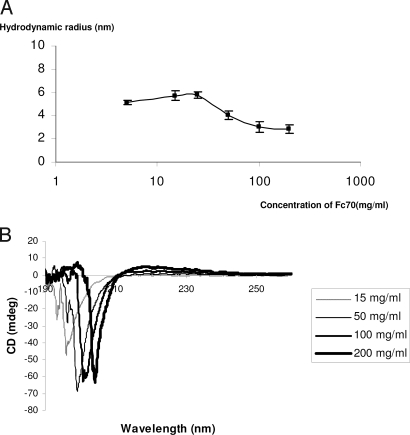
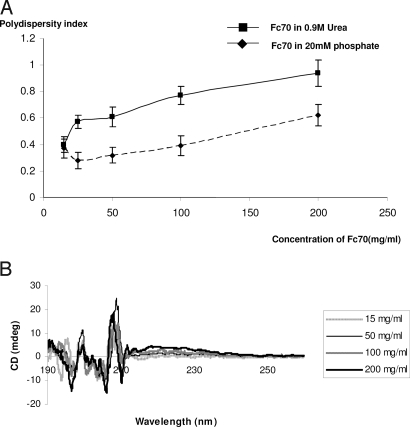
Fascinating evidence was presented recently that monocrowding with Ficoll 70 (Fc70) improves the refolding speed of VlsE that was unfolded in urea (1). This demonstrated the power of macromolecular crowding as present in cellular interiors. However, we noted that the Fc70 concentrations used in this study (1) are high enough to cause self-crowding (2), meaning compaction of the crowder, thus diminishing its crowding capacity. In fact, as evidenced with dynamic light scattering (DLS), the hydrodynamic radii of Fc molecules begin to shrink with increasing crowder concentrations (Fig. 1A), and CD spectroscopy reveals structural changes (Fig. 1B). Furthermore, DLS in 0.9 M urea-phosphate buffer (1) suggests a heterogeneous size distribution of Fc, increasing with its concentration (Fig. 2A). Correspondingly, CD shows urea-dependent structural changes of Fc70 (Fig. 2B). It is conceivable that in the system used to study protein folding kinetics (1) concentrations below the self-crowding threshold would give equally dramatic, or greater, effects as shown earlier by us in another context (3). These lower concentrations would still represent fractional volume occupancies in the biological range (4). Because monocrowding is practically absent in biological systems, we have previously applied mixed macromolecular crowding (each component below its self-crowding concentration) to PCR. We observed a stabilization of Fc70 in such a mixture. Furthermore, we found Taq polymerase to be protected against heat denaturation and its catalytic activity augmented (3). Our experience suggests that mixed macromolecular crowding as outlined above would allow an even greater appreciation of the impact of macromolecular crowding on biological processes.
Fig. 1.
Size and structural changes in Fc70. (A) Decrease of hydrodynamic radius with increasing concentrations of Fc70 in 20 mM phosphate buffer. (B) CD spectra demonstrate shift of the maximum absorbance to the right with increasing Fc concentrations.
Fig. 2.
Urea-induced effects on Fc70. (A) Large increase in polydispersity of Fc70 molecules in 0.9 M urea/20 mM phosphate buffer deduced from DLS. (B) CD spectra indicate significant effects of urea on Fc70 structure.
Footnotes
The authors declare no conflict of interest.
References
- 1.Homouz D, Perham M, Samiotakis A, Cheung MS, Wittung-Stafshede P. Crowded, cell-like environment induces shape changes in aspherical protein. Proc Natl Acad Sci USA. 2008;105:11754–11759. doi: 10.1073/pnas.0803672105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harve KS, Lareu RR, Rajagopalan R, Raghunath M. Macromolecular crowding in biological systems: Dynamic light scattering (DLS) to quantify the excluded volume effect. Biophys Rev Lett. 2006;1:317–325. [Google Scholar]
- 3.Lareu RR, Harve KS, Raghunath M. Emulating a crowded intracellular environment in vitro dramatically improves RT-PCR performance. Biochem Biophys Res Commun. 2007;363:171–177. doi: 10.1016/j.bbrc.2007.08.156. [DOI] [PubMed] [Google Scholar]
- 4.Ellis RJ. Macromolecular crowding: Obvious but under-appreciated. Trends Biochem Sci. 2001;26:597–604. doi: 10.1016/s0968-0004(01)01938-7. [DOI] [PubMed] [Google Scholar]