Abstract
Suppression of T-cell responses by host-derived regulatory factors is a key event leading to viral persistence. Antibody blockade of either IL-10 or programmed death-ligand 1 (PD-L1) during viral persistence enhances T-cell function and reduces viral titers. Because blockade of these immunoregulatory networks represents a powerful approach to establish immune control during persistent infection, it is important to determine whether these immunoinhibitory factors act independently or jointly and if combined blockade of these factors further enhances T-cell immunity and viral clearance. Herein, we demonstrate that the IL-10 and PD-L1 immunosuppressive pathways are mechanistically distinct. As a result, simultaneous blockade of IL-10 and PD-L1 was significantly more effective in restoring antiviral T-cell responses than blockade of either alone, and led to substantially enhanced control of an established persistent viral infection. Thus, combinatorial blockade of multiple immune-regulatory molecules may ultimately restore the T-cell responses required to tip the balance from viral persistence to immune-mediated control or elimination of persistent infection.
Keywords: T-cell exhaustion, virus persistence, functional restoration, immune regulation, immunosuppression
Clearance of viral infections is dependent on functional T-cell responses. However, following some viral infections, T cells rapidly lose their ability to proliferate, to lyse virally infected cells, and to produce multiple effector molecules (1–4). The loss of these polyfunctional T-cell responses (termed “exhaustion”) directly leads to the failure to eliminate the virus and terminate the infection. Sustained T-cell activity prevents persistent viral infection. Prolonged T-cell responses strongly correlate with acute clearance of hepatitis C virus (HCV) infection and transfer of functional virus-specific CD4 and CD8 T cells completely eliminates an established persistent viral infection (5–10). In addition to viral components (11), the host-derived suppressive cytokine IL-10 and the programmed death-1 (PD-1)/PD-ligand (L) 1 inhibitory pathways actively suppress T-cell responses, allowing viral persistence (9, 12, 13). Antibody blockade to relieve IL-10 or PD-L1-mediated inhibition in vivo rapidly enhances T-cell function and reduces viral replication(9, 12, 13). Thus, the re-establishment of functional T-cell responses leads to increased control of persistent viral infection, but in many cases an even further enhancement of immunity is required to completely eliminate infection (5, 9, 12–14).
The suppressive environment maintained by IL-10 and PD-L1 not only limits T-cell responses to infection, but also inhibits the ability to stimulate immunity through vaccination (15, 16). Yet, despite the profound impact IL-10 and PD-L1 have on T-cell immunity, little is known about the actual mechanisms used to suppress the immune response. Consequently, whether these suppressive factors induce each other and function through a linear pathway or whether they are invoked and function through distinct mechanisms is unclear. Defining these mechanisms of suppression is necessary to determine whether simultaneous blockade of IL-10 and PD-L1 will further enhance T-cell immunity and more effectively control persistent viral replication. Here, we demonstrate that IL-10 and PD-L1 suppress antiviral T-cell activity via separate pathways and consequently, simultaneous blockade of IL-10 and PD-L1 dramatically increases T-cell responses over that seen by neutralizing either molecule alone. The combinatorial blockade of both IL-10 and PD-L1 rapidly eliminates persistent virus infection.
Results
Distinct Mechanisms of IL-10 and PD-1/PD-L1 Immunosuppression During Persistent Viral Infection.
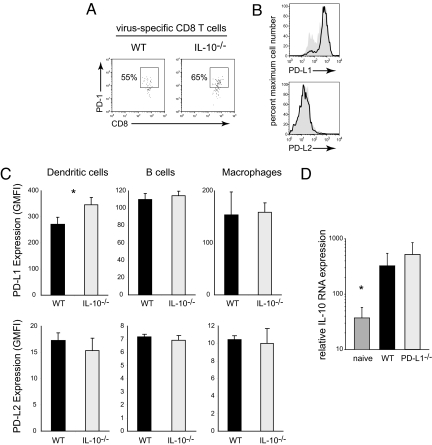
To determine the functional relationship between IL-10 and the PD-1/PD-L1 pathways during persistent viral infection, WT C57BL/6 mice or C57BL/6 mice genetically deficient in IL-10 (IL-10 KO) or PD-L1 (PD-L1 KO) were infected with lymphocytic choriomeningitis virus (LCMV) Clone 13 (Cl 13). Infection with LCMV-Cl 13 generates a persistent infection that rapidly aborts T-cell function by inducing the expression of host-immunosuppressive factors (9, 12, 13). To evaluate if the increased expression of IL-10 triggered the up-regulation of PD-1 on virus-specific CD8 T cells or PD-L1 on antigen-presenting cells (APC) (i.e., dendritic cells, B cells, macrophages), WT C57BL/6 and IL-10-deficient mice were infected with LCMV. Because IL-10-deficient mice rapidly clear the otherwise persistent LCMV-Cl 13 infection, PD-1/PD-L1 levels were analyzed 5 days after LCMV-Cl 13 infection at a time when T-cell responses and viral titers are similarly high in WT mice and IL-10-deficient mice (serum viral titers in WT: 2.5 × 104 ± 2.2 × 104 versus IL-10 KO: 7.1 × 103 ± 4.9 × 103; n = four mice per group; P = 0.22). The similar virus titers enable differentiation of factors induced by IL-10 versus those triggered in response to heightened virus replication despite the acute clearance of LCMV-Cl 13 in IL-10 KO mice (9, 13). Five days after infection, PD-1 expression was similar on virus-specific CD8 T cells in WT and IL-10-deficient mice (Fig. 1A), indicating that IL-10 is not required for the up-regulation of PD-1 on T cells during viral infection.
Fig. 1.
IL-10 and PD-L1 act through distinct pathways to suppress antiviral immunity. (A) WT and IL-10 KO mice were infected with LCMV-Cl 13 and the level of PD-1 expression on virus-specific CD8 T cells was quantified on day 5 after infection (at a time when viral titers were similarly high in the two mouse strains). Flow plots are gated on CD8 and LCMV-GP33–41 tetramer+ cells to specifically identify virus-specific T cells. Data are representative of four mice per group and of two experiments. (B) PD-L1 (Upper) and PD-L2 (Lower) expression on dendritic cells on day 5 after LCMV-Cl 13 infection of WT (gray) and IL-10 KO (black line) mice. (C) Bar graphs demonstrate the geometric mean fluorescence intensity (GMFI) of PD-L1 (Upper) and PD-L2 (Lower) expression on dendritic cells, B cells, and macrophages in WT and IL-10 KO mice. Bars indicate the average ± SD of four to five mice per group. *, P < 0.05. (D) IL-10 RNA expression was quantified in the spleens of uninfected WT mice (naïve) and in WT and PD-L1 KO mice on day 5 after LCMV-Cl 13 infection (at a time when viral titers were equivalent in the WT and knockout mice). Bars indicate the average ± SD of five to six mice per group. *, P < 0.05 compared to LCMV-Cl 13 infected mice.
In addition to increased PD-1 on T cells, expression of its ligand PD-L1 is an important determinant of immunosuppression (12). To determine whether the IL-10 produced in response to infection induces PD-L1 up-regulation, we assessed PD-L1 expression on multiple APC subsets during LCMV-Cl 13 infection. In the absence of IL-10, PD-L1 expression was modestly elevated on dendritic cells (DCs) (Fig. 1B) and expressed similarly by B cells and macrophages compared to WT mice (see Fig. 1 B and C), suggesting a slight potential compensation by one immune-regulatory pathway in the absence of another. Although PD-L1 is the dominant suppressive ligand for PD-1 during persistent infection, we additionally measured expression of the second PD-1 ligand, PD-L2. PD-L2 expression was minimal by APC, but was nonetheless expressed at the same level in WT mice and IL-10-deficient mice (see Fig. 1 B and C), indicating that PD-L1 and PD-L2 expression are not initially increased because of the high levels of IL-10 following infection.
PD-1/PD-L1 signaling may instead trigger increased IL-10 expression to suppress T-cell immunity. To test the contribution of PD-L1 signaling on IL-10 induction, IL-10 RNA expression was quantified in PD-L1 KO mice. Similar to IL-10-deficient mice, on day 5 after LCMV-Cl 13 infection virus titers are similar in PD-L1 KO mice and WT mice (serum viral titers in WT: 2.5 × 104 ± 2.2 × 104 versus PD-L1 KO: 6.3 × 103 ± 3 × 103; n = four mice per group; P = 0.23). The absence of PD-L1 signaling did not significantly alter IL-10 expression in the spleen (Fig. 1D). Thus, in response to infection with a persistent virus, IL-10 and PD-1/PD-L1 represent separate pathways of immunosuppression.
Dual IL-10R/PD-L1 Blockade Further Enhances Polyfunctional T-Cell Activity.
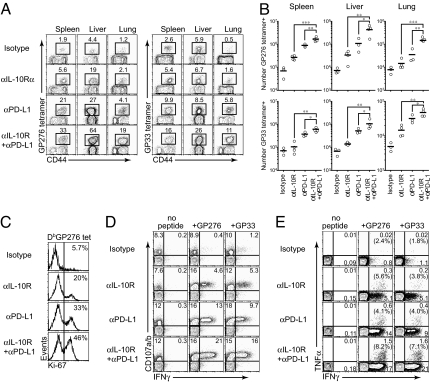
The distinct pathways of IL-10 and PD-1/PD-L1 during persistent viral infection suggest that inhibition of both suppressive factors could substantially boost T-cell immunity. To determine whether IL-10R and PD-L1 coblockade further enhances antiviral T-cell activity compared to either treatment alone, WT mice were depleted of CD4 T-cells and infected with LCMV-Cl 13 to generate a persistent infection. Beginning on day 49 after infection, blocking-antibody treatment was initiated. Mice were treated with either (i) isotype control antibody, (ii) IL-10R-blocking treatment antibody alone, (iii) PD-L1-blocking treatment alone, or (iv) dual therapy with both IL-10R and PD-L1-blocking antibodies. Mice received antibody every 3 days for a total of five treatments (i.e., spanning a period of 12 days). Antiviral T-cell responses were then quantified 3 days after the completion of therapy. Compared with isotype, individual treatment with either anti-IL-10R or anti-PD-L1-blocking antibodies significantly elevated the frequency and number of tetramer-positive (virus-specific) CD8 T cells in lymphoid (spleen) and nonlymphoid (liver and lung) tissues (Fig. 2 A and B). Even more impressive, the simultaneous administration of IL-10R and PD-L1-blocking antibodies further increased the frequency and the absolute number of tetramer+, virus-specific CD8 T cells in multiple tissue compartments and against both dominant (GP33–41) and subdominant (GP276–286) LCMV epitopes (see Fig. 2 A and B). Furthermore, dual blockade increased the frequency of actively proliferating Ki67+ virus-specific CD8 T cells when compared to isotype control or either antibody treatment alone (Fig. 2C), suggesting that the increased number of virus-specific CD8 T cells following IL-10 or PD-L1 blockade is because of enhanced proliferation.
Fig. 2.
Blockade of multiple immune regulatory molecules further enhances T-cell immunity in the absence of CD4 T-cell help when compared to either treatment alone. (A) Mice were depleted of CD4 T cells immediately before LCMV-Cl 13 infection and the frequency of LCMV-GP276–286 and LCMV-GP33–41 tetramer-positive cells was measured in the indicated tissues 3 days following the final antibody treatment. Dot plots are gated on CD8 T cells and the number in each plot indicates the frequency of tetramer+ cells in the spleen, liver, or lung. Data are representative of three to four mice per group and of two experiments. (B) Total number of LCMV-GP276–286 and LCMV-GP33–41 tetramer-positive cells in the indicated tissues was calculated based on (A). *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) The mice treated with antibodies were sacrificed at 1 week after initial treatment. Ki-67 expression was observed on DbGP276–286 tetramer-positive cells. The numbers in each plot indicate the frequency of Ki-67-positive cells. Data are representative of two independent experiments. (D) The ability of LCMV-specific CD8+ T cells to produce IFNγ and degranulate was measured 3 days following the completion of therapy. Splenocytes were stimulated with the indicated peptides in the presence of αCD107a/b antibodies and then costained for IFNγ. Dot plots are gated on CD8 T cells and the numbers represent the frequency of positive cells in each quadrant. Data are representative of two independent experiments. (E) Flow plots illustrate the frequency of TNFα and IFNγ producing GP33–41 and GP276–286 specific CD8+ T cells in the spleen 3 days after the cessation of therapy. Dot plots are gated on CD8 T cells and the numbers represent the frequency of positive cells in each quadrant. The numbers in parentheses indicate the frequency of cells that express both TNFα and IFNγ. Data are representative of two independent experiments.
In addition to increasing the total number of virus-specific cells, dual blockade also increased T-cell function. When administered in combination, IL-10R/PD-L1 coblockade increased the frequency of IFNγ-producing CD8 T cells compared to either treatment alone or with isotype control antibody (Fig. 2D). Importantly, dual blockade elevated the frequency of polyfunctional CD8 T cells able to simultaneously produce both IFNγ and TNFα (Fig. 2E). In these studies, CD4 T cells were depleted before infection to enhance the magnitude of CD8 T-cell exhaustion and provide a situation wherein persistent Cl 13 infection is never resolved and is maintained for life (17, 18). This enabled analyses of the ability of each treatment to restore T-cell function in a harsher immune environment (i.e., prolonged and elevated viral replication) and after extended periods of exhaustion that not achievable in non-CD4-depleted mice (12). However, similar results were observed in non-CD4-depleted mice when antibody treatments were initiated on day 25 after infection (data not shown). Thus, combination blockade of IL-10 and PD-L1 activity effectively increased the amount and functionality of virus-specific CD8 T cells during persistent infection.
IL-10/PD-L1 Coblockade Restores Function to Previously Exhausted T-Cells.
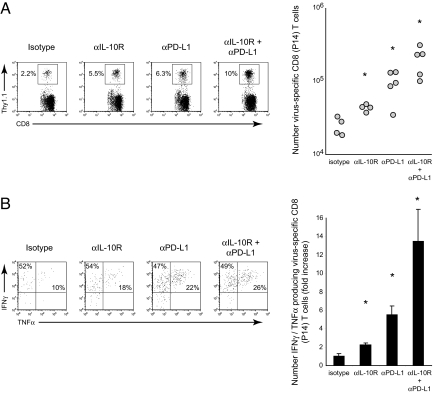
Although functional antiviral T-cell responses are clearly elevated by dual IL-10R/PD-L1 blockade, whether activity is being resurrected from previously exhausted T-cells or de novo activation of new thymic emigrants is unclear. To address this issue, naive Thy1.1+ TcR tg LCMV-GP33–41-specific CD8 T-cells were adoptively transferred into Thy1.2+ C57BL/6 mice before infection. Two days following cell transfer, the mice were subsequently infected with LCMV-Cl 13 and treated with single or dual antibody therapy, beginning on day 25 after infection. The cotransfer enabled analysis of LCMV-specific T cells that were present from the beginning of infection and that subsequently became exhausted during viral persistence. Importantly, the transgenic LCMV-specific CD8 T cells could be distinguished from endogenous (i.e., host-derived) antiviral T cells based on Thy1.1 versus Thy1.2 expression, excluding analysis of new thymic emigrants. Similar to their endogenous counterparts (see Fig. 2), dual IL-10R/PD-L1 blockade further increased the frequency and number of previously exhausted virus-specific T cells (Fig. 3A). All antibody blockade treatments substantially increased the frequency of IFNγ/TNFα-producing cells compared to isotype antibody treatment, although similar increases in the percentage of IFNγ/TNFα-producing cells was observed following IL-10R and PD-L1 blockade alone or in combination (Fig. 3B). However, combined with the increase in the number of virus-specific T cells following dual IL-10R/PD-L1 blockade, there was a dramatic increase in the absolute number of polyfunctional, IFNγ/TNFα-producing cells by over 12-fold, compared to isotype treatment, and over 2.5- to 6-fold compared with PD-L1 or IL-10R treatment alone, respectively (see Fig. 3B). Thus, dual IL-10R/PD-L1 blockade restores multiple antiviral functions to previously exhausted T-cells.
Fig. 3.
Dual IL-10R/PD-L1 blockade restores function to previously exhausted T cells. (A) LCMV-Cl 13-infected mice were treated with (i) isotype control antibody, (ii) anti-IL-10R-blocking antibody alone, (iii) anti-PD-L1-blocking antibody alone, or (iv) cotreated with anti-IL-10R and anti-PD-L1-blocking antibodies. Antibody therapy was initiated on day 25 after infection and administered every 3 days for a total of five treatments. The flow plots illustrate the frequency of Thy1.1+ tg virus-specific CD8 T cells (P14 cells) in the spleen on day 40 after LCMV-Cl 13 infection (i.e., day 3 after treatment). The graph on the right illustrates the number of P14 cells following each treatment regimen. Circles represent individual mice within each group and the line indicates the average for each group. *, P < 0.05 compared to all other treatment groups. Data are representative of four to five mice per group and of two experiments. (B) Flow plots illustrate the percentage of IFNγ- and TNFα-producing P14 cells in the spleen on day 40 after LCMV-Cl 13 infection (i.e., day 3 after treatment). The bar graph represents the average fold increase ± SD in the number of TNFα-producing P14 cells in each treatment group compared to isotype treatment (which is set to 1). *, P < 0.05 compared to all other treatment groups. Data are from the same experiment as shown in (A) and are representative of four to five mice per group and of two experiments.
Elimination of Persistent Viral Infection by Dual IL-10R/PD-L1 Blockade.
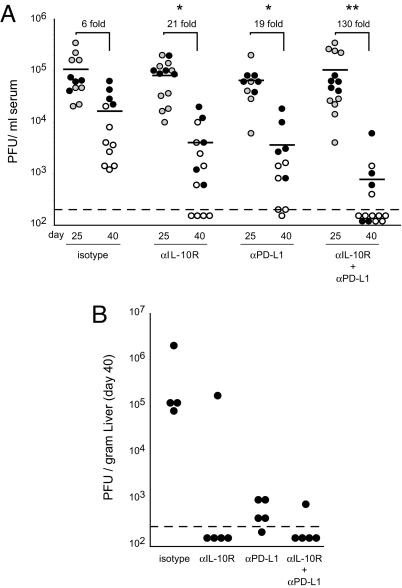
Based on the critical role functional T cells play in controlling persistent viral infection (5), we next determined if the heightened T-cell responses induced by dual IL-10R/PD-L1 blockade also led to an enhanced ability to control persistent virus replication. Before treatment, serum virus titers were similar in all groups (Fig. 4A). Following the 2-week therapeutic regimen, individual IL-10R or PD-L1 therapies each led to a 21-fold and 19-fold reduction, respectively, in virus titers compared to isotype treatment (see Fig. 4A). Even more impressive, coadministration of IL-10R and PD-L1 blockade induced a highly significant 130-fold decrease in serum virus titers, with the majority of mice having undetectable levels of viral replication (see Fig. 4A). Similarly, with the exception of one mouse that did not respond to anti-IL-10R treatment alone, viral titers were reduced by over two to three logs in the livers of antibody-treated animals compared to isotype control treatment (Fig. 4B), indicating systemic control of virus replication. Thus, dual IL-10R/PD-L1 blockade enables rapid and enhanced control of persistent infection.
Fig. 4.
Enhanced control of persistent viral infection following dual IL-10R/PD-L1 blockade. (A) LCMV-Cl 13-infected mice were treated with the indicated antibodies and serum viral titers were quantified on day 25 (gray circles, before treatment) and day 40 (white circles, 3 days following cessation of therapy). Each circle represents a single mouse within each group and the graph contains data from three experiments. The black circles indicate mice in which liver viral titers were quantified in parallel, as shown in (B). The dashed line indicates the level of detection of the assay (200 PFU/ml serum). The number above each group represents the average fold decrease in virus titers between day 25 and day 40. *, significant (P < 0.05) decrease in viral titers compared to isotype treatment. **, significant (P < 0.05) decrease in virus titers compared to all other treatment groups. (B) Liver virus titers were quantified on day 40 after LCMV-Cl 13 infection (3 days following the completion of antibody therapies).
Discussion
Understanding the complex mechanisms that regulate T-cell immunity is crucial for the design of T-cell regenerative therapies to purge an established persistent viral infection. Herein, we make the discovery that two of the dominant factors that suppress antiviral immunity during viral persistence operate through distinct pathways to restrain T-cell activity. Interestingly, in IL-10-deficient mice, PD-L1 expression was increased on DCs, suggesting that in the absence of one regulatory network the immune system may compensate by increasing the expression of other regulatory factors to suppress errant immune responses and autoimmunity. Alternatively, in the absence of IL-10, the elevated level of immune activation (i.e., type I and type II interferons) following LCMV-Cl 13 infection may enhance PD-L1 on DCs (19). The precise factors that differentially regulate IL-10 and PD-L1 expression are under investigation. However, as a result of using different pathways, combination therapy that neutralized both of these immunosuppressive pathways dramatically restored and boosted antiviral T-cell activity to control an established, persistent viral infection as compared to blockade of either pathway alone.
Virus-specific T cells proliferate and simultaneously produce multiple effector molecules in response to viral infection. The ability of T cells to simultaneously possess multiple antiviral effector functions is an indicator of the efficacy of the immune response and the loss of these polyfunctional T cells is a key event on the path to viral persistence (3, 9, 13, 20). The level of polyfunctionality in the virus-specific T-cell repertoire is correlated with acute clearance of HCV infection and enhanced control of HIV replication (21–24), suggesting that therapeutic strategies that restore polyfuntionality to T cells may be more effective in fighting viral infection. One of the most striking impacts of dual IL-10R/PD-L1 blockade is the enhanced frequency and particularly the increased number of polyfunctional virus-specific T cells able to proliferate and produce multiple effector cytokines in response to viral antigen. It is interesting that the ultimate increase in the number of virus-specific CD8 T cells following IL-10R and PD-L1 blockade alone or in combination was consistent with the level of proliferation induced by each treatment (i.e., Ki67 staining) (see Fig. 3). On the other hand, IL-10R and/or PD-L1 blockade only modestly increased the frequency of TNFα-producing cells within the total virus-specific CD8 T-cell population (i.e., IFNγ-producing cells), suggesting that the IL-10 and PD-L1 regulatory pathways function primarily by inhibiting T-cell proliferation to antigen stimulation. By alleviating IL-10 and PD-L1-mediated immunosuppression, T cells are able to proliferate, thereby increasing the size of the polyfunctional virus-specific T-cell population. Thus, the dual blockade allowed the rapid re-establishment of a large antiviral effector population, well equipped to fight and control infection. It is particularly impressive that following only 2 weeks of the dual blockade therapy (i.e., from the initiation to the completion of therapy), previously exhausted T-cells expanded and regained a level of functional activity capable of in many cases decreasing viral replication to undetectable levels. Thus, restoring T-cell immunity can occur rapidly and effectively once the dominant suppressive factors are identified and then neutralized.
The rapid restoration of T-cell function following dual IL-10R/PD-L1 blockade suggests that the antiviral immune response is constantly maintained on a fine line between exhaustion and productive immunity. Neutralization of a single factor restores T-cell function to control virus replication to some degree, but the entire potential of the T-cell response is still being restrained. Because multiple distinct immunoregulatory pathways are implemented to suppress T-cell activity, therapies that target different suppressive factors can be combined to further unleash T-cell responses. Importantly, the resulting increase in T-cell functionality induces a potentially greater ability to control and eliminate virus replication. One concern that arises by inhibiting these dominant immunoregulatory pathways is that they may also unleash undesired T-cell responses and autoimmunity. These pathways are instituted to prevent tissue destruction by self-reactive T cells that may have escaped thymic deletion, and mice lacking IL-10 or PD-1 can develop various forms of autoimmune disease (25–27). Blocking the negative regulation implemented by IL-10 and PD-L1 restores antiviral immunity to control persistent virus replication, but the suppression of self-reactive T cells may also be alleviated. In our studies, no overt signs of autoimmunity were observed following IL-10R or PD-L1 blockade, likely because of the short duration of treatment, and treated mice lived normal life-spans. Thus, future strategies to treat established, persistent viral infections by blocking the negative immune-regulatory pathways need to weigh the potential acceptable risk with the benefit to controlling viral replication.
Because IL-10 and PD-L1 are increased during many persistent viral infections, our data suggest that future therapies incorporating blockade of multiple suppressive factors may rapidly reconstitute immunity and control persistent viral infections. An exciting application for immunomodulatory therapies is chronic HCV infection, in which both IL-10 and PD-1 levels are increased, clearance relies on robust antiviral T-cell activity, and no known latent reservoir for the virus exists (28). Indeed, in vitro blockade of either IL-10 or PD-L1 increased HCV-specific T-cell responses (29–31), suggesting that these therapies may effectively restore and amplify antiviral T-cell activity to a level sufficient to purge infection. Even in situations such as HIV, CMV, and EBV, where a long-lived, latent reservoir does exist and IL-10/PD-1 levels are elevated (28), a similar therapeutic approach targeting multiple host-derived regulatory factors could restore and sustain T-cell activity, in effect generating a large effector T-cell population and prolonged control of viral replication. Because these therapies do not directly target a specific pathogen, they are amenable to fight multiple persistent infections and would be less susceptible to virus mutation, escape, and selection, a problem for therapies that directly target viral gene products.
Our finding that immunosuppressive factors function through multiple pathways suggests that they may also function distinctly on different cell types (i.e., CD4 versus CD8 T cells, B cells, DCs, and so forth) to comprise the whole of immunosuppression. PD-L1 is likely directly targeting virus-specific T cells to inhibit their function and prevent viral clearance (32). IL-10 is produced primarily by DCs and B cells following persistent LCMV infection (9); however the precise cell type (i.e., T cell, DC, B cell) that is being targeted and how IL-10 is altering this cell's function to suppress T-cell activity is unclear. Determining how different immune subsets are suppressed by IL-10 and PD-L1 during persistent viral infection is currently being explored, and will be important for the design of effective vaccine/adjuvant combinations to specifically restore antiviral T-cell function. Through these approaches, it may ultimately be possible to devise targeted-vaccine strategies to restore T-cell immunity and purge established persistent viral infections in humans.
Materials and Methods
Mice and Virus.
C57BL/6 mice were from the Rodent Breeding Colony at the Scripps Research Institute or purchased from The Jackson Laboratory. IL-10-deficient mice were obtained from The Jackson Laboratory. PD-L1-deficient mice were provided by Dr. Arlene Sharpe at the Harvard Medical School, Boston, MA. The LCMV-GP33–41-specific CD8+ TcR transgenic (P14) mice have been described previously (33). All mice were housed under specific pathogen-free conditions. Mouse handling conformed to the requirements of the National Institutes of Health, The Scripps Research Institute Animal Research Committee, and Emory University Institutional Animal Care and Use Committee guidelines. Mice were infected intravenously with 2 × 106 plaque forming units of LCMV- LCMV-Cl 13. To deplete CD4+ T cells, mice were given 500-μg of anti-mouse CD4 antibody (GK1.5) (Bioexpress) i.p. on days −1 and 0 after infection with LCMV-Cl 13. Virus stocks were prepared and viral titers were quantified as described (34). For analysis of liver viral titers, the mice were perfused with 25 to 30 ml of 0.9% saline by direct cardiac injection to remove blood from the tissue.
T-Cell Isolation and Transfer.
CD8 T cells were purified from the spleens of naïve P14 mice by negative selection (StemCell Technologies). Following purification, 2,000 cells were cotransferred i.v. into C57BL/6 mice. We avoided the problems associated with using large, nonphysiologic numbers of transferred transgenic T cells (35) by only transferring low numbers of transgenic T cells. These transgenic T cells behave similarly to their endogenous (i.e., host-derived) T-cell counterparts, based on tetramer analysis and intracellular cytokine staining (ref. 4 and data not shown). The number of P14 cells in the spleen was determined by multiplying the frequency of Thy1.1+ cells (determined flow cytometrically) by the total number of splenocytes.
Quantitative RT-PCR.
RNA from total splenic mononuclear cells was obtained and amplified as previously noted (9). Briefly, RNA expression was normalized by input concentration and amplified using the Qiagen One-step RT-PCR kit (Qiagen). The Assays-on-Demand Real-Time IL-10 expression kit (Applied Biosystems) was used to amplify IL-10 RNA. The RT-PCR did not amplify genomic DNA (data not shown). To quantify IL-10 RNA, a standard curve was generated by 10-fold serial dilutions of total splenic RNA (1 μg to 1 pg total RNA, standard curve: r2>0.99) from Cl 13-infected splenocytes and a relative number of IL-10 RNA determined. Amplifications were performed on the ABI7700 (Applied Biosystems).
Intracellular Cytokine Analysis and Flow Cytometry.
Splenocytes were stained directly ex vivo with LCMV-GP33–41 or LCMV-GP276–286 tetramers and for surface expression of CD8 (clone 53–6.7, Caltag), PD-1 (clone J43), PD-L1 (clone MIH1), Ki-67 (B56), CD107a (clone1D4B), and CD107b (clone ABL-93). Antigen-presenting cells were identified by staining with subset specific antibodies: DC (CD45+, CD3−, NK1.1−, CD11c+), B cells (CD45+, CD3−, NK1.1−, CD11c−, B220+), and macrophages (CD45+, CD3−, NK1.1−, CD11c−, CD11b+).
To analyze cytokine expression, splenocytes were stimulated for 5 h with 2 μg/ml of the MHC class I restricted LCMV-GP33–41 or LCMV-GP276–286 peptide (>99% pure; Synpep,) in the presence of 50-U/ml recombinant murine IL-2 (R&D Systems) and 1-mg/ml brefeldin A (Sigma). Following surface staining, cells were fixed, permeabilized, and stained with antibodies to TNF-α (clone MP6-XT22) and IFN-γ (clone XMG1.2). For Ki-67 staining, cells were permeabilized and incubated with antibody to Ki-67. To detect degranulation, splenocytes were stimulated for 5 h in the presence of brefeldin, monensin, anti-CD107a-FITC, and anti-CD107b-FITC. All antibodies were purchased from BD PharMingen, unless otherwise noted. Flow cytometric analysis was performed using the Digital LSR II (Becton Dickinson). The absolute number of virus-specific T cells was determined by multiplying the frequency of tetramer+ or cytokine+ cells by the total number of cells in the spleen.
In Vivo IL-10R and PD-L1-Specific Antibody Treatment.
C57BL/6 mice received 250 μg per mouse per injection, i.p. of anti-IL-10R-specific antibody (clone 1B1.3a, provided by Schering-Plough) or 200 μg per mouse per injection, i.p. anti-PD-L1-specific antibody (clone 10F.9G2) (36), or rat IgG1 isotype control antibody [clone KM1.GL113 (anti-E. coli β-galactosidase), provided by Schering-Plough] beginning on day 25 or day 49 after infection and continuing every 3 days for five treatments.
Statistical Analysis.
Student's t-tests and Mann-Whitney Rank Sum tests were performed using SigmaStat 2.0 software (Systat Software Inc.).
Acknowledgments.
This is publication number 19361 from the Viral Immunobiology Laboratory, Department of Immunology and Microbial Sciences at The Scripps Research Institute. This work was supported by National Institutes of Health Grants AI077012 (to D.G.B.), AI009484, AI045927 (to M.B.A.O.), P01 AI056299 (to A.H.S. and G.J.F.), R37 AI038310 (to A.H.S.), and a postdoctoral fellowship from the Korean Engineering and Science Foundation (to S-J.H.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Zajac AJ, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallimore A, Dumrase T, Hengartner H, Zinkernagel RM, Rammansee HG. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks DG, McGavern DB, Oldstone MB. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J Clin Invest. 2006;116:1675–1685. doi: 10.1172/JCI26856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oldstone MB, Blount P, Southern PJ, Lampert PW. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986;321:239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- 6.Berger DP, Homann D, Oldstone MB. Defining parameters for successful immunocytotherapy of persistent viral infection. Virology. 2000;266:257–263. doi: 10.1006/viro.1999.0074. [DOI] [PubMed] [Google Scholar]
- 7.Folgori A, et al. Early impairment of hepatitis C virus specific T cell proliferation during acute infection leads to failure of viral clearance. Gut. 2006;55:1012–1019. doi: 10.1136/gut.2005.080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thimme R, et al. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks DG, et al. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moss P, Rickinson A. Cellular immunotherapy for viral infection after HSC transplantation. Nat Rev Immunol. 2005;5:9–20. doi: 10.1038/nri1526. [DOI] [PubMed] [Google Scholar]
- 11.Oldstone MB. Viral persistence. Cell. 1989;56:517–520. doi: 10.1016/0092-8674(89)90573-4. [DOI] [PubMed] [Google Scholar]
- 12.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2005;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 13.Ejrnaes M, et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matter M, Odermatt B, Yagita H, Nuoffer JM, Ochsenbein AF. Elimination of chronic viral infection by blocking CD27 signaling. J Exp Med. 2006;203:2145–2155. doi: 10.1084/jem.20060651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J Exp Med. 2008;205:533–541. doi: 10.1084/jem.20071948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ha SJ, et al. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Battegay M, et al. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lichterfeld M, et al. HIV-1-specific cytotoxicity is preferentially mediated by a subset of CD8(+) T cells producing both interferon-gamma and tumor necrosis factor-alpha. Blood. 2004;104:487–494. doi: 10.1182/blood-2003-12-4341. [DOI] [PubMed] [Google Scholar]
- 21.Badr G, et al. Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells. J Virol. 2008;82:10017–10031. doi: 10.1128/JVI.01083-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almeida JR, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204:2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Betts MR, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rehr M, et al. Emergence of polyfunctional CD8+ T cells after prolonged suppression of human immunodeficiency virus replication by antiretroviral therapy. J Virol. 2008;82:3391–3404. doi: 10.1128/JVI.02383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura H, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 28.Blackburn S, Wherry EJ. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15:143–146. doi: 10.1016/j.tim.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Rigopoulou EI, Abbott WG, Haigh P, Naoumov NV. Blocking of interleukin-10 receptor–a novel approach to stimulate T-helper cell type 1 responses to hepatitis C virus. Clin Immunol. 2005;117:57–64. doi: 10.1016/j.clim.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Urbani S, et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radziewicz H, et al. Liver infiltrating lymphocytes in chronic human HCV infection display an exhausted phenotype with high PD-1 and low CD127 expression. J Virol. 2006;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7–1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur J Immunol. 1998;28:390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 34.Borrow P, Evans CF, Oldstone MB. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marzo AL, et al. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodig N, et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]