Abstract
The modular components, or subcircuits, of developmental gene regulatory networks (GRNs) execute specific developmental functions, such as the specification of cell identity. We survey examples of such subcircuits and relate their structures to corresponding developmental functions. These relations transcend organisms and genes, as illustrated by the similar structures of the subcircuits controlling the specification of the mesectoderm in the Drosophila embryo and the endomesoderm in the sea urchin, even though the respective subcircuits are composed of nonorthologous regulatory genes.
Keywords: Drosophila development, regulatory subcircuits, genomic regulatory logic, sea urchin development
Developmental gene regulatory networks (GRNs) provide the specific causal links between genomic regulatory sequences and the processes of development (1–3). They consist of the regulatory and signaling genes that drive any given process of development and the functional interactions among them. The design features of the GRN directly explain why the events of a given process of development occur; for example, why a given set of cells becomes specified to a given fate, why it emits particular signals to adjacent cells, and why it differentiates in a given direction. The architecture of a GRN is mandated by the cis-regulatory sequences of the enhancers that control each gene of the network. These sequences determine what inputs affect expression of each gene, and how these inputs operate in a combinatorial fashion.
The individual components of a complex developmental process are in general controlled by GRN subcircuits, and it is their architecture that illuminates the basic logic of development. In this Perspective, we summarize several types of subcircuits that are used in development and focus on those used for similar functions. The structural features, or architectures, of subcircuits provide new insights into the basic developmental processes they control. Table 1 lists several examples.
Table 1.
Network logic: Commonly encountered subcircuits and the complex developmental jobs they do
| GRN subcircuit design feature | Developmental control logic |
|---|---|
| Double negative gate | Exclusive spatial derepression and repression |
| Intraterritorial repression | Exclusion of alternative regulatory states |
| Ligand gene response to own signal transduction system | Community effect: enforce transcriptional conformity within territory |
| Auto and cross regulatory feedback | Dynamic regulatory state lockdown |
| Regulatory auto-repression | Temporal expression peak/oscillation |
| Regulatory auto-repression controlling expression of signal ligand genes | Dynamic spatial wave of signaling |
On the left are structural design features found in diverse GRNs. On the right are elements of developmental processes that are generated by these subcircuits.
Double-Negative Gate
We first consider the double-negative gate (see Fig. 1A). This is a counterintuitive feature of network design that is a critical component of both the sea urchin and Drosophila GRNs controlling early embryogenesis. It is used to install a specific regulatory state in a specific region of the developing embryo. Instead of employing a developmental control gene as principal activator, it operates by relieving an otherwise global inhibitor through localized transcriptional repression of the inhibitor in a specified domain. Here are some examples:
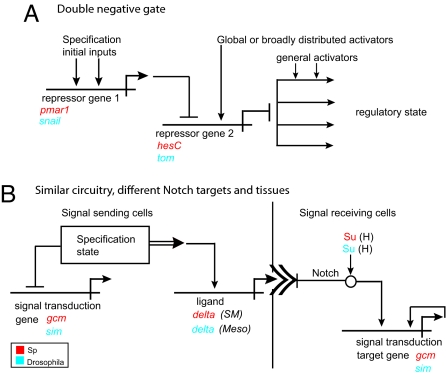
Fig. 1.
GRN subcircuits used in both Drosophila and sea urchin development. Horizontal lines indicate the relevant cis-regulatory modules of the indicated genes, and the bent arrows attached represent the transcription unit; incident vertical arrows indicate positive transcription factor inputs into these cis-regulatory systems, and barred stems indicate negative inputs; double sideways “v” indicates the Notch receptor for the Delta ligand; “Regulatory State” represents the sum of expressed transcription factors at the given time and place. (A) The double negative gate. (B) Notch signaling circuitry. In Drosophila, the delta ligand is expressed in mesoderm (Meso), and Notch signaling occurs in adjacent neurogenic ectoderm causing expression of the midline gene sim; in the sea urchin, the Delta ligand is expressed in the skeletogenic mesoderm (SM), and Notch signaling occurs in adjacent nonskeletogenic mesoderm, causing expression of the pigment cell regulator gcm.
In the specification of the skeletogenic mesoderm of the sea urchin embryo the double negative gate consists of activation of genes encoding a lineage-specific repressor, Pmar-1, which prevents expression of a global transcriptional repressor of the genes that establish the skeletogenic regulatory state (3, 4). Interestingly, the same basic regulatory logic is used in the oral ectoderm of this embryo. Here, the domain-specific repressor Goosecoid is used to repress expression of an otherwise pan-ectodermal transcriptional repressor, thereby permitting a set of regulatory genes to be activated only in the oral ectoderm.
In the Drosophila embryo, the mesodermal Snail repressor prevents transcription of Tom, which inhibits processing of the Notch signaling ligand, Delta (5). This results in mesoderm-specific expression of the Delta ligand, with the consequence that Notch signaling is constrained to the adjacent cells where it activates sim and other genes required for the specification of the ventral midline of the neurogenic ectoderm (2, 5). Although not explicitly described, the entire anterior–posterior patterning network of the Drosophila embryo is also composed of a series of double-negative gates, in which broadly distributed gap gene repressors establish domains of pair-rule gene expression by cross-repressive interactions among themselves (6).
What is the functional advantage of this unexpected design feature compared with simple regional transcriptional activation? The double-negative gate appears to be an effective mechanism for ensuring spatially restricted patterns of gene expression, and for actively preventing these genes from functioning elsewhere in the embryo by sequence-specific transcriptional repression. In addition, this mechanism might relieve the regulatory complexity of highly pleiotropic genes such as those encoding Delta and other cell signaling molecules. For example, Delta is used in many different cell types at numerous stages in the development of virtually all animal embryos. It might not be feasible to dedicate a separate enhancer for every aspect of Delta activity. The double-negative gate provides a means for producing cell-specific regulation without the need for a distinct and dedicated enhancer.
Transcriptional Exclusion of Alternative States
A second design feature of GRN subcircuits is the cryptic, intraterritorial repression of other possible regulatory states during the process of specification (Table 1). We say “cryptic” because such functions are not revealed until they are experimentally interrupted, with the consequence that the state of the specification switches because of the absence of the repressor. Often, the function is to prevent a set of cells from responding to a signal that they are exposed to. For example, in the sea urchin embryo the skeletogenic mesoderm emits a Delta ligand, which in consequence of Notch signaling in the adjacent mesoderm cells activates transcription of the gcm regulatory gene (Fig. 1B). The skeletogenic cells are prevented from responding to Notch signaling themselves by the intraterritorial repression of gcm.
An exactly parallel mechanism is used for the localized expression of sim and the specification of the ventral midline of the neurogenic ectoderm. As discussed earlier, the Snail repressor leads to localized synthesis of the Delta ligand in the ventral mesoderm of the early Drosophila embryo via a double negative gate. This triggers Notch signaling in adjacent cells. In principle, the ventral mesoderm could respond to the Delta signal as well, but the target gene of Notch signaling in the neurogenic ectoderm, sim, is specifically repressed by the intraterritorial Snail repressor (Fig. 1B) (2). As in the case of the double-negative gate, we see an example of a regulatory design that is used in very different embryos to implement a similar job.
Such exclusion functions are a widespread feature of GRNs, and examples have been noted in Xenopus, Caenorhabditis elegans, and mouse, as well as Drosophila and sea urchin (7). This is one of several GRN design features that contribute to the remarkable stability and reproducibility of animal development.
There is a further exact parallel between the wiring leading to mesodermal gcm expression in the sea urchin and to neuronal sim expression in Drosophila. After their initial activation by a double-negative gate that induces the mediator of Notch signaling (SuH), both genes lock down their respective states of expression by means of positive autoregulation (Fig. 1B) (8, 9).
Other General Subcircuit Design Features
Table 1 includes additional GRN subcircuit design features that are responsible for the execution of specific developmental functions.
The “community effect” (1) is an intraterritorial signaling function, in which all cells of the territory produce and respond to the same signal ligand. The underlying circuit design feature is that the gene encoding the signal ligand is activated by the same signal transduction system that it triggers in adjacent cells. The general consequences of community effect circuitry are: (i) establishment of a regulatory state through the activation of a set of regulatory genes downstream of the signal transduction system in all of the cells of the territory; (ii) averaging, that results in installation of approximately the same levels of expression in all of the cells within the territory; (iii) maintenance of the regulatory state so that, if signaling is interrupted, the regulatory state is lost.
There are many examples. In the sea urchin embryo, specification of the oral ectoderm depends on a community effect where the ligand gene is nodal (10, 11). Similarly in the specification of the endomesoderm a community effect obtains where the ligand gene is wnt (12, 13). Within the ventrolateral mesodermal territory of the Xenopus embryo the gene encoding eFGF similarly responds to a Brachyury input (14), but brachyury gene is activated in consequence of the FGF signaling pathway (15).
As extensively discussed in refs. 1–3, one of the most widespread and important equivalences between GRN topology and developmental function noted in Table 1 is the use of intra and intergenic feedback loops for stabilizing transcriptional regulatory states. We have already seen examples of autoregulatory feedback (Fig. 1B), and many cases of cross-regulatory intergenic feedback have come to light in GRNs from sea urchin, mouse, Drosophila, and other developmental systems. These typically appear in the network architecture just downstream of the transient signals or other initial states that trigger the first transcriptional events in a specification process. They consist of sets of 2 or 3 coexpressed regulatory genes, the cis-regulatory systems of which use as positive inputs the transcription factors encoded by other gene(s) of the interacting set. Thus, these transcriptional feedback subcircuits function as “dynamic state lockdown” motifs; once the participant genes are under each other's transcriptional influence, the regulatory state cannot turn off, and these genes together with others they control represent the regulatory state defining that specification event. Thus, paradoxically, a stabilization function depends on continuing dynamic transcription. Other mechanisms of transcriptional state lockdown, such as those operating at the chromatin level, lie downstream of these GRN subcircuits.
Dynamic Aspects of Regulatory State Change in Space and Time
Just as dynamically functioning transcriptional circuits can produce stable regulatory states, the static genome can produce dynamic spatial and/or temporal transcriptional patterns. Such processes are also mandated by the design features of specific GRN subcircuits. One well-defined example is the generation of pulses of gene expression, which rise and then fall because of regulatory autorepression. The same regulatory design produces either peaks of gene expression or successive oscillations mediated by cis-regulatory autorepression (Table 1). Several examples have been discovered in the sea urchin and sea star embryo GRNs (16).
The blimp1 gene presents an interesting autorepression system of this kind in the sea urchin embryo. blimp1 turns itself off after some hours of expression when its product accumulates to high levels, and then acts as a repressor. However, blimp1 also provides a required input into the gene encoding the diffusible signaling ligand, Wnt8, which in turn is locked into a feedback relationship with blimp1: The Wnt8 ligand induces Tcf to activate blimp1 expression. The consequence of this unexpected subcircuit design is that an expanding torus of coordinately expressed and coordinately silenced wnt8 and blimp1 expression sweeps across the endomesoderm of the embryo. It begins in the centrally located skeletogenic mesoderm, then as Wnt8 ligand diffuses outward, it extends to the surrounding nonskeletogenic mesoderm and finally to the peripheral endoderm, while, in each previously active domain, the expression of both is extinguished due to blimp1 autorepression (13).
Additional regulatory genes are also entrained in the expanding torus subcircuit (17). In an accompanying article in this Special Feature, this GRN subcircuit is shown to control both Wnt and Notch signaling across the entire endomesoderm (18). The subcircuit not only includes autorepression but also transrepression of notch by high levels of Blimp1 and of another gene that repressively controls delta expression. It combines in one almost all of the individual subcircuit functions/architectures shown in Table 1. Its overall developmental role is to ensure a dynamic, Boolean march of successive and exclusive domains of Wnt8 and Notch signal transduction that moves peripherally from one fate domain to the next as development proceeds.
GRNs in Metazoan Development and Evolution
Here, we have focused on examples of the ways in which GRN subcircuits mandate developmental logic. The design features we have considered are devices used to drive the development of all animal embryos, and as the parallelism illustrated in Fig. 1 shows, disparate organisms, in different tissues, using different genes, nonetheless execute similar developmental decisions with the same circuit designs. We believe that in the near future a repertoire of such GRN subcircuits will be revealed, a repertoire that has been assembled in countless combinations throughout the evolution of diverse body plans among the Metazoa.
This PNAS Special Feature contains 10 articles covering a variety of contemporary topics relevant to the role of gene regulatory networks in animal development and evolution. The first 2 articles, by Hobert (19) and Hong et al. (20), respectively, provide Perspectives on two long-standing problems in metazoan development. Hobert discusses recent advances in our understanding of the gene regulatory networks responsible for the specification of individual neuronal cell types in C. elegans (19). Hong et al. (20) summarize the use of postgenome technologies in determining how different concentrations of the Dorsal transcription factor produce a variety of gene expression patterns in the early Drosophila embryo.
The next 4 articles are original research papers that present new insights into our understanding of how gene regulatory networks control different aspects of embryonic and postembryonic development, as well as changes in body patterning during animal evolution. The articles from Tumpel et al. (21), Nikitina et al. (22), Ochoa-Espinosa et al. (23), and Smith and Davidson (18) describe advances in basic embryonic patterning processes, including the specification of the posterior hindbrain in vertebrates, the specification of neural crest progenitors in lampreys, the combinatorial control of A/P patterning of the Drosophila embryo, and the specification of the endomesoderm territory in the sea urchin embryo.
Two more articles are devoted to one of the major challenges in developmental biology, namely, unraveling the complex regulatory networks underlying the formation of postembryonic tissues and organs. The article by Ririe et al. (24) examines vulva development in C. elegans, with an emphasis on how gene networks coordinate individual cells to produce a complex organ. Georgescu et al. (25) examine the fascinating problem of T cell specification and diversification in the mammalian immune system. Evidence is presented for dynamic networks that are generally more plastic and reversible than those seen in hard-wired developmental processes such as endomesoderm specification in the sea urchin.
The final 2 research articles address problems in the evolutionary diversity of animal morphology. Gross et al. (26) explore the genome organization of the Mexican cavefish, Astyanax mexicanus, in an effort to understand the basis for its peculiar mode of adaptation, including the loss of eyes. Finally, Usui et al. (27) investigate the large sensory bristles (macrochaetae) of the adult fruitfly as a paradigm for understanding the evolution of morphological diversity.
Acknowledgments.
We thank Susan Marty for superb administrative assistance and David Stopak for his editorial oversight. This work was supported by the National Academy of Sciences Sackler Colloquium program and National Institutes of Health Grants HD37105 (to E.H.D.) and GM34431 (to M.L.).
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Gene Networks in Animal Development and Evolution,” held February 15–16, 2008, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS web site at http://www.nasonline.org/SACKLER_Gene_Networks.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Davidson EH. The Regulatory Genome. Gene Regulatory Networks in Development and Evolution. San Diego: Academic; 2006. [Google Scholar]
- 2.Levine M, Davidson EH. Gene regulatory networks for development. Proc Natl Acad Sci USA. 2005;102:4936–4942. doi: 10.1073/pnas.0408031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliveri P, Tu Q, Davidson EH. Global regulatory logic for specification of an embryonic lineage. Proc Natl Acad Sci USA. 2008;105:5955–5962. doi: 10.1073/pnas.0711220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Revilla-i-Domingo R, Oliveri P, Davidson EH. A missing link in the sea urchin embryo gene regulatory network: hsC and the double negative specification of micromeres. Proc Natl Acad Sci USA. 2007;104:12383–12388. doi: 10.1073/pnas.0705324104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Renzis S, Yu J, Zinzen R, Wieschaus E. Dorsal-ventral pattern of Delta trafficking is established by a Snail–Tom–Neuralized pathway. Dev Cell. 2006;10:257–264. doi: 10.1016/j.devcel.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Surkova S, et al. Characterzation of the Drosophila segment determination morphome. Dev Biol. 2008;313:844–862. doi: 10.1016/j.ydbio.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveri P, Davidson EH. Development. Built to run not fail. Science. 2007;315:1510–1511. doi: 10.1126/science.1140979. [DOI] [PubMed] [Google Scholar]
- 8.Nambu JR, Lewis JO, Wharton KA, Crews ST. The Drosophila single-minded gene encodes a helix–loop–helix protein that acts as a master regulator of CNS midline cells. Cell. 1991;67:1157–1167. doi: 10.1016/0092-8674(91)90292-7. [DOI] [PubMed] [Google Scholar]
- 9.Ransick A, Davidson EH. cis-regulatory processing of Notch signaling input to the sea urchin glial cells missing gene during mesoderm specification. Dev Biol. 2006;297:587–602. doi: 10.1016/j.ydbio.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 10.Nam J, Su YH, Lee PY, Robertson AJ, Coffman JA, Davidson EH. Cis-regulatory control of the nodal gene, initiator of the sea urchin oral ectoderm gene network. Dev Biol. 2007;306:860–869. doi: 10.1016/j.ydbio.2007.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Range RC, Glenn TD, Miranda E, McClay DR. LvNumb works synergistically with Notch signaling to specify non-skeletal mesoderm cells in the sea urchin embryo. Development. 2008;135:2445–2454. doi: 10.1242/dev.018101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minokawa T, Wikramanayake AH, Davidson EH. Regulatory inuts of the wnt8 gene in the sea urchin endomesoderm network. Dev Biol. 2005;288:545–558. doi: 10.1016/j.ydbio.2005.09.047. [DOI] [PubMed] [Google Scholar]
- 13.Smith J, Theodoris C, Davidson EH. A gene regulatory network subcircuit drives a dynamic pattern of gene expression. Science. 2007;318:794–797. doi: 10.1126/science.1146524. [DOI] [PubMed] [Google Scholar]
- 14.Casey ES, O'Reilly MA, Conlon FL, Smith JC. The T-box transcription factor Brachyury regulates expression of eFGF through binding to a non-palindromic response element. Development. 1998;125:3887–3894. doi: 10.1242/dev.125.19.3887. [DOI] [PubMed] [Google Scholar]
- 15.Latinkic BV, et al. The Xenopus Brachyury promoter is activated by FGF and low concentrations of activin and suppressed by high concentrations of activin and by paired-type homeodomain proteins. Genes Dev. 1997;12:1240–1250. doi: 10.1101/gad.11.23.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinman VF, Nguyen AT, Cameron RA, Davidson EH. Developmental gene regulatory network architecture across 500 million years of echinoderm evolution. Proc Natl Acad Sci USA. 2003;100:13356–13361. doi: 10.1073/pnas.2235868100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith J, Davidson EH. A new method, using cis-regulatory control, for blocking embryonic gene expression. Dev Biol. 2008;318:360–365. doi: 10.1016/j.ydbio.2008.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith J, Davidson EH. Gene regulatory network subcircuit controlling a dynamic spatial pattern of signaling in the sea urchin embryo. Proc Natl Acad Sci USA. 2008;105:20089–20094. doi: 10.1073/pnas.0806442105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hobert O. Regulatory logic of neuronal diversity: Terminal selector genes and selector motifs. Proc Natl Acad Sci USA. 2008;105:20067–20071. doi: 10.1073/pnas.0806070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong J, Hendrix D, Papatsenko D, Levine M. How the Dorsal Gradient Works: Insights From Post-Genome Technologies. Proc Natl Acad Sci USA. 2008;105:20072–20076. doi: 10.1073/pnas.0806476105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tumpel S, Cambronero F, Sims C, Wiedemann L, Krumlauf R. A regulatory module embedded in the coding region of Hoxa2 controls expression in rhombomere 2. Proc Natl Acad Sci USA. 2008;105:20077–20082. doi: 10.1073/pnas.0806360105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikitina N, Sauka-Spengler T, Bronner-Fraser M. Dissecting early regulatory relationships in the Lam-prey neural crest gene regulatory network. Proc Natl Acad Sci USA. 2008;105:20083–20088. doi: 10.1073/pnas.0806009105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochoa-Espinosa A, Yu D, Tsirigos A, Stuffi P, Small S. Anterior-posterior positional information in the absence of a strong Bicoid gradient. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0807878105. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ririe T, Fernandes J, Sternberg P. The Caenorhabditis elegans vulva: A post-embryonic gene regulatory network controlling organogenesis. Proc Nat Acad Sci. 2008;105:20095–20099. doi: 10.1073/pnas.0806377105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Georgescu C, et al. A gene regulatory network armature for T-lymphocyte specification. Proc Natl Acad Sci USA. 2008;105:20100–20105. doi: 10.1073/pnas.0806501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross J, et al. Synteny and candidate gene prediction using an anchored linkage map of Astyanax mexicanus. Proc Natl Acad Sci USA. 2008;105:20106–20111. doi: 10.1073/pnas.0806238105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Usui K, Goldstone C, Gibert J-M, Simpson P. Redundant mechanisms mediate bristle patterning on the Drosophila thorax. Proc Natl Acad Sci USA. 2008;105:20112–20117. doi: 10.1073/pnas.0804282105. [DOI] [PMC free article] [PubMed] [Google Scholar]