Abstract
The minichromosome maintenance protein (MCM) complex is an essential replicative helicase for DNA replication in Archaea and Eukaryotes. Whereas the eukaryotic complex consists of 6 homologous proteins (MCM2–7), the archaeon Sulfolobus solfataricus has only 1 MCM protein (ssoMCM), 6 subunits of which form a homohexamer. Here, we report a 4.35-Å crystal structure of the near-full-length ssoMCM. The structure shows an elongated fold, with 5 subdomains that are organized into 2 large N- and C-terminal domains. A near-full-length ssoMCM hexamer generated based on the 6-fold symmetry of the N-terminal Methanothermobacter thermautotrophicus (mtMCM) hexamer shows intersubunit distances suitable for bonding contacts, including the interface around the ATP pocket. Four unusual β-hairpins of each subunit are located inside the central channel or around the side channels in the hexamer. Additionally, the hexamer fits well into the double-hexamer EM map of mtMCM. Our mutational analysis of residues at the intersubunit interfaces and around the side channels demonstrates their critical roles for hexamerization and helicase function. These structural and biochemical results provide a basis for future study of the helicase mechanisms of the archaeal and eukaryotic MCM complexes in DNA replication.
Keywords: DNA replication, replicative helicase, nucleic-acid motor, β-hairpin, cancer
The minichromosome maintenance proteins (MCMs) are essential for initiation and elongation during DNA replication in cells. These proteins serve as the replicative helicase at the replication origin and DNA fork (1–3). The recruitment and subsequent activation of MCM at the replication origin are tightly regulated through the ordered stepwise assembly of multiple factors (such as ORC and Cdc6) to ensure DNA replication occurs once per cell cycle (4–6). Failure to recruit MCM proteins to the replication origin results in loss of origin firing and G1 phase arrest.
The MCM proteins in eukaryotes consist of a subgroup of 6 homologous proteins (MCM2–7) that belong to AAA+ ATPase family (7). These 6 MCM proteins can form heterohexamers, and the presence of all 6 MCM proteins is necessary for entry and completion of S phase (5, 8). However, some archaeal MCM proteins, such as Sulfolobus solfataricus MCM (ssoMCM) and Methanothermobacter thermautotrophicus MCM (mtMCM), are encoded by a single mcm gene. Both ssoMCM and mtMCM can form homooligomers (9–11). The N-terminal region is poorly conserved among MCM proteins from archaea to eukaryotes. However, the C-terminal region shares a highly similar stretch of amino acids, referred to as the MCM box (12), for the binding and hydrolysis of ATP.
The crystal structures of the poorly conserved N-terminal portion of mtMCM (N-mtMCM) and ssoMCM (N-ssoMCM) reveal that this region can form dodecamers and hexamers (11, 13). The monomeric fold and the assembled hexamer structures of the N-ssoMCM and N-mtMCM are highly conserved (11, 13). A β-hairpin structure present in the N domain of the both MCM proteins protrudes into the central hexameric channel to form the narrowest point within the channel, possibly for interacting with DNA at a certain stage of MCM function (11, 14).
Comprehension of the molecular mechanisms of the MCM helicase has been limited by the lack of 3-dimensional structures of a full-length (FL) MCM protein. Here, we report the crystal structure of ssoMCM, which is an X-ray analysis of a near-FL MCM. The structure reveals how the different domains of ssoMCM are organized and allows a detailed analysis of how subunits oligomerize into a functional hexamer. Our structure-based mutagenesis analysis provides insights into the structural and functional relationship of ssoMCM helicase function.
Results
Structural Features of the Near-FL SsoMCM.
We crystallized the FL (residues 1–686) and a C-terminal truncation (T612,residues 1–612) of ssoMCM [Fig. 1A and supporting information (SI) Fig. S1]. Se-SAD phasing was used to solve the structures of the FL construct and the T612 construct. The molecular models built on the electron density maps of the 2 constructs reveal a similar structure, both containing the N-terminal domain and the C-terminal AAA+ domain, with 1 monomer per asymmetric unit (Fig. 1B). Even though low σ level density for the winged-helix domain (WH) at the C terminus is clearly present in the SAD map of the FL structure, we could not build the WH domain because of the poorly defined electron density. As a result, the final ssoMCM model contains residues 7–601, missing the C-terminal 85-residue WH domain.
Fig. 1.
Overall features of the monomeric and hexameric ssoMCM structural models. (A) Diagram depicting the domains of ssoMCM. N-C, N to C domain linker; α/β, α/β-domain of ATPase core; α/β-α, linker between subdomains in the ATPase core; α, α-domain. WH, winged helix domain (disordered in our structure). (B) Fold of ssoMCM monomer. Domains and linkers are colored as in A. Helices are shown as cylinders and β-strands as arrows. Zinc atoms are shown as red spheres. β-Hairpins are labeled as follows: NT-hp, N-terminal hairpin; H2I-hp, helix-2 insert hairpin; PS1-hp, presensor 1 hairpin; EXT-hp, external hairpin. (C) Ribbon diagram showing the top and side views of a hexamer model of ssoMCM.
The ssoMCM monomer structure is elongated and can be divided into N and C domains (Fig. 1 A and B). The N domain structure is similar to that of N-mtMCM (11, 13), however, the N-terminal β-hairpin (NT hairpin) of N-ssoMCM is longer than that of the N-mtMCM because of a 5-residue insertion in the hairpin sequence of ssoMCM (13). Between the N and C domains is a 40-residue linker (N-C linker, in red in Fig. 1 A and B) that is well-structured, forming a long connection with 2 consecutive helices at its C terminus that appear to be an integral part of the C domain. The C domain consists of an AAA+ ATPase/helicase domain (the ATPase core), which is composed of 2 clearly separable subdomains: a canonical α-helical/β-strand region (α/β-domain, colored in cyan in Fig. 1 A and B) and a nontypical 3 α-helical bundle domain (α-domain, in purple in Fig. 1 A and B; see SI Text for a more detailed comparison with other known AAA+ protein structures). There are a total of 5 main β-strands and 5 main α-helices in the α/β-domain and 3 α-helices in the α-domain (Fig. 1B and Fig. S1). Connecting the α/β-domain and the α-domain is a 47-residue linker (α/β-α linker, in blue in Fig. 1 A and B). This α/β-α linker folds into 2 long α-helices spaced with a loop in the middle. Interestingly, the N-C linker and the distal α/β-α linker wrap around each other like 2 interlocking index fingers (Fig. 1B). Through such interlocking interactions, the 2 long linkers can stabilize not only the conformations of each other, but also the relative positions of the N domains and the C-terminal α/β- and α-domains.
Other noticeable structural features in the C domain include 3 β-hairpin structures protruding from the surface (labeled H2I-hp, PS1-hp, and EXT-hp in Fig. 1B). The most N-terminal β-hairpin, named H2I-hairpin (or H2I-hp, residues 374–390), protrudes farthest into the central channel of the hexamer model presented below and has its β-hairpin tip structured into a small 310-like helix. This β-hairpin is formed from a sequence motif called “helix-2 insert” (H2I) that is unique to MCM and other H2I subfamily members of the AAA+ ATPase family (15). Next to the H2I hairpin is a shorter β-hairpin structure (residues 424–439), which is formed from the sequence just ahead of (or N-terminal to) the sensor-1 asparagine (15). As a result, this β-hairpin is named the presensor-1 β-hairpin (PS1-hp in Fig. 1B). Comparable in length with the H2I hairpin but more recessed from the central channel, this PS1hairpin is equivalent to the major β-hairpin structure of the AAA+ domain of SV40 Large T antigen (LTag) hexameric helicase (16, 17). The third β-hairpin (residues 319–333) contains a VLED sequence motif similar to that of the acidic pin of RuvA helicase (18). This β-hairpin is located on the exterior of the hexamer model and is thus named EXT-hairpin (or EXT-hp in Fig. 1B). The mutational data reported here and from previous reports (14, 19) demonstrate the critical role of these 3 hairpins for the helicase function.
Hexamerization of SsoMCM.
SsoMCM exists mostly in hexameric form in solution with medium salt concentration (see below). The structures of the N domains of ssoMCM and mtMCM show a similar hexamerization interface (11, 13). These two align well with each other (Fig. S2A). However, N-ssoMCM has a narrower central channel largely because of a longer β-hairpin finger extending into the channel (Fig. S2 B and C) (13). Based on these 2 hexamer structures, we generated hexamer models of ssoMCM by applying the 6-fold symmetry of either the N-ssoMCM or the N-mtMCM structures. Significantly, the N-mtMCM 6-fold symmetry [Protein Data Bank (PDB) ID code 1LTL] immediately yielded a hexamer that has reasonable bonding distances between neighbors not only for the N domain, but also for the majority of the C-terminal AAA+ domain (Fig. 1C). No clashes between neighboring monomers are present in the hexamer structure. However, the hexamer model generated by using the N-ssoMCM hexameric symmetry (PDB ID code 2VL6) has some clashes at the C-terminal domain. Thus, it seems that ssoMCM may have 2 or more conformations differing slightly in the angles between N and C domains, with at least 1 of such conformations (as the one reported here) that can assemble a hexamer following the hexameric symmetry of the N-mtMCM.
The ssoMCM hexamer model has a short dumbbell appearance, with a large N domain ring and C domain ring on both ends, and a “slim waist” around the middle portion of the hexamer (Fig. 1C, Side view). The hexamer is 103 Å in length along the hexameric axis and 138 Å in width. The hexamer has a wide central channel (Fig. 1C, Top view), narrowing toward the N terminus. Clear side channels (11-Å opening, between main-chain atoms) are present, which are comparable with the side-channel dimensions observed in the SV40 large T hexamer (12-Å opening, between main-chain atoms) (16, 17). Side channels are also visualized by electron microscopic (EM) reconstruction of mtMCM (20, 21). These channels were proposed to be potential exits for unwound ssDNA in the LTag double hexameric “looping” model of DNA unwinding (see Discussion) (16, 17).
Fitting the Hexamer into the mtMCM EM Map.
Although ssoMCM has been characterized mainly as a hexamer, there is some evidence that it may form a double hexamer (22). Double-hexamer structures are observed for the homolog mtMCM. We fitted the ssoMCM hexamer model to the available EM map of the double-hexameric mtMCM (20). The overall topology of the ssoMCM hexamer model fits snugly into the EM map (Fig. 2A). Many major structural features agree between the ssoMCM hexamer structure model and the EM map, including the striking slim waist formed between the N- and C- terminal domains, the side channels, and even the surface contour inside the central channel.
Fig. 2.
Structural features of the ssoMCM hexamer. (A) Double hexameric EM map of mtMCM with the ssoMCM hexamer model fitting snugly inside the map (20). The PS1 hairpin is located near the side channels of the EM map (indicated by an arrow). (B) Side and top views of the ssoMCM hexamer model. Subunits are labeled a–f. Two subunits in the front are removed in the side view to reveal the interior. The 4 β-hairpins located inside the central and side channels are colored. (C) Close-up view of subunits a and b in the back side of the hexamer in B (side view). β-Hairpins are labeled as in Fig. 1B. The opening between the 2 neighboring subunits at the C terminus (side channel) is indicated. (D) Close-up top view as in B, showing the radial and helical nature of the 4 β-hairpins.
Structural Features Within the Main Channel.
The narrowest point of the hexameric central channel is formed by the 6 NT-hairpins within the N-terminal domains (Fig. 2 B–D). The next narrowest point is in the C-terminal helicase domain, which is formed by the H2I hairpin (Fig. 2 B–D and Fig. S2B). This hairpin was predicted to be located in the central channel (19, 21), and the position may be relevant to its critical role in helicase activity (19).
In contrast to the H2I hairpins that protrude into the central channel, the 6 PS1 hairpins are somewhat recessed from the central channel (Fig. 2 B–D), as anticipated before (21). This is reminiscent of the RuvB PS1 hairpin, which is recessed from the central channel and forms contacts to RuvA (23). Unlike the RuvB PS1 hairpin, which likely does not interact with DNA (24), the MCM PS1 hairpin is involved in DNA binding and helicase activity (14). Interestingly, the equivalent β-hairpin of LTag protrudes into the hexameric central channel (16, 17) and is not recessed.
The 6 PS1 hairpins of ssoMCM are also located near the C-terminal side channel entrance that connects to the main channel. Such a PS1 hairpin location makes it accessible from both the main channel and the side channel (Fig. 2 B–D) and may have functional implications for DNA unwinding.
Structure Features Around the Side Channels.
In addition to the PS1 hairpin on the interior entrance of the side channel, the acidic β-hairpin (or EXT hairpin) is present on the exterior exit of the side channel (Fig. 2 B–D). Unlike the PS1 hairpin that has positively charged residues on the hairpin tip, the EXT hairpin has 2 hydrophobic and 2 acidic residues (VLED324–327) on the tip. A β-hairpin with a similar motif in RuvA, called the acidic pin, is involved in DNA fork unwinding during branch migration (18). This acidic pin is situated on the inside of the RuvA tetramer, as opposed to the exterior location of the EXT hairpin in MCM (see Discussion). SsoMCM D327 on the EXT hairpin is well conserved among archaeal MCMs and semiconserved in eukaryotic MCMs (alignment not shown). The presence of this acidic EXT hairpin and the PS1 hairpin around the exit and the entrance of the side channel, suggests a potential role of the 2 β-hairpins for helicase function, potentially interacting with and translocating DNA through the side channel (see Discussion).
The residues from 199 to 211 form a well-structured loop (L207) in the N-ssoMCM and N-mtMCM structures. This loop points toward the C-terminal domain. In our ssoMCM hexamer model, it would abut against the PS1 hairpin and helix Cα3 (Fig. S1) from a neighboring subunit around the side channel (Fig. S3C). Mutational analysis of this loop has been recently performed (25). This loop was proposed to act as a medium for transmitting signals from ATP hydrolysis to the N-terminal domain. Our hexamer structure indicates that these residues are within bonding distance with the α/β-domain of the same subunit (in cis) and the PS1 hairpin/Cα3 region of a neighboring subunit (in trans) (Fig. S3).
Nucleotide-Binding Pocket at the Interface.
The ATP-binding pocket, with its Walker A and B motifs on the ATP-bound subunit and the arginine finger from the next subunit (in trans), lies at the interface between 2 neighboring subunits near the C terminus of the ssoMCM hexamer model. There is no nucleotide in the crystal structure, and the ATP-binding pocket configuration of the ssoMCM hexamer model is similar to that of the empty site of the LTag hexamer structure (Fig. S4) (17). The close resemblance of the ATP pocket configuration to that of the LTag apo form provides further evidence that supports the hexamer model presented here.
Like LTag helicase, the ATP-binding pocket is C-terminal to the side channel. The ring formed by the C-terminal domains of the hexamer model appears loose, a characteristic of the apo-LTag hexamer. The LTag hexamer tightens up upon ATP binding to the ATP pocket at the interface between subunits (17); the conformation of ssoMCM hexamer model suggests that ATP binding should tighten the interactions between 2 adjacent subunits in a ssoMCM hexamer.
Structure-Guided Mutational Analysis of SsoMCM.
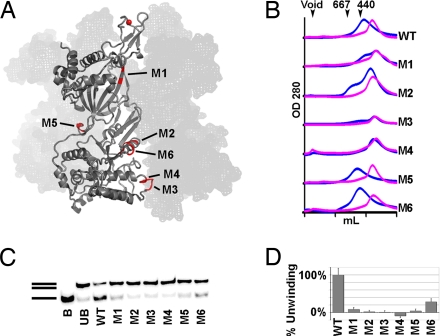
We constructed 6 mutants of ssoMCM (M1–M6) based on the crystal structure of ssoMCM to validate the structural model of the hexamer (Table 1 and Fig. 3). The locations of the mutated residues on the structure are shown in Fig. 3A. Among the 6 mutants, the residues mutated in M1–M4 are located at the interface between neighboring subunits in the hexameric model.
Table 1.
Summary of ssoMCM mutational studies
| Mutant no. | Mutation | Location | 250 mM | 1 M | Helicase |
|---|---|---|---|---|---|
| WT | Hex | Mon | + | ||
| M1 | L189D, D191R | Interface (N terminal) | Mon* | Mon | −− |
| M2 | A416R, A420R | Interface (side channel) | Mon* | Mon | −− |
| M3 | TPDSP550GGGGG | Interface (α-domain) | Mon* | Mon | −− |
| M4 | ILI555DSD | Interface (α-domain) | Mon | Mon | −− |
| M5 | EEV202GGG | Loop 207 (side channel) | Hex | Mon | −− |
| M6 | ED326AA, R329A | EXT hairpin | Hex | Mon | − |
The poly-Ala model was converted to a model with full side chains to aid mutational design. For mutants with 3 or more residues mutated, such as M3 that replaces residues 550–554 with glycine, only the 1st mutated residue is numbered. WT, wild type; Hex, hexamer; Mon, monomer; Mon*, predominantly monomer with a small hexameric component; +, WT activity; −, significantly reduced activity; −−, near abrogation of activity. M1–M4 are mutated residues at the intersubunit interface, and M5 and M6 are functional mutants.
Fig. 3.
Structure-based mutagenesis and functional analysis of the mutants (also see Tables 1 and 2). (A) Location of mutations on the ssoMCM monomer structure. (B) Superose-6 size exclusion FPLC analysis of ssoMCM mutants in 0.25 M (blue line) and 1.0 M (pink line) NaCl. The molecular marker positions are indicated. The calculated molecular mass of ssoMCM monomer is 77 kDa, hexamer 462 kDa. (C) Representative helicase assay. B, Boiled dsDNA; UB, unboiled dsDNA; double lines, dsDNA; single lines, ssDNA. (D) Quantitative analysis of the helicase assay of the mutants, shown as the percentage of WT activity. Error bars represent the standard error from 3 experiments.
Purified wild-type (WT) ssoMCM shows elution peaks consistent with the molecular mass of a hexamer by gel filtration chromatography in a buffer containing 0.25 M NaCl (Fig. 3B), agreeing with previous reports (14, 22, 26–29). Interestingly, ssoMCM can shift to a smaller peak consistent with a monomer in 1.0 M NaCl. In medium salt conditions (0.25 M NaCl), where WT protein exists as hexamers, mutants M1–M4 all eluted predominantly in the monomer peak, providing strong evidence supporting the critical role of these residues in intersubunit interactions for hexamerization. Helicase assays revealed that M1–M4 mutants had essentially undetectable unwinding activity (Fig. 3 C and D), suggesting that these residues are important not only for hexamerization, but also for helicase activity. Mutant M5 mutated residues on the 310-like helix in the N-domain L207 near the side channel (Fig. S3C). Mutant M6 examines the functional role of the 2 acidic residues (ED) and the arginine on the tip of the acidic EXT hairpin located at the exit of the side channel. Both M5 and M6 formed hexamers comparable with WT in the 2 salt concentrations (Fig. 3B), suggesting that they maintained structural integrity. However, the helicase activity of both mutants was greatly reduced (Fig. 3 C and D). This result suggests a critical role of both L207 and the acidic EXT-hairpin around the side channel for helicase function.
To examine further the roles these mutations play in helicase function, we performed DNA-binding assays by fluorescence polarization anisotropy and ATPase assays with the Enzchek phosphate release assay system (Table 2). Both ssDNA and Y-shaped DNA were used for the binding assays. Among all 6 mutants, only mutant M4 had greatly compromised DNA binding and ATPase activities, which may correlate with the fact that M4 is also the only mutant that has completely lost the hexamerization ability under the tested conditions. In addition, M1 displayed very low level of binding to Y-DNA, and M2 had greatly reduced ATPase activity. Perhaps the most interesting mutants are M3 and M5, which showed DNA binding and ATPase activity comparable with those of the WT. Thus, the loss of helicase activity of these 2 mutants are not likely the result of the change of properties in DNA binding and ATP hydrolysis, and mutations in the 2 mutants somehow decoupled the DNA binding and ATP hydrolysis from the strand separation of the dsDNA substrate.
Table 2.
Kinetic parameters of ssoMCM mutants
| Mutant no. | ATPase activity |
ATPase activity + Y-DNA |
ssDNA binding |
Y-DNA binding |
|||
|---|---|---|---|---|---|---|---|
| kcat, min−1 | Km, nM | kcat, min−1 | Km, nM | (Kd), nM | Kd, nM | Hill | |
| WT | 3.1 ± 0.2 | 280 ± 50 | 4.1 ± 1.2 | 1,900 ± 1,200 | 800 ± 200 | 1,000 ± 100 | 2.5 ± 0.7 |
| M1 | 1.8 ± 0.4 | 1,200 ± 700 | 5.4 ± 0.9 | 13,000 ± 2,000 | 2,900 ± 1,400 | Large Kd | |
| M2 | Large Km | Large Km | 1,200 ± 500 | 4,200 ± 1,900 | 1.5 ± 0.5 | ||
| M3 | 3.1 ± 0.5 | 400 ± 200 | 2.8 ± 1.3 | 2,500 ± 2,400 | 170 ± 10 | 1,200 ± 200 | 2.1 ± 0.6 |
| M4 | Large Km | No detectable activity | Large Kd | Large Kd | |||
| M5 | 2.8 ± 0.5 | 220 ± 100 | 3.3 ± 0.7 | 1600 ± 800 | 200 ± 70 | 1100 ± 300 | 1.3 ± 0.4 |
| M6 | 2.7 ± 0.7 | 4,400 ± 1,900 | Large Km | 900 ± 300 | 1,400 ± 200 | 2.1 ± 0.6 | |
Large Km, kcat not informative under the conditions tested because of a high Km value for the mutant, although some activity was detected. Large Kd, >20 μM. Values are per monomer. Standard error is given for each value. Hill, Hill cooperativity coefficient.
Discussion
The crystal structure of ssoMCM reported here reveals the multidomain organization of the molecule and several unique structural features, including: 4 β-hairpins, 2 long interlocking interdomain linkers, and the direct contacts between N and C domains, both within a subunit (intrasubunit) and between subunits (intersubunit) in a hexamer model. A hexamer generated based on the 6-fold symmetry of the N-mtMCM (11) reveals intersubunit distances suitable for bonding throughout the N and C domains, including the conformations of the ATP-binding pocket at the interface between 2 subunits. Additionally, the hexameric model fits into the hexamer/double hexamer 3-dimensional EM map of FL mtMCM and matches the surface contour and the striking side-channel openings of the EM map (20). Furthermore, mutagenesis of the residues at the intersubunit interfaces and around the side channels shows the critical role of these residues for hexamerization and for helicase function.
Mechanisms of N- and C-Terminal Communication.
Previous structural and sequence analysis suggested a long linker bridging the N domain and the C-terminal helicase domain (11). However, biochemical evidence suggests that the N domain of archaeal MCM communicates and interacts with the C-terminal helicase domain (19, 22). This interdomain interaction is implied by the observation that the separately purified N-ssoMCM and the C-ssoMCM cooperate in DNA binding and in helicase function (22). Additionally, an R98A mutation in the mtMCM N domain (ssoMCM R110) reduces the DNA-stimulated ATPase activity of the C domain (19). Further, mutations on L207 by us and others (25) were shown to affect ATPase and helicase activity of ssoMCM and mtMCM. Our ssoMCM structure models reveal that the N and C domains interact with each other, not only within a single subunit (cis-N-C interactions, Fig. S3B), but also between 2 subunits within a hexamer (trans-N-C interactions, Fig. S3C), despite a long 40-residue linker region between the N and C domains.
Forming potential cis-N-C interactions within a subunit, the N-domain strand Nβ4 is positioned next to the C domain H2I hairpin, such that the N domain β-sheet appears to be expanded to the 2 strands of the H2I hairpin (Fig. S3B; see Fig. S1 for helix and strand naming). N domain L207 is directed such that several of the tested mutations could interact with the α/β-domain of the same subunit. Possible trans-N-C interactions in the hexamer model include L207 with both helix Cα3 and the PS1 hairpin from the C domain of the next subunit (Fig. S3C). Additionally, mtMCM R98 in the N domain (R110 in ssoMCM) is located within bonding distance for interacting with the PS1 hairpin of the C domain from the next subunit (Fig. S3C). These cis- and trans-N-C interactions revealed by the hexameric MCM model provide a molecular explanation for the observed cooperation of separately purified N and C domains, the reduced ATPase activity of the mtMCM R98 N-terminal mutation, and the variety of L207 mutations on the N domain that affect ATPase activity and helicase activity (19, 22, 25).
Location of C-Terminal WH Domain.
The C-terminal WH domain was not modeled in our crystal structure. The poorly defined electron density for this WH domain suggests a flexible domain position. Indeed, evidence indicates different locations for the WH domain in the context of a hexamer. FRET analysis suggests that the WH domain is on the side of ssoMCM hexamer and can move toward the N terminus to contact the N domain (14). This proposed WH location would occupy the “valley” (or the waist) on the side of the hexamer (Fig. 1C). However, the obvious valley is not occupied in the EM map of the double hexameric mtMCM (20, 21), suggesting a different location for the WH domain. An alternative WH location is suggested from the EM study of the DNA-bound mtMCM, which shows that the double hexamer has a C-terminal “cap,” possibly consisting of the WH domain, at only 1 of the hexamers (21). Evidence suggests a third possible location for the WH domain. The EM maps of the mtMCM double hexamer without DNA bound reveals weak density at the very C terminus of the central channel (20). If the WH were to be modeled into our FL ssoMCM low-σ level density map, it would also be located around the very C terminus of the hexameric channel (data not shown). Taken together, these data suggest that the WH domain is likely a flexible appendage that adopts different positions.
Multiple β-Hairpin Structural Elements in SsoMCM.
One striking feature of the ssoMCM structure is that there are 4 β-hairpin structural elements located throughout the N and C domains (Fig. 1B), with 3 hairpins (NT hairpin, H2I hairpin, and PS1 hairpin) located in the main channel and 2 hairpins (PS1 hairpin and EXT hairpin) located near the side channels (Fig. 2 B–D). Interestingly, the 3 central channel β-hairpins from 1 subunit are not arranged in a straight line along the hexameric channel; rather, they are offset in such a way that the NT hairpin reaches over to the top of the H2I hairpin of the next subunit, forming a helical arrangement (Fig. 2 B–D), which may have implications in their interactions with helical DNA substrates.
The PS1 hairpin is located at the intersection between the main and side channels. In sequence alignments, this PS1 hairpin aligns with the lone β-hairpin in the central channel of SV40 LTag (16, 30). We have shown that the β-hairpin protruding into the central channel of LTag moves along the channel in response to ATP binding and hydrolysis (16, 17), which are likely coupled to DNA translocation and unwinding. Additional experiments will be required to determine whether the PS1 hairpin or other β-hairpins of ssoMCM will also have a similar “power-stroke” for DNA translocation and unwinding. However, similar to the LTag β-hairpin, the ssoMCM PS1 and H2I hairpins connect to the ATP-binding site through strands 2, 3, and 4 of the AAA+ core and border the ATP site of the neighboring subunit. This structural arrangement suggests that ATP binding/hydrolysis may also be able to trigger the movement of the PS1 hairpin and the H2I hairpin for helicase function. Indeed, mutation of residues on the PS1 hairpin in ssoMCM or deletion within the H2I hairpin sequence in mtMCM abolishes helicase activity (14, 19), suggesting a critical role in helicase function for these 2 β-hairpins (19, 31, 32).
The acidic β-hairpin located at the exit of the side channels is also directly connected to the ATP P loop through β-strand 1. Therefore, the EXT hairpin may also respond to ATP binding/hydrolysis. Our mutational data demonstrated that the residues at the tip of the EXT hairpin are vital for helicase activity (mutant M6; Fig. 3 and Table 2) but not DNA binding. Additionally, an arginine mutation at the base of the EXT hairpin (R331A) removes ATPase and helicase activity (27), which also suggests the important role of the EXT hairpin for function. The location of the EXT hairpin at the side-channel exit, together with the PS1 hairpin at the side-channel entrance from the central channel, suggests an intriguing possibility that these 2 β-hairpins may work together to interact with DNA passing through the side channels during unwinding.
Potential DNA-Unwinding Modes by SsoMCM.
Based on the structural and biochemical data of ssoMCM, 2 possible DNA-unwinding modes for the hexameric ssoMCM are represented by simple diagrams in Fig. 4. The locations of the 4 β-hairpins of ssoMCM are schematically shown in a 2-dimensional hexamer diagram in Fig. 4A. One unwinding model (Fig. 4B) is similar to the “wedge” (or steric exclusion) model proposed for DnaB (1, 33, 34), with 1 DNA strand passing through the central channel, and the other being excluded from the channel. The PS1 hairpin seems less accessible in this unwinding mode, but the EXT hairpin may directly participate in coordinating the position of the 5′ strand, perhaps disengaging the 5′ strand during unwinding, providing a basis for the “opposite-strand interaction” model proposed recently (34).
Fig. 4.
Two possible DNA unwinding modes by MCM helicase. (A) Schematic representation of a MCM hexamer helicase. The 4 β-hairpins (NT, H2I, PS1, and EXT hairpins) are represented by short solid bars; the central channel and the side channels are in darker shades. (B) Steric exclusion model for a single-hexameric MCM helicase. (C) Side-channel extrusion model, showing ssDNA extruding from the side channel. DNA is shown as black lines. Arrows indicate direction of helicase movement.
The second unwinding model in Fig. 4C shows a hexameric helicase binding a dsDNA region ahead of the fork, extruding ssDNA strands from a side channel. In this model, the 3 β-hairpins in the helicase domain all interact with DNA directly during unwinding, as does the NT hairpin. The unwinding modes presented in Fig. 4 B and C can be adapted to suit a double-hexamer helicase. The validation of these models requires further studies.
In this report, we describe the crystal structure of near-FL ssoMCM, which reveals several new structural features and uncovers the multidomain organization of FL MCM, both as an individual subunit and in a hexameric model. Moreover, our structure-based mutational data provide experimental evidence supporting the important role of several key structural features, including that of the MCM hexamerization interface for helicase function. These structural and biochemical data provide a foundation for future investigation of the functional role of archaeal and eukaryotic MCM complexes in DNA replication.
Materials and Methods
Crystallization, Data Collection, and Structural Determination.
The FL MCM construct (residues 1–686) and a truncation mutant T612 (residues 1–612) have been crystallized (see SI Text for details), and native and Se-Met diffraction data were collected (Table S1). Experimental phases to 4.6 Å and 4.35 Å resolution were determined for both constructs using SAD data. The phases were further improved by density modification using solvent flattening and histogram matching. The improved electron density maps from both FL and T612 are very similar to each other, with the T612 map having more featured helices because of slightly higher resolution. Secondary structure elements and domain organization are clearly recognizable in most parts of the density map, as expected for the resolution range of the crystallographic map. The N-ssoMCM crystal structure (PDB ID code 2VL6) was immediately docked into the N domain of the map by automated phased-translation searches. The α- and β-core domain (α/β-domain) taken from the bchI and cdc6 AAA+ domain (PDB ID codes 1G8P and 1FNN) (35, 36) was subsequently docked into the map automatically by using the phased-translation searches and rebuilt. Other helices and loops were built by using the graphics program COOT. To the best of our knowledge, the amino acid registry is correct; however, chain-trace registry errors may still be present because of the polyalanine nature of the structure. A more detailed description of structural determination is included in the SI Text and Fig. S5.
For experimental details on molecular cloning, protein purification, and the functional assays of helicase, DNA binding, and ATPase activities, please see SI Text.
Supplementary Material
Acknowledgments.
We thank Dr. U. Sen for help with crystallization and data collection, Dr. R. Zhang at 19-ID beamline in Argonne National laboratory, and the staff at the 8.2.1 beamline at the Berkeley Advanced Light Source for assistance with data collection.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 3F9V).
This article contains supporting information online at www.pnas.org/cgi/content/full/0808037105/DCSupplemental.
References
- 1.Lee JK, Hurwitz J. Processive DNA helicase activity of the minichromosome maintenance proteins 4, 6, and 7 complex requires forked DNA structures. Proc Natl Acad Sci USA. 2001;98:54–59. doi: 10.1073/pnas.98.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishimi Y. A DNA helicase activity is associated with an MCM4, -6, and -7 protein complex. J Biol Chem. 1997;272:24508–24513. doi: 10.1074/jbc.272.39.24508. [DOI] [PubMed] [Google Scholar]
- 3.You Z, Komamura Y, Ishimi Y. Biochemical analysis of the intrinsic Mcm4–Mcm6–mcm7 DNA helicase activity. Mol Cell Biol. 1999;19:8003–8015. doi: 10.1128/mcb.19.12.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendez J, Stillman B. Perpetuating the double helix: Molecular machines at eukaryotic DNA replication origins. Bioessays. 2003;25:1158–1167. doi: 10.1002/bies.10370. [DOI] [PubMed] [Google Scholar]
- 5.Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- 6.Bowers JL, Randell JC, Chen S, Bell SP. ATP hydrolysis by ORC catalyzes reiterative Mcm2–7 assembly at a defined origin of replication. Mol Cell. 2004;16:967–978. doi: 10.1016/j.molcel.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 7.Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- 8.Tye BK. MCM proteins in DNA replication. Annu Rev Biochem. 1999;68:649–686. doi: 10.1146/annurev.biochem.68.1.649. [DOI] [PubMed] [Google Scholar]
- 9.Kelman Z, Lee JK, Hurwitz J. The single minichromosome maintenance protein of Methanobacterium thermoautotrophicum DeltaH contains DNA helicase activity. Proc Natl Acad Sci USA. 1999;96:14783–14788. doi: 10.1073/pnas.96.26.14783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chong JP, Hayashi MK, Simon MN, Xu RM, Stillman B. A double-hexamer archaeal minichromosome maintenance protein is an ATP-dependent DNA helicase. Proc Natl Acad Sci USA. 2000;97:1530–1535. doi: 10.1073/pnas.030539597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher RJ, et al. The structure and function of MCM from archaeal M. Thermoautotrophicum. Nat Struct Biol. 2003;10:160–167. doi: 10.1038/nsb893. [DOI] [PubMed] [Google Scholar]
- 12.Kelman Z, White MF. Archaeal DNA replication and repair. Curr Opin Microbiol. 2005;8:669–676. doi: 10.1016/j.mib.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Liu W, Pucci B, Rossi M, Pisani FM, Ladenstein R. Structural analysis of the Sulfolobus solfataricus MCM protein N-terminal domain. Nucleic Acids Res. 2008;36:3235–3243. doi: 10.1093/nar/gkn183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGeoch AT, Trakselis MA, Laskey RA, Bell SD. Organization of the archaeal MCM complex on DNA and implications for the helicase mechanism. Nat Struct Mol Biol. 2005;12:756–762. doi: 10.1038/nsmb974. [DOI] [PubMed] [Google Scholar]
- 15.Iyer LM, Leipe DD, Koonin EV, Aravind L. Evolutionary history and higher order classification of AAA+ ATPases. J Struct Biol. 2004;146:11–31. doi: 10.1016/j.jsb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Li D, et al. Structure of the replicative helicase of the oncoprotein SV40 large tumour antigen. Nature. 2003;423:512–518. doi: 10.1038/nature01691. [DOI] [PubMed] [Google Scholar]
- 17.Gai D, Zhao R, Li D, Finkielstein CV, Chen XS. Mechanisms of conformational change for a replicative hexameric helicase of SV40 large tumor antigen. Cell. 2004;119:47–60. doi: 10.1016/j.cell.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Ingleston SM, Sharples GJ, Lloyd RG. The acidic pin of RuvA modulates Holliday junction binding and processing by the RuvABC resolvasome. EMBO J. 2000;19:6266–6274. doi: 10.1093/emboj/19.22.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkinson ER, Chong JPJ. Minichromosome maintenance helicase activity is controlled by N- and C-terminal motifs and requires the ATPase domain helix-2 insert. Proc Natl Acad Sci USA. 2006;103:7613–7618. doi: 10.1073/pnas.0509297103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gómez-Llorente Y, Fletcher RJ, Chen XS, Carazo JM, San Martín C. Polymorphism and double hexamer structure in the archaeal minichromosome maintenance (MCM) helicase from Methanobacterium thermoautotrophicum. J Biol Chem. 2005;280:40909–40915. doi: 10.1074/jbc.M509760200. [DOI] [PubMed] [Google Scholar]
- 21.Costa A, et al. Structural basis of the Methanothermobacter thermautotrophicus MCM helicase activity. Nucleic Acids Res. 2006;34:5829–5838. doi: 10.1093/nar/gkl708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barry ER, McGeoch AT, Kelman Z, Bell SD. Archaeal MCM has separable processivity, substrate choice and helicase domains. Nucleic Acids Res. 2007;35:988–998. doi: 10.1093/nar/gkl1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada K, et al. Crystal structure of the RuvA–RuvB complex: A structural basis for the Holliday junction migrating motor machinery. Mol Cell. 2002;10:671–681. doi: 10.1016/s1097-2765(02)00641-x. [DOI] [PubMed] [Google Scholar]
- 24.Ohnishi T, Hishida T, Harada Y, Iwasaki H, Shinagawa H. Structure–function analysis of the three domains of RuvB DNA motor protein. J Biol Chem. 2005;280:30504–30510. doi: 10.1074/jbc.M502400200. [DOI] [PubMed] [Google Scholar]
- 25.Sakakibara N, et al. Coupling of DNA binding and helicase activity is mediated by a conserved loop in the MCM protein. Nucleic Acids Res. 2008;36:1309–1320. doi: 10.1093/nar/gkm1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carpentieri F, De Felice M, De Falco M, Rossi M, Pisani FM. Physical and functional interaction between the minichromosome maintenance-like DNA helicase and the single-stranded DNA-binding protein from the crenarchaeon Sulfolobus solfataricus. J Biol Chem. 2002;277:12118–12127. doi: 10.1074/jbc.M200091200. [DOI] [PubMed] [Google Scholar]
- 27.Moreau MJ, McGeoch AT, Lowe AR, Itzhaki LS, Bell SD. ATPase site architecture and helicase mechanism of an archaeal MCM. Mol Cell. 2007;28:304–314. doi: 10.1016/j.molcel.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Pucci B, De Felice M, Rossi M, Onesti S, Pisani FM. Amino acids of the Sulfolobus solfataricus minichromosome maintenance-like DNA helicase involved in DNA binding/remodeling. J Biol Chem. 2004;279:49222–49228. doi: 10.1074/jbc.M408967200. [DOI] [PubMed] [Google Scholar]
- 29.Pucci B, et al. Modular organization of the Sulfolobus solfataricus minichromosome maintenance protein. J Biol Chem. 2007;282:12574–12582. doi: 10.1074/jbc.M610953200. [DOI] [PubMed] [Google Scholar]
- 30.Shen J, Gai D, Patrick A, Greenleaf WB, Chen XS. The roles of the residues on the channel β-hairpin and loop structures of simian virus 40 hexameric helicase. Proc Natl Acad Sci USA. 2005;102:11248–11253. doi: 10.1073/pnas.0409646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi TS, Wigley DB, Walter JC. Pumps, paradoxes and ploughshares: Mechanism of the MCM2–7 DNA helicase. Trends Biochem Sci. 2005;30:437–444. doi: 10.1016/j.tibs.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Chong JP. Learning to unwind. Nat Struct Mol Biol. 2005;12:734–736. doi: 10.1038/nsmb0905-734. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan DL, Davey MJ, O'Donnell M. MCM4,6,7 uses a “pump in ring” mechanism to unwind DNA by steric exclusion and actively translocate along a duplex. J Biol Chem. 2003;278:49171–49182. doi: 10.1074/jbc.M308074200. [DOI] [PubMed] [Google Scholar]
- 34.Rothenberg E, Trakselis MA, Bell SD, Ha T. MCM forked substrate specificity involves dynamic interaction with the 5′-tail. J Biol Chem. 2007;282:34229–34234. doi: 10.1074/jbc.M706300200. [DOI] [PubMed] [Google Scholar]
- 35.Fodje MN, et al. Interplay between an AAA module and an integrin I domain may regulate the function of magnesium chelatase. J Mol Biol. 2001;311:111–122. doi: 10.1006/jmbi.2001.4834. [DOI] [PubMed] [Google Scholar]
- 36.Liu J, et al. Structure and function of Cdc6/Cdc18: Implications for origin recognition and checkpoint control. Mol Cell. 2000;6:637–648. doi: 10.1016/s1097-2765(00)00062-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.