Abstract
The prokaryotic KcsA channel is gated at the helical bundle crossing by intracellular protons and inactivates at the extracellular selectivity filter. The C-terminal transmembrane helix has to undergo a conformational change for potassium ions to access the central cavity. Whereas a partial opening of the tetrameric channel is suggested to be responsible for subconductance levels of ion channels, including KcsA, a cooperative opening of the 4 subunits is postulated as the final opening step. In this study, we used single-channel fluorescence spectroscopy of KcsA to directly observe the movement of each subunit and the temporal correlation between subunits. Purified KcsA channels labeled at the C terminus near the bundle crossing have been inserted into supported lipid bilayer, and the fluorescence traces analyzed by means of a cooperative or independent Markov model. The analysis revealed that the 4 subunits do not move fully independently but instead showed a certain degree of cooperativity. However, the 4 subunits do not simply open in 1 concerted step.
Keywords: gating, ion channel, single-molecule
The opening of the ion-conducting pore has been investigated in great detail although the exact mechanisms remain to be elucidated. In the crystal structure of KcsA (1), the helical bundle crossing formed by the TM2 helices is closed, and it is generally agreed that it has to open to allow ion conduction. Recently, Shimizu and coworkers (2) demonstrated a rotational movement of TM2 during gating by using X-ray diffraction, which may be responsible for pore opening. However, the ion-conducting pore of KcsA can also be gated (inactivated) at the selectivity filter (3, 4). The pH dependence in KcsA, as well as the voltage-dependent gating in the voltage-sensitive channels, is thought to be mediated by the helical bundle crossing (3–7), whereas voltage dependence of KcsA occurs at the selectivity filter (8). Because the pore region consists of a tetramer, it is still a matter of debate whether the 4 subunits open in 1 cooperative movement, or whether all 4 subunits open and close independently.
The main indications for independent movement of the 4 subunits came from subconductance levels that were found in different families of channels, including KcsA (5, 9–11) and Shaker (12, 13). It has been suggested that the subconductance levels are caused by a partial (i.e., not all subunits) opening of the pore. Chapman and VanDongen (14) demonstrated for voltage-gated channels that in heterotetramers with subunits of different voltage dependence, the channel enters frequently into subconductance levels at intermediate activation voltage. Although these results suggest an independent action of the subunits, their models improved significantly when introducing a cooperativity factor for the pore opening. One also has to keep in mind that, in voltage-gated channels, the opening of the pore is coupled to the movement of the voltage sensor. The reason for the partial opening in the drk1 K+ channel may therefore be the different energy barriers for the activation of the voltage sensors, and the energy from the sensors may have been sufficient to uncouple any cooperativity between subunits in the C terminus of S6. Therefore, it remains unknown whether the pore itself—if uncoupled from the voltage sensors—would still open cooperatively.
Although subconductance levels have also been found in 2TM K+ channels [KcsA (5, 9–11) and KIR (15–17)], only a little is known about the underlying mechanism. In KIR2.1, Xie et al. (17) suggested that the binding of a single PIP2 leads to opening of subconductance levels and full opening of the channel. For KcsA, Kariev et al. (18) suggested a charge interaction at the helical bundle crossing in the protonated channel as a possible reason for the pH dependence. Opening of a single subunit would disturb the coordination of the interaction and facilitate opening of further subunits. Therefore, in the present work, we set out to directly measure the movement of single subunits to determine their temporal coordination with the aim of determining whether the channel opens in 1 cooperative step or if each subunit moves independently. Our method is based on previous work where we showed that labeling of the helical bundle crossing of KcsA (position G116C or Q119C) allows the monitoring of the channel's opening with fluorescence (4). We formed supported bilayers including these mutants and imaged the fluorescence of single channels over time.
Results
Fluorescence Represents the Subunits of KcsA.
To monitor the conformational changes of the KcsA channel, we labeled them with tetramethylrhodamine-5-maleimide (TMRM) at the helical bundle crossing at positions G116C and Q119C in the KcsA-E71A background. We have shown previously that TMRM attached to these positions follows the movement of the helical bundle crossing: opening of the channel results in decreased fluorescence intensity (4). The E71A background was chosen because the opening of the helical bundle crossing most likely corresponds directly to ion conduction in the absence of inactivation of the selectivity filter (3). We also labeled position L90C as a control.
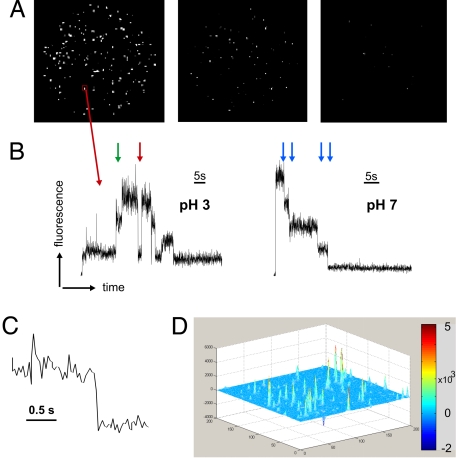
We resorted to single-channel fluorescence measurements, because in an ensemble measurement, we would not be able to distinguish whether 2 signals originate from 2 subunits of the same channel or from neighboring channels. Any modification of the subunits to make them distinct from one another would disturb the symmetry between them and may affect their eventual cooperativity. To observe the single channels, we formed a supported bilayer on a glass surface with the lipid-to-protein mass ratio adjusted in a manner such that single spots would be evenly distributed and spatially distinct in the field of view (Fig. 1A). The fluorescence was recorded with a frame rate of 33 s−1 (30-ms exposure time). For quantitative analysis of the fluorescence intensity, we chose a square region of 3 × 3 pixels around the center of the spots and determined their average intensity over time (Fig. 1B). To ensure that we are actually observing single KcsA channels, we analyzed the bleaching behavior of the spots and found it to be step-like (Fig. 1B). The step-like bleaching behavior is caused by the quantitized photobleaching of single fluorophores and indicates that only a few fluorophores were present within the observed spots. Supported bilayers without any added fluorophore showed a low number of spots (background fluorescence). These spots did not show an ordered step-like bleaching behavior, but mainly very fast fluctuations (data not shown). Some of these spots were also found in our analysis of the measurements, but were not taken into consideration for further analysis. Addition of free fluorophores (TMRM) to the vesicles before bilayer formation resulted in rapid movement of fluorescent spots, and we found no clear static spots such as the ones shown in Fig. 1D. Therefore, by only considering static spots, we ensured that all fluorescent spots that we analyzed originated from fluorophores bound to the channel protein.
Fig. 1.
Single-channel imaging of KcsA-Q119C-E71A labeled with TMRM. (A) Distribution of channels in the field of view (Ø ≈ 90 μm). Over time the spots are photobleaching until the last frame (Right) is almost completely bleached. Shown are frames taken with brightfield (BF) at 0.75 s, 24 s, and 60 s. (B) The intensity changes over time are measured within a 3 × 3 pixel square region (red box) around the center of the spot. Shown are 2 time traces at pH 3 and pH 7, respectively. In the left trace, channel activity can be observed. High intensity reflects a closed subunit (4). The subunits may act independently (green arrow) or cooperatively (red arrow). In the right trace (pH 7), no activity was observed. The blue arrows indicate the bleaching steps. (C) Multiple transition step in B shown on an expanded time scale. (D) Three-dimensional representation of the entire image intensity averaged over the first one-third of the series, from which the intensity averaged over the last one-third of the image series is subtracted. The spots appear as strong peaks, indicating that the channels are not diffusing in the supported bilayer (moving spots would disappear in the average and not form peaks). It also indicates that the majority of spots are bleached during the recording (bleached spots are positive in the first third, but zero in the last third).
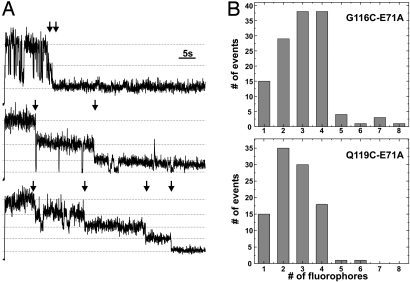
To further corroborate that the spots represent the single KcsA channels, we counted the bleaching steps (Fig. 2A). As the tetrameric KcsA consists of 4 identical subunits, each channel had 4 cysteines as possible labeling positions for the thiol-reactive fluorophore. Consequently, the spots should be bleaching in 4 equidistant intensity steps, and counting the number of bleaching steps should give the number of subunits (19). Fig. 2B shows histograms of the number of fluorophores per spot in different preparations, the first from G116C-E71A and the second from Q119C-E71A. In both cases (as well as in the L90C mutant, data not shown) most spots bleached in up to 4 steps. Only a few spots were found, which showed >4 bleaching steps, up to 8. We assume that, in these cases, 2 channels were present at the same spot in the image. The lower number of steps per spot at position 119 as compared with 116 indicates that we had less visible fluorophores per channel. This may be due to incomplete labeling efficiency (oxidation of cysteines, limited accessibility of cysteine) or to prior photobleaching of attached fluorophores.
Fig. 2.
Subunit counting. (A) Because of its tetrameric structure, cysteine-modified KcsA can bind up to 4 thiol-reactive fluorophores. We analyzed the time traces with respect to how many bleaching steps are observed. Here, 3 different time traces recorded with TIR at pH 5 are shown with 2, 3, and 4 bleaching steps (arrows). (B) Histograms of G116C-E71A (Upper) and Q119C-E71A (Lower) obtained from counting the bleaching steps from time traces. The probability of a subunits being fluorescent was 0.72 and 0.59 for G116C and Q119C, respectively.
Fluctuation Changes Show Characteristics of KcsA Gating.
In the fluorescence versus time traces, we found step-like fluctuations in the intensity before the actual bleaching (Fig. 1B and 2A). Based on our previous results (4), we interpreted these fluctuations as caused by the gating of the subunits. However, in single-molecule fluorescence measurements, “blinking” of the fluorophore itself must also be considered. Blinking—intensity fluctuations in different time ranges—is an intrinsic property of the fluorophore. It is suggested to be a result of transient transitions into triplet states (20), formation of conformational isomers (21), or interaction with radicals (22, 23). To establish the amount of blinking of the fluorophore, we used the L90C control mutant. In this mutant, the label is located near the p loop, where no large conformational changes are expected. We previously showed that the fluorescence intensity of this mutant is independent of the pH (4). Therefore, the intensity changes that we found in these mutants are likely caused by intrinsic blinking of the bound fluorophores. Intensity changes in L90C occurred much less frequently (13% ± 8% compared with 83% of Q119C-E71A at pH 3). However, blinking may influence our results, and we have to keep this in mind when interpreting the quantitative results (see below).
Thompson and coworkers (5) demonstrated that the pH sensor of KcsA is located at the helical bundle crossing. Because it is located in close proximity to the fluorescent probes, is it also possible that the pH sensor is directly responsible for the fluorescence change? It may be that the pH sensor influences the fluorophores attached close to their location. However, this would be a static change, once the pH has been altered, and would influence the overall fluorescence intensity (the transition rates of fluorophores lie in the nanosecond range). For the fluorescence steps that we were analyzing, it would only slightly change the height of the steps while leaving untouched the kinetics between the 2 states of the channel/fluorophore. In fact, we found a slight increase of the step size as a function of pH, which was opposite to the pH dependence observed in the kinetics. But a mere alteration in the absolute fluorescence values at the bright or dark level would not influence the transition rates between the 2 states, and thus the single-channel fluorescence kinetics remain independent of such influences.
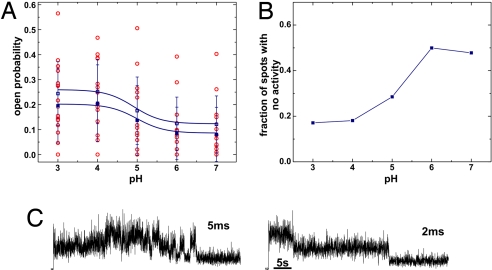
Given that the fluctuations of the C-terminal mutants are caused by the opening and closing of the channel, they should follow the gating behavior of KcsA. And, because the opening of the bundle crossing is the pH-dependent step (3–5), the fluorescence changes should be pH-dependent. We estimated the open probability of the single subunits directly from the fluorescence changes by separating those sections of the time course that included different numbers of visible subunits (Fig. 3). We will refer to this method as the direct determination of single-subunit open probability. The subunit open probability estimated in this manner followed the pH dependence of KcsA well, with a pK of 4.9. The values for the subunit open probability varied widely (0.1–0.56 for pH 3, 0.01–0.4 for pH 7) as a result of the low observation time caused by the fast bleaching. As expected, the values varied most widely at pH 5 where KcsA undergoes most gating events.
Fig. 3.
Open probability determined directly from fluorescence time traces. (A) Open probability of 1 subunit determined from the time traces (BF) considering that the closed subunit is bright and the open subunit is dark. The red spots are single values. Mean and SD are shown in blue. Filled symbols are mean of all values, open symbols are corrected values, which excluded all time traces with no activity (Fig. 3B). P = 0.014/0.006 (ANOVA). (B) pH dependence of fraction of spots showing no activity. (C) Image series (BF) were recorded with higher time resolution (5 and 2 ms, respectively). Whereas the signal-to-noise ratio decreased, no additional information was observed.
Whereas the absolute value at high pH (0.12) would fit well with the low open probability of KcsA in this range, the value for the low pH (0.25) is too low compared with previously published values for the channel open probability of this mutant measured in patch-clamp recordings (0.8–0.95) (3, 24). (Note that it is assumed that all 4 subunits have to open for the channel to conduct.) Nevertheless, in our electrophysiological recordings of labeled Q119C-E71A in planar lipid bilayer with the same lipid composition as we used for formation of supported bilayers (phosphatidylcholine, PC), we found a lower open probability than published previously (0.49 ± 0.25 at pH 3; pK = 4.7, Fig. 4E). We suppose that the difference in the open probability may be caused by the different lipid composition used for the bilayer as compared with the previous recordings [PE:PG = 3:1 (5)]. The PC was essential for the formation of stable supported bilayers. We also cannot exclude that the mutations of the positions close to the pH sensor (5) have an influence on the open probability. Labeled mutant channels showed a slightly lower open probability compared with unlabeled ones (0.5 vs. 0.65 at pH 3).
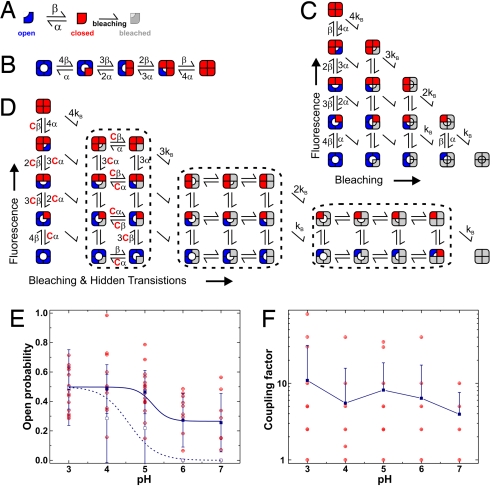
Fig. 4.
Markov model fitting of KcsA fluorescence time traces. (A) Each subunit may be in the fluorescent (closed, red) or nonfluorescent (open, blue) state. After bleaching, the states fuse into the bleached, nonfluorescent state (gray), where we cannot determine the state of the channel anymore. (B) If the subunits are independent, the rate constants for opening of a single subunit are multiplied by the number of closed subunits and so on. This model indicates the independent, nonbleaching channel. (C) Introducing irreversible bleaching into the model results in a 15-state model, where the bleached subunits (gray) have an undefined state of the bundle crossing. (D) If the subunits are not independent, we have to introduce an additional coupling constant (see text for details) so that the bleached subunits have an influence on the rate constants of the fluorescent subunits. (E) Open probability of a single subunit calculated from the rate constants α and β. Shown are the actual values (red) and mean ± SD (blue filled; P = 0.001 ANOVA). For comparison the channel open probability of KcsA-E71A-Q119C labeled with TMRM is given (blue open symbol). (F) Coupling constant resulting from Markov model fits of the time traces. Shown are single values (red) as well as mean ± SD (blue; neg. SD omitted in logarithmic scale).
KcsA-WT channel switches between gating modes with very different open probabilities (0.16–0.8) (24). But these gating modes and the voltage dependence of KcsA-WT, which reduces the open probability at 0 mV (in the supported bilayer) to 0.33, are caused by conformational changes of the selectivity filter, not the helical bundle crossing, and are largely removed in the KcsA-E71A mutant (3, 24). One should keep in mind, however, that the published values are apparent open probabilities (NPo), whereas we are directly measuring the single-subunit open probability.
We tested whether insufficient exchange of pH on the trans side of the supported bilayer might be responsible for the low open probability at low pH. Nevertheless, the results did not change when preparing the vesicles and supported bilayer at pH 3 and then increasing the pH stepwise to pH 7, as compared with starting at pH 7 and decreasing it stepwise to pH 3. Also forming a new bilayer for each pH did not alter our findings. Another possible reason for the low open probability may be that some of the channels become immobilized on the glass surface. Fig. 3B shows the pH dependence of the fraction of spots that do not exhibit any fluctuations in the fluorescence intensity. Whereas at pH 6–7 these spots mainly represent those channels that remain closed during the observation time, at pH 3 the fraction with no activity (17%) likely represents channels locked in the closed position. For the calculation of the “corrected” values in Fig. 3A, all spots that do not show any activity have been excluded. The “true” curve would start with the normal values at pH 7 and approach the corrected curve at pH 3.
We also investigated the dwell times in the open and closed state based on our measurements. We found that the dwell times were relatively long with values of 2.4 ± 1.5 s and 2.2 ± 1.3 s for the open and closed state, respectively. These long dwell times suggest that the movement of the lower bundle crossing is switching from the active to the resting state, whereas faster gating is caused by movement in the selectivity filter, consistent with previous findings (3, 4, 24). Nevertheless, the value for the mean open time is longer than values determined previously by using single-channel recordings (burst length, 0.16–0.06 s for pH 3 and 7, respectively). Also here, mutation or labeling of the helical bundle crossing may have an influence on the dwell time distribution. Because the long dwell times could also be the result of poor time resolution, we looked for faster events with a higher time resolution. However, with increased frame rates of 50, 200, and 500 s−1 (Fig. 3C), we did not detect additional gating events, but under these recording conditions our signal-to-noise ratio was reduced significantly.
Simultaneous Movement of KcsA Subunits.
While observing the fluorescence versus time traces, we noticed that frequently, 2 or more subunits changed their state simultaneously (Fig. 1B and 2A). We found that on average 37 ± 29% of all transitions were multiple transitions. If we calculate the probability for such a double transition in a 2-subunit system with the dwell times observed (see above), the probability is <1%. For a subsequent simultaneous opening and closing, it is even lower [<0.25%; for details see supporting information (SI) Text and Fig. S1]. The transitions, therefore, do not occur independently, but have to be coupled by an interaction. An interaction of an external quencher with several fluorophores simultaneously would be difficult to imagine as a possible cause for the simultaneous events. Self-quenching or homo-transfer between the fluorophores attached to different subunits of the channel should occur constantly, considering that the transition time constants of fluorophores lie in the nanosecond range. In this case, we would thus not see independent intensity changes or changes after bleaching of 1 fluorophore. We therefore set out to investigate more closely whether or not the simultaneous events could be explained by cooperative gating of the 4 subunits.
We fitted the fluorescence time traces to Markov models. Chakrapani et al. (24) used a cooperative final transition for the opening to fit their KcsA currents similar to the cooperative opening of the voltage-gated channels. Here, however, we assumed a more basic model, in which each subunit can exist in only 2 states: open and closed. We are excluding an a priori cooperative final step, because we are observing the movement of single subunits and not the actual conductance state of the channel. If the movement that we are observing with the fluorescence involved a single cooperative step, we would never observe the single subunits. Our model results in 3 fluorescence states: Open (dark), closed (bright), and bleached (dark). In the latter, we cannot determine the physical state of the channel (Fig. 4A). A channel with 4 subunits will have 5 equidistant fluorescence levels (see above and Fig. 4B). If one considers bleaching, the model expands to 15 states (Fig. 4C).
In the independent model, the channel is described by 3 parameters: the opening and closing rates α and β and the bleaching rate kB. The model was fitted to the time traces by using the QUB software (25) as described below. To increase the convergence of the model, we compared the bleaching rates found by the fitting routine with the time constant obtained from exponential fitting of the photobleaching time course from the entire field of view. We found a good agreement between the values (Table S1) so that we could subsequently fix the bleaching rate kB to the value obtained from the exponential fit. Nevertheless, this exponential fit had to be done for each image series, because the bleaching rate would be dependent on laser intensity, solutions (presence of reducing agents, oxygen saturation), or focusing. Although fixing kB increased the speed of the fitting and convergence, the other rate constants did not change significantly (see SI Text and Fig. S2).
Kinetic Analysis of Subunit Movement.
From the results of the QUB fits, we calculated the open probability of 1 subunit (Fig. 4E, filled symbols). As in the direct determination, the values had a relatively wide distribution due to the low number of events. At pH 6 and 7, the open probabilities calculated from the rate constants are overestimated. The reason is that at high pH values there are many traces with zero or very few events other than bleaching (Fig. 3B) because the channel is almost constantly closed, so that very few conformational changes are observed before bleaching of the fluorophores attached to the channel. Because the number of events was not sufficient to lead to a convergent fit, they could not contribute to the value of the open probability here. Nevertheless, from the direct determination, we know that the open probability of the channels without activity is close to zero.
We found a similar pH dependence as determined directly from the time traces, but with higher absolute open probability. Considering that pH 6 and 7 are overestimated, these values correspond well to the channel open probability that we determined for KcsA-E71A-Q119C from electrophysiological recordings in a PC bilayer (Fig. 4E, open symbols). One has to keep in mind, however, that the subunit open probability in the independent case has to be taken to the 4th power to calculate the channel open probability. For a highly cooperative case, the subunit and channel open probability are nearly identical.
If we calculate the dwell time constants from the transition rates α and β, we find very similar values as in the direct determination (4.9 ± 2.9 s and 3.6 ± 2 s for closed and open dwell time, respectively, at pH 5) confirming the slow gating of the bundle crossing. This shows that the model is accurately describing the channel kinetics.
Cooperative Gating Events.
Many traces could not be fitted with the independent model (Fig. 4C) largely because of the simultaneous events. To include a cooperativity between the subunits, we introduced a coupling constant C. This coupling constant increases the rate constant of opening and closing for all but the initial subunit (Fig. 4D). By using this coupling constant, the energy difference between 2 states was decreased by a factor ΔΔG = kT ln(C). This is, in other words, the coupling energy that the first subunit to open would have to overcome additionally. The subsequent steps would occur with equal rate constants.
The data were fitted with the independent model first (C = 1), and then with increasing value for the coupling constant (C = 1, 2.5, 5, 10, …) to determine the optimal coupling constant. This way, we did not change the number of free parameters so that we could directly compare the quality of the fit. Importantly, the introduction of the coupling constant led to convergence of time traces, for which we did not find a solution with the independent model. For time series that did converge with the independent model, introduction of a coupling factor improved the fit significantly. We found coupling factors that varied over the range from 1 to 80 with a mean value of 8.3 ± 14 for pH 3–5 (Fig. 4F). They showed slight pH dependence: Whereas the coupling remains constant between pH 4 and pH 6, it seems to be slightly stronger at pH 3 and weaker at pH 7. Considering the variance of the values and the low numbers of events per trace, we are reluctant to dwell into a more detailed discussion about pH dependence. However, these results indicate clearly that there is a direct interaction between the subunits that leads to cooperative opening and closing.
Gating Is Not Fluorophore Specific.
To increase the observation time and to verify that the observed effects are not fluorophore specific, we used a different dye, ATTO 565-maleimide (ATTO-565M), which turned out to be much more resistant against photobleaching. By using this dye, we increased the observation time ≈4- to 5-fold with a similar signal-to-noise ratio. The results from both the direct analysis of the time series and the model fits with ATTO-565M were very similar to the results we obtained with TMRM. The open probability of a subunit varied from 0.08 to 0.23 with a pK of 5.2 (Fig. S3a). The coupling constant varied from 1 to 50 with a mean value of 15.4 ± 18.3 for pH 3–4 (Fig. S3b). Because of the slow kinetics, the 4-fold increase of the observation time did not significantly decrease the variance. The results ensure, however, that our findings are not specifically generated by the fluorophore TMRM, but are also true for a completely different dye with different inherent properties (e.g., blinking).
Discussion
By fluorescently labeling the purified KcsA channel at the C terminus of TM2, we were able to monitor the opening and closing of the channel using single-molecule fluorescence spectroscopy. In our analysis of the movement of the 4 subunits, we required a coupling factor to explain the data, demonstrating that there is an energetic coupling between the subunits. Independent movement of single subunits, which was also observed, does not contradict an interaction between the subunits but shows that the coupling energy is not extremely high. We found a value for the coupling energy of up to 3.9–4.4 kT with a mean of 2–2.7 kT. One should keep in mind that the results include some blinking of the fluorophores (13%), but, because blinking is an intrinsic property of the single fluorophore, the 4 fluorophores blink independently. Therefore, if anything, the coupling constant would be decreased.
What could be the cause for the cooperative action of the subunits that we observed? In the literature, 2 different mechanisms have been identified that could lead to cooperative opening of the channels. Kariev et al. (18) suggested a water basket built from electrostatic interactions between the glutamines (Q119) near the helical bundle crossing. However, cysteine mutation and/or subsequent labeling of Q119C could destroy the water basket. Similarly, it is likely that labeling G116C could interfere with water basket formation. We did not find a significant difference between the Q119C-E71A and G116C-E71A mutants used in this study. Because both these labeled cysteine mutants do show cooperativity, this suggests 2 explanations: (i) our labeled mutants did not fully prevent water basket formation or (ii) water basket formation was fully prevented but it is not the only underlying mechanism of cooperativity. The second possibility is supported by the fact that we found cooperativity also at low pH, although basket formation is prevented by protonation of the glutamines. The disturbance of this proposed mechanism may also be responsible for the lower open probability that we observed.
Thompson et al. (5) identified in the pH sensor of KcsA (E118, E120, H25) an intersubunit interaction that may lead to cooperative opening of the channel. At low pH, protonation of H25 disturbs the interaction between E118 and E120 with R121 and R122 and reduces the energy barrier for subunit opening. The authors observed an increased occurrence of subconductance levels on mutating the pH sensor. Although this interaction is in agreement with the cooperative behavior that we found, it cannot fully explain it. In their model, the closed state is stabilized by electrostatic interaction between the glutamates (E118, E120) and arginines (R121, R122). Lowering of the pH would lead to an abolition of the interaction and should thus leave the subunits to act independently. Nevertheless, we find a slight decrease, if any, of the coupling constant at pH 7. Furthermore, whereas removal of the interaction by mutation increases the occurrence of subconductance levels (5), lowering of pH does not have the same effect, although the interaction would also be expected to be removed. We therefore have to postulate another intersubunit interaction that stabilizes the open position. One or more of the identified positions (E118, E120, H25) may still be involved in the putative interaction.
The cooperativity described here for the KcsA channels has been postulated and demonstrated for voltage-dependent channels, too. There, the 4 voltage sensors are activated followed by 1 cooperative opening. Although it has been suggested that it is actually the voltage sensors that act cooperatively (26), it may, in fact, be that the interaction between the S5 or S6 (TM1 or TM2) C termini persists in Shaker and that this indirectly restrains the S4 from entering its final position. Once the interaction is broken, the S4 are free to move, which could be the cooperative step described by Pathak et al. (26).
In our study, we did find both behaviors, independent as well as cooperative movement of the 4 subunits. It has been suggested that the occurrence of a partial opening of the channel is the cause for subconductance levels, and recently, the removal of an intersubunit interaction at the C terminus has been shown to increase occurrence of subconductance levels (5). Based on our findings, it is not correct to assume that every time only 1 subunit opens, the channel conducts, because in the electrophysiological recordings of KcsA the occurrence of subconductance levels is lower than observed here. It is possible, however, that at least 2 or 3 subunits must open to create sufficient space for ion conduction. Another possibility is that the movement of the helical bundle crossing is coupled to the activation/inactivation of the selectivity filter (4). The selectivity filter is open after a pH step, because the open probability at that instant is highest, but after opening of the bundle crossing, it evolves into the inactivated state (3, 27). This indicates that the movement of the bundle crossing influences the state of the selectivity filter. Thus, it may be that the selectivity filter is in a deactivated state and that several subunits have to open before the selectivity filter becomes conductive. Finally, we should also keep in mind that some of the channels in our preparations may be immobilized on the glass (Fig. 3B) and therefore reduce the observation of opening and cooperative movement. Such a possible influence of the glass surface would be removed in a black lipid bilayer.
Conclusions
Single-molecule fluorescence offers a powerful tool to uncover information that is hidden in an ensemble measurement. By direct observation of the subunit movement of KcsA using single-channel fluorescence spectroscopy, we found in this study that the subunits of KcsA act cooperatively with a coupling constant of 2–2.7 kT. To finally answer the question, whether a partial opening (just 1–3 subunits) causes subconductance levels, the present technique will have to be combined with electrophysiology so that the single-channel current and the single-channel fluorescence are measured simultaneously in a bilayer system that allows electrical control of the membrane potential.
Methods
Protein Purification and Reconstitution.
Escherichia coli strain M15 was transformed with KcsA-G116C-E71A-pQE32, KcsA-119C-E71A-pQE32, or KcsA-L90C-pQE32 and single colonies grown in 3-ml cultures for 7 h in the presence of 100 μg/ml Ampicillin and 25 μg/ml Kanamycin. Of the cultures, 0.5 ml was added to 1 L of LB containing the same antibiotics and grown overnight. Channel expression was induced by addition of 0.5 mM IPTG (Sigma-Aldrich) at T = 20 °C. The cells were harvested, lysed by passage through a French press at 20,000 psi, and the membranes isolated by using centrifugation (4, 28). The membranes were solubilized by using 2% N-dodecyl-β-maltoside (DDM) for 1 h at 4 °C and centrifuged at 100,000 × g for 30′ to remove any nonsolubilized particles. The channels were purified by metal affinity chromatography (Talon Superflow, Clontech). Before elution, the cysteines were reduced by 1 mM tris(2-carboxyethyl)phosphine (TCEP) (Pierce). The channels were labeled with TMRM (Invitrogen) or ATTO-565M (ATTO-TEC GmbH) in 10-fold molar excess overnight at 4 °C. Excess label was removed by washing on the metal affinity column. POPC or DPhPC (Avanti Polar Lipids) vesicles were prepared from chloroform stock by evaporating the solvent under a steady N2 stream and forming an emulsion in the desired buffer solution in the presence of 0.02% DDM by vigorous sonication. The lipids were mixed with the protein solution in a mass ratio of 100:1 at a final lipid concentration of 10 mg/ml. The detergent was removed by treatment with biobeads (SM2, Bio-Rad) for 3 successive rounds (2 h at room temperature, 2 h at room temperature, and 12 h at 4 °C). We used 100 mM KCl + 10 mM K3PO4 pH-adjusted with H3PO4 for all measurements.
Fluorescence Measurements of Supported Bilayer.
Borosilicate glass coverslips were washed by sonication for 30′ in a staining jar in the following order: Alconox (Fischer Scientific), KOH (1 M), acetone, methanol (filtered). Between each step, the coverslips were rinsed with H2O (MilliQ, filtered at 0.2 μm). Coverslips were stored in H2O and rinsed and dried under a steady N2 stream right before use. At the intensities used in the recordings (<1 mW), almost no background fluorescence was detected in the presence of buffer only. To adjust the protein concentration, 1.4 μl of the proteoliposome solution was mixed with an emulsion of 225 μl of POPC or DPhPC (2.5 mg/ml in KCl solution at the specified pH) and sonicated. The mixture was subject to 5 freeze–thaw cycles, to ensure homogenous mixing. 4 μl of that solution was then added to 400 μl of buffer on a clean coverslip. After 30–90 min, the vesicles formed a supported bilayer on the coverslip, and excess vesicles were carefully washed off with KCl buffer by using a micropipette.
Fluorescence was measured by using an inverted microscope (Axiovert 200, Zeiss). For excitation a 30-mW, 532-nm laser (World Star Tech) was used. Excitation light (<1 mW) was defocused to a diameter of ≈60–90 μm. Emission was collected with a 63× N.A. 1.4 objective (Zeiss) and filtered by a 610/75 nm emission filter in combination with a Z532 dichroic mirror (Chroma Technologies). Images were recorded with an EMCCD Camera (iXon+ 860BV, Andor Technology). For total internal reflection (TIR) measurements, a similar setup based on an Olympus microscope (IX71) was used. Emission was collected with a 100× N.A. 1.45 or 1.65 objective (Olympus America). For TIR measurements with the 1.65 N.A. objective, N-SF11 coverslips (no.1) were used to match the objective's higher index of refraction.
Data Analysis.
Images were first analyzed by using the AndorIQ program and an in-house routine written in Matlab (Mathworks). The spots were selected from the “averaged difference” (Fig. 1D). No further signal conditioning was applied. The averaged intensity of a 3 × 3 pixel square around the spot's center was plotted over time and further analyzed whether it met the selection criteria: only spots that showed a clear bleaching step at the end were considered to ensure that only single channels were observed. Occasionally very intense spots were found, which bleached in a simple exponential decay. These spots contained many fluorophores; therefore, we assumed they were aggregates in our preparation and we excluded them from further analysis. For kinetic analysis of the time courses, the QUB suite (25) was used. The discrete levels of the bleaching steps were found by eye by using the “grab” function. Open probability and dwell time histograms were obtained from the “Stat” routine. Before analyzing the open probability, the time course was divided into sections between each bleaching step. The subunit open probability Po was calculated from the probability of resting in the all closed state Pac: Po = 1 − Pac1/N (N = number of unbleached subunits). For further analysis the time courses were fitted to a hidden Markov model taking an eventual coupling constant and the bleaching of the single subunits into account (see below). The time series was idealized by using the IB realization routine. The idealized time series was then fitted to the model with the MIL (maximum interval likelihood) routine.
Design of Hidden Markov Model with Bleaching and Coupling.
To analyze whether coupling between the single subunits of the KcsA tetramer exists, the time courses were fitted to a hidden Markov model. This had to take into consideration the bleaching of the single subunits and a possible coupling constant. Fig. 4C shows the basic model that only convolutes the bleaching with 4 independently acting subunits. Each subunit (Fig. 4A) may rest in the closed (fluorescent) or open (dark) conformation, which are assigned different, equidistant fluorescence levels. Thus, the “unbleached” channel has 5 different fluorescence levels and rate constants as shown in Fig. 4B. Each subunit may undergo bleaching at a rate kB. If the 4 subunits are independent, bleaching of 1 subunit would merely reduce the number of available fluorescence levels and therefore also reduce the rate constants, as if only 3 subunits were present and so forth (Fig. 4C).
Interaction between the subunits was considered by introducing a coupling constant C in the model, which changes the rate constants in the partly opened channel (Fig. 4D). The coupling constant reduces the energy for opening of all subunits but the first by kT ln(C) (see below). The coupling is thus removed by the opening of the first subunit [water basket (18)]. In contrast to the independent model, the state of the bleached subunit has to be taken into account when considering the remaining subunits. If, for instance, 1 subunit is already bleached and closed, the opening probability for any of the other 3 would be 3·α (Fig. 4D). If, however, the bleached subunit is already open, the probability for the other 3 would increase by the coupling factor C (3·α·C). The model expands to 31 states.
The coupling energy was determined from the difference of energy the first and the second subunit have to overcome:
Supplementary Material
Acknowledgments.
We thank Mireille Marsolais and Élise Faure for technical assistance. This work was supported by the Canadian Institutes of Health Research Grant MOP-81351 (to R.B.), Natural Sciences and Engineering Research Council of Canada Grant 327201DG (to R.B.), Cancer Research Chair 202965 (to R.B.), and National Institutes of Health Grants GM30376 and MH078822 (to F.B.). Infrastructure was supported by the Canada Foundation for Innovation Grant 202965 (to R.B.). H.M. is supported by the Fonds québécois de la Recherche sur la Nature et les Technologies. H.C.H. is supported by a National Institutes of Health Fellowship F31NS054532.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807056106/DCSupplemental.
References
- 1.Doyle DA, et al. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu H, et al. Global twisting motion of single molecular KcsA potassium channel upon gating. Cell. 2008;132:67–78. doi: 10.1016/j.cell.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 3.Cordero-Morales JF, et al. Molecular determinants of gating at the potassium-channel selectivity filter. Nat Struct Mol Biol. 2006;13:311–318. doi: 10.1038/nsmb1069. [DOI] [PubMed] [Google Scholar]
- 4.Blunck R, Cordero-Morales JF, Cuello LG, Perozo E, Bezanilla F. Detection of the opening of the bundle crossing in KcsA with fluorescence lifetime spectroscopy reveals the existence of two gates for ion conduction. J Gen Physiol. 2006;128:569–581. doi: 10.1085/jgp.200609638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson AN, Posson DJ, Parsa PV, Nimigean CM. Molecular mechanism of pH sensing in KcsA potassium channels. Proc Natl Acad Sci USA. 2008;105:6900–6905. doi: 10.1073/pnas.0800873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeuchi K, Takahashi H, Kawano S, Shimada I. Identification and characterization of the slowly exchanging pH-dependent conformational rearrangement in KcsA. J Biol Chem. 2007;282:15179–15186. doi: 10.1074/jbc.M608264200. [DOI] [PubMed] [Google Scholar]
- 7.Miloshevsky GV, Jordan PC. Open-state conformation of the KcsA K+ channel: Monte Carlo normal mode following simulations. Structure. 2007;15:1654–1662. doi: 10.1016/j.str.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Cordero-Morales JF, Cuello LG, Perozo E. Voltage-dependent gating at the KcsA selectivity filter. Nat Struct Mol Biol. 2006;13:319–322. doi: 10.1038/nsmb1070. [DOI] [PubMed] [Google Scholar]
- 9.Cuello LG, Romero JG, Cortes DM, Perozo E. pH-dependent gating in the Streptomyces lividans K+ channel. Biochemistry. 1998;37:3229–3236. doi: 10.1021/bi972997x. [DOI] [PubMed] [Google Scholar]
- 10.Meuser D, Splitt H, Wagner R, Schrempf H. Exploring the open pore of the potassium channel from Streptomyces lividans. FEBS Lett. 1999;462:447–452. doi: 10.1016/s0014-5793(99)01579-3. [DOI] [PubMed] [Google Scholar]
- 11.Splitt H, Meuser D, Borovok I, Betzler M, Schrempf H. Pore mutations affecting tetrameric assembly and functioning of the potassium channel KcsA from Streptomyces lividans. FEBS Lett. 2000;472:83–87. doi: 10.1016/s0014-5793(00)01429-0. [DOI] [PubMed] [Google Scholar]
- 12.Mathur R, Zheng J, Yan Y, Sigworth FJ. Role of the S3–S4 linker in Shaker potassium channel activation. J Gen Physiol. 1997;109:191–199. doi: 10.1085/jgp.109.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng J, Sigworth FJ. Intermediate conductances during deactivation of heteromultimeric Shaker potassium channels. J Gen Physiol. 1998;112:457–474. doi: 10.1085/jgp.112.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapman ML, VanDongen AM. K channel subconductance levels result from heteromeric pore conformations. J Gen Physiol. 2005;126:87–103. doi: 10.1085/jgp.200509253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chauhan-Patel R, Spruce AE. Characterization of single inward rectifier potassium channels from embryonic Xenopus laevis myocytes. J Membr Biol. 1997;158:265–274. doi: 10.1007/s002329900263. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda H, Stanfield PR. Single inwardly rectifying potassium channels in cultured muscle cells from rat and mouse. J Physiol. 1989;414:111–124. doi: 10.1113/jphysiol.1989.sp017679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie LH, John SA, Ribalet B, Weiss JN. Phosphatidylinositol-4,5-bisphosphate (PIP2) regulation of strong inward rectifier Kir2.1 channels: Multilevel positive cooperativity. J Physiol. 2008;586:1833–1848. doi: 10.1113/jphysiol.2007.147868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kariev AM, Znamenskiy VS, Green ME. Quantum mechanical calculations of charge effects on gating the KcsA channel. Biochim Biophys Acta. 2007;1768:1218–1229. doi: 10.1016/j.bbamem.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulbrich MH, Isacoff EY. Subunit counting in membrane-bound proteins. Nat Methods. 2007;4:319–321. doi: 10.1038/NMETH1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ha T, Enderle T, Chemla DS, Selvin PR, Weiss S. Quantum jumps of single molecules at room temperature. Chem Phys Lett. 1997;271:1–5. [Google Scholar]
- 21.Widengren J, Schwille P. Characterization of photoinduced isomerization and back-isomerization of the cyanine dye Cy5 by fluorescence correlation spectroscopy. J Phys Chem A. 2000;104:6416–6428. [Google Scholar]
- 22.Zondervan R, Kulzer F, Orlinskii SB, Orrit M. Photoblinking of rhodamine 6G in poly(vinyl alcohol): Radical dark state formed through the triplet. J Phys Chem A. 2003;107:6770–6776. [Google Scholar]
- 23.Sauer M, Drexhage KH, Lieberwirth U, Muller R, Nord S, Zander C, et al. Dynamics of the electron transfer reaction between an oxazine dye and DNA oligonucleotides monitored on the single-molecule level. Chem Phys Lett. 1998;284:153–163. [Google Scholar]
- 24.Chakrapani S, Cordero-Morales JF, Perozo E. A quantitative description of KcsA gating II: Single-channel currents. J Gen Physiol. 2007;130:479–496. doi: 10.1085/jgp.200709844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin F, Milescu L, Qiong F, Nicolai C, Bannen J. QUB suite. Buffalo, NY: State University of New York; 1997. [Google Scholar]
- 26.Pathak M, Kurtz L, Tombola F, Isacoff E. The cooperative voltage sensor motion that gates a potassium channel. J Gen Physiol. 2005;125:57–69. doi: 10.1085/jgp.200409197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakrapani S, Cordero-Morales JF, Perozo E. A quantitative description of KcsA gating I: Macroscopic currents. J Gen Physiol. 2007;130:465–478. doi: 10.1085/jgp.200709843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson J, et al. Distance measurements reveal a common topology of prokaryotic voltage-gated ion channels in the lipid bilayer. Proc Natl Acad Sci USA. 2006;103:15865–15870. doi: 10.1073/pnas.0607532103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.