecewise regression: A tool for identifying ecological thresholds. Ecology. 2003;84:2034–2041. [Google Scholar]
- 43.Hastie TJ, Tibshirani RJ. Generalized Additive Models. New York: Chapman & Hall; 1990. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.