Abstract
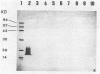
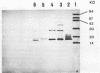
A monoclonal antibody to Serpulina hyodysenteriae 8930 was produced and was used to probe pronase-treated cell lysates of S. hyodysenteriae isolates in immunblots. The results showed that the monoclonal antibody was specific for only five closely related S. hyodysenteriae isolates: 8930, 5380, 70A, RMIT 88, and RMIT 97.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum D. H., Joens L. A. Serotypes of beta-hemolytic Treponema hyodysenteriae. Infect Immun. 1979 Sep;25(3):792–796. doi: 10.1128/iai.25.3.792-796.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding J. W. Antibody production by hybridomas. J Immunol Methods. 1980;39(4):285–308. doi: 10.1016/0022-1759(80)90230-6. [DOI] [PubMed] [Google Scholar]
- Hampson D. J., Mhoma J. R., Combs B. G., Lee J. I. Serological grouping of Treponema hyodysenteriae. Epidemiol Infect. 1990 Aug;105(1):79–85. doi: 10.1017/s0950268800047671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson D. J., Mhoma J. R., Combs B., Buddle J. R. Proposed revisions to the serological typing system for Treponema hyodysenteriae. Epidemiol Infect. 1989 Feb;102(1):75–84. doi: 10.1017/s0950268800029708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson D. J. Slide-agglutination for rapid serological typing of Treponema hyodysenteriae. Epidemiol Infect. 1991 Jun;106(3):541–547. doi: 10.1017/s0950268800067601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. L., Glock R. D., Christensen C. R., Kinyon J. M. Inoculation of pigs with Treponema hyodysenteriae (new species) and reproduction f the disease. Vet Med Small Anim Clin. 1972 Jan;67(1):61–64. [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mapother M. E., Joens L. A. New serotypes of Treponema hyodysenteriae. J Clin Microbiol. 1985 Aug;22(2):161–164. doi: 10.1128/jcm.22.2.161-164.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Songer J. G., Kinyon J. M., Harris D. L. Selective medium for isolation of Treponema hyodysenteriae. J Clin Microbiol. 1976 Jul;4(1):57–60. doi: 10.1128/jcm.4.1.57-60.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. J., Alexander T. J. The production of dysentery in swine by feeding cultures containing a spirochaete. Br Vet J. 1971 Nov;127(11):58–61. doi: 10.1016/s0007-1935(17)37282-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]