Abstract
Oscillatory rhythms in different frequency ranges mark different behavioral states and are thought to provide distinct temporal windows that coherently bind cooperating neuronal assemblies. However, the rhythms in different bands can also interact with each other, suggesting the possibility of higher-order representations of brain states by such rhythmic activity. To explore this possibility, we analyzed local field potential oscillations recorded simultaneously from the striatum and the hippocampus. As rats performed a task requiring active navigation and decision making, the amplitudes of multiple high-frequency oscillations were dynamically modulated in task-dependent patterns by the phase of cooccurring theta-band oscillations both within and across these structures, particularly during decision-making behavioral epochs. Moreover, the modulation patterns uncovered distinctions among both high- and low-frequency subbands. Cross-frequency coupling of multiple neuronal rhythms could be a general mechanism used by the brain to perform network-level dynamical computations underlying voluntary behavior.
Keywords: amplitude modulation, gamma, theta
Oscillations in neural population voltage activity are universal phenomena (1). Among brain rhythms, theta oscillations in local field potentials (LFPs) recorded in the hippocampus are prominent during active behaviors (2–5), and these have long been intensively analyzed in the rodent in relation to spatial navigation (6), memory (7), and sleep (8). Theta-band rhythms (4–12 Hz) are now known to occur in other cortical (9–12) and subcortical (12–15) regions, however, including the striatum (14–17), studied here. Gamma oscillations (30–100 Hz) have also received special attention because of their proposed role in functions such as sensory binding (18), selective attention (19–21), transient neuronal assembly formation (22), and information transmission and storage (23–25). The existence of physiologically meaningful neocortical oscillations at even higher frequencies, above the traditional gamma range, has been reported as well (10, 26–28). In rodents, for example, brief sharp-wave associated ripples (120–200 Hz) appear in the hippocampal formation during slow wave sleep, immobility and consummatory behavior, characteristically in the absence of theta waves (2, 29).
The oscillatory activities conventionally assigned to different frequency bands are not completely independent (2–4, 9, 10, 30). In one type of interaction, the phase of low-frequency rhythms modulates the amplitude of higher-frequency oscillations (9, 10, 30). For example, theta phase is known to modulate gamma power in rodent hippocampal and cortical circuits (2–4, 31), and the phase of theta rhythms recorded in the human neocortex can modulate wide-band (60–200 Hz) high-frequency oscillations (10). Such theta–gamma nesting is thought to play a role in sequential memory organization and maintenance of working memory, and more generally in “phase coding” (25, 31). Based on evidence suggesting that theta rhythms in hippocampal and striatal memory circuits are coordinated in rats during learning and performance of a conditional T-maze task (14), we asked whether theta phase modulates cooccurring high-frequency oscillations in the striatum as well as in the hippocampus, and if so whether such cross-frequency effects occur between the 2 structures, and whether the phase–amplitude coupling is related to specific behavioral performance. We demonstrate here that distinct bands of high-frequency oscillations are modulated by ongoing low-frequency rhythms, both within and across the striatum and hippocampus. We further show that the strength of these cross-frequency interactions changes dynamically, and differentially, during different epochs of behavioral performance requiring decision and action. These findings suggest that the cross-frequency interactions reflect behaviorally relevant simultaneous activation of synchronized striatal and hippocampal memory circuits.
Results
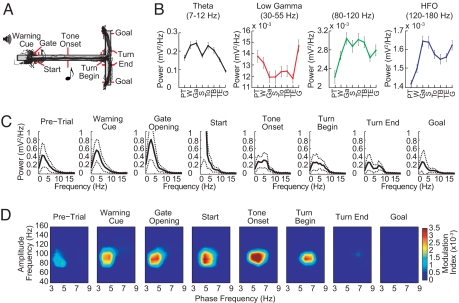
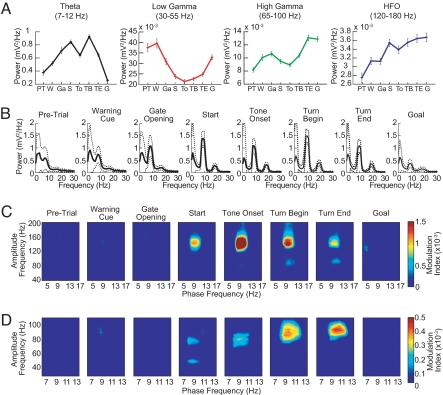
We analyzed the LFP oscillatory activity recorded in the dorsal caudoputamen and the CA1 field of the dorsal hippocampus as rats (n = 6) navigated a T-maze in which they turned right or left in response to auditory instruction cues indicating which of the 2 end arms was baited with chocolate (14, 15) (Fig. 1A). In both the striatum and the hippocampus, theta power increased as the rats left the start zone, peaked as the animals traversed the maze, and diminished as the rats approached the goal [Figs. 1 B and C and 2 A and B and supporting information (SI) Fig. S1]. By contrast, low gamma power (LG, 30–60 Hz) diminished during the middle of the task, and high gamma (HG, 60–100 Hz) and high-frequency oscillations (HFO, >100 Hz) powers increased throughout the maze runs (Figs. 1 B and C and 2 A and B and Fig. S1). Notably, these modulations in power had different time courses in the 2 structures (see Figs. 1 B and C and 2 A and B and Fig. S1).
Fig. 1.
Dynamic amplitude modulation of fast LFP rhythms by theta phase in the striatum during maze runs. (A) T-maze with task events and run trajectories from a representative session with 39 trials. Red markers show photobeam positions. (B) Average power of striatal oscillations for successive event windows (1 s) over 4 frequency ranges of interest. Error bars represent SEM. Event labels: PT, pre-trial; W, warning cue; Ga, gate opening; S, start; To, tone onset; TB, turn begin; TE, turn end; G, goal reaching. (C) Mean power spectra (solid lines) showing characteristic changes in the power peak during perievent windows. Dashed lines represent ±SD. (D) Phase-to-amplitude comodulograms plotted for each task-event window. Pseudocolor scale represents modulation index values shown at right. Positive values indicate a statistically significant (P < 0.01) phase-to-amplitude cross-frequency coupling (see SI Text). Results illustrated in B–D were obtained from a striatal tetrode in a representative rat by analyzing all trials in the session shown in A.
Fig. 2.
Phase-to-amplitude modulation in the hippocampus. (A) Average power of hippocampal oscillations recorded in CA1 and plotted for each task-event window over 4 frequency ranges of interest. Events are labeled as in Fig. 1B. (B). Mean power spectra recorded during the successive task epochs. (C and D) Phase-to-amplitude comodulograms obtained from LFPs recorded during the distinct task-event windows shown. Plots in C show amplitude modulation of rhythms over the entire high-frequency range studied (40–200 Hz). Plots in D show modulation in the high frequency range focused on gamma, from 30 to 110 Hz. Results illustrated in A–D were obtained from a tetrode in the superficial layer of CA1 in a representative rat by analyzing all trials (n = 40) in a session.
To determine whether interactions across these frequency ranges occurred, we developed a cross-frequency measure to analyze phase-to-amplitude modulation in limited-time datasets (modulation index, see SI Text). This method allowed us to examine phase–amplitude modulation for successive event epochs during the maze runs. Phase-to-amplitude comodulograms were constructed by applying this measure to multiple frequency band pairs made up of “phase frequency” and “amplitude frequency” bands stepped through task time (Figs. 1D and 2 C and D and Fig. S2).
In both the striatum and the hippocampus, phase–amplitude couplings characteristically emerged as the animals traversed the maze and tended to disappear at goal reaching (Figs. 1D and 2 C and D). In the striatum, theta-band oscillations dynamically modulated a narrow band of higher-frequency oscillations (≈80–120 Hz), and the modulation tended to occur at low (≈3–8 Hz) theta frequencies (Fig. 1D). Theta phase in the hippocampus modulated the amplitude of a wider range of high frequencies (≈40–350 Hz), with the strongest coupling occurring at the HFO frequency band (Figs. 2C and 4D and Fig. S3). The theta-band oscillations responsible for phase–amplitude coupling in the hippocampus were in a narrower band and generally higher in frequency (≈8–12 Hz) than those in the striatum (Figs. 1D and 2 C and D, but see Fig. S4). Phase-to-amplitude modulation in the hippocampus was found in all 6 rats studied; modulation in the striatum was found in 4 of the 6 rats studied.
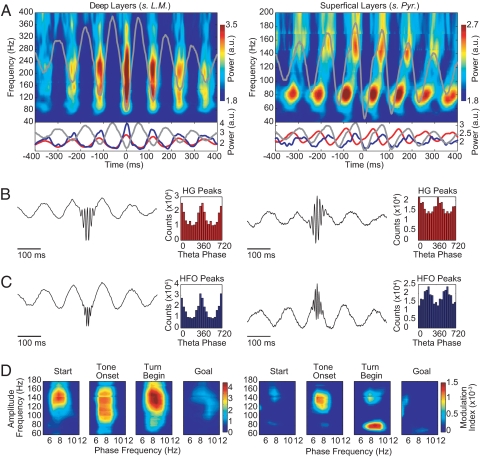
Fig. 4.
The theta phase modulation of high-frequency hippocampal LFP oscillations differs for rhythms in different frequency bands and layers in the CA1 region of the dorsal hippocampus. (A) (Upper) Time–frequency plots of the mean normalized power time-locked to the theta (7–12 Hz) trough for the deep (Left) and superficial (Right) CA1 layers recordings. (Lower) Plots showing the mean normalized power at 80 Hz (HG, red line) and at 160 Hz (HFO, blue line). The theta trough-locked averaged raw signal is shown in gray in all plots. (B and C) Averaged raw signal obtained by centering the LFP traces at the peaks of the HG (B) or HFO (C) and the corresponding histograms of the theta phases at which the peaks occurred. (D) Phase-to-amplitude comodulograms showing differential theta modulations of HG and HFO rhythms. Results are shown for simultaneous recordings from 1 deep (left column) and 1 superficial (right column) CA1 layer tetrode in a representative rat.
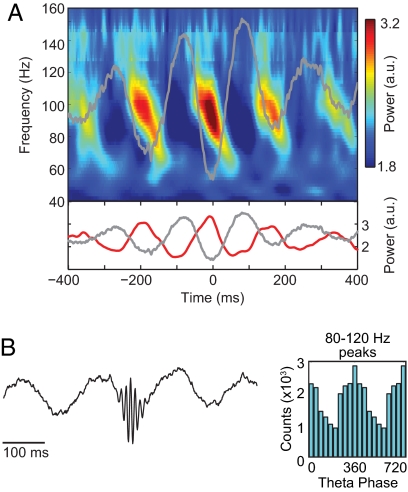
Within both structures, the couplings exhibited consistent patterns. In the striatum, 80- to 120-Hz oscillations peaked at the troughs of the theta wave (Fig. 3), a pattern similar to that reported for electrocorticogram recordings of oscillatory activity in the human neocortex (10). In the hippocampus, phase–amplitude interactions depended on both the frequency band and the CA1 layer in which the recordings were made. The HG and HFO amplitudes peaked at the trough of the theta oscillation at the deep recording sites (Fig. 4 A–C Left and Fig. S5). At the superficial recording sites, the HG power peaked on the rising phase of theta, and the HFO power was maximal near the peak of the theta wave (Fig. 4 A–C Right and Fig. S5). The opposite preferred theta phase for the HFO powers recorded at the deep and superficial layers* likely relates to the well-established phase-reversal of the theta rhythm across the CA1 layers also evident in our recordings (Fig. S5; see also ref. 3). However, this reversal does not account for the differences in preferred phases between the HFO and HG frequency bands in the superficial CA1 layers.
Fig. 3.
The amplitude of striatal 80–120 Hz LFP oscillations is maximal at the troughs of cooccurring striatal theta oscillations. (A) (Upper) Time–frequency plot of the mean normalized power time-locked to the theta (5–8 Hz) trough. (Lower) Plot showing the mean normalized power at 100 Hz (red line). The theta trough-locked averaged raw signal is shown in both Upper and Lower plots as a gray line. (B) (Left) Averaged raw signal obtained by aligning the LFP traces at the peaks of the 80- to 120-Hz oscillation (see SI Text). (Right) The histogram of the theta phases at which the peaks occurred. Results were obtained from the same animal and experimental session as in Fig. 1.
The amplitude modulation of high-frequency rhythms by cooccurring theta was correlated with the power of theta both in the striatum and in the hippocampus, with stronger modulation occurring at greater theta powers (Figs. S6 and S7). The strongest comodulations thus occurred during the middle parts of the maze runs. However, the presence of the theta rhythm per se did not guarantee the existence of the cross-frequency phase-to-amplitude coupling. The peak of theta power in the striatal LFPs did not always match the peak of the cross-frequency modulation observed in the striatum (e.g., Fig. 1 C and D). In the hippocampal LFPs, clear theta peaks occurred during the pre-trial, warning cue, gate-opening, and goal-reaching periods, when cross-frequency coupling was typically not observed (see Fig. 2 B–D). Moreover, comparable levels of hippocampal theta were associated with different modulation index values (e.g., compare the “Gate Opening” and “Tone Onset” events in Fig. 2).
Multiple regression analysis demonstrated that for any given high-frequency rhythm, the amplitude modulation depended partly on its own power and partly on the power of other high-frequency rhythms, in addition to the power of the cooccurring theta rhythm (Fig. S7). Likewise, although the animals' running speeds increased up to the middle of the maze runs and then decreased toward goal reaching, the relation between the intensity of the cross-frequency coupling and the animals' speed was not a straightforward one: Speed-controlled analyses demonstrated that changes in speed alone cannot account for the distinct levels of modulation among the task events (Fig. S8).
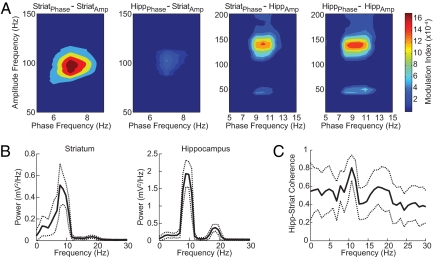
The presence of phase-amplitude coupling during the midrun period suggested that the coupling might be related to the presence of heightened coherence between the striatal and the hippocampal rhythms, which also tends to occur during this epoch (14). As shown in Fig. 5 for the “Tone Onset” period, cross-structure coupling did occur. The phase–amplitude couplings between the striatal theta phase and the amplitude of the hippocampal fast oscillations were very prominent (Fig. 5A, third image from the left). The hippocampal theta modulation of striatal 80- to 120-Hz oscillations was much weaker (Fig. 5A, second image from the left). Notably, the striatal–hippocampal cross-structure coupling was strongest at the high theta-band frequencies (8–12 Hz), frequencies that within the hippocampus modulated hippocampal gamma and HFO rhythms, and frequencies at which the striatal and hippocampal theta rhythms were most highly coherent (Fig. 5C, see also ref. 14). By contrast, it was the low-frequency striatal theta band (3–8 Hz) that modulated the striatal 80- to 120-Hz oscillations, frequencies at which the striatal and hippocampal theta rhythms were less coherent (Fig. 5C). The cross-structure coupling patterns were thus consistent with the patterns of coherence between the theta rhythms in the striatum and hippocampus. Cross-structure phase–amplitude interactions could therefore occur, but they were constrained by subbands within the traditional theta range. It is striking that the frequency constraints for intrastriatal phase–amplitude coupling are different from the constraints for striatal–hippocampal coupling.
Fig. 5.
Phase-amplitude couplings occur between simultaneously recorded striatal and hippocampal oscillations. (A) Phase-to-amplitude comodulograms obtained during a 1-s interval around the Tone Onset task event. Results are shown for all phase–amplitude combinations as labeled. Note that the theta phase in each structure modulates the amplitude of oscillations in the other structure. (B) Mean power spectrum (solid line) of the LFPs recorded in each brain region during the same task period (Tone Onset), showing a peak in the theta band in both regions. Dashed lines represent ±SD. (C) Coherence spectrum (solid line) between the striatal and the hippocampal oscillations during the same task period showing a peak of coherence at ≈10 Hz. Results were obtained from a representative animal during a session (different rat than in Fig. 1).
A relation between behavioral learning and patterns of striatal–hippocampal theta coherence has been suggested because high levels of cross-structure theta coherence were found in rats that learned the T-maze task used here but not in rats that failed to learn the task (14). In our analysis, we found clear examples of correlations between the phase-amplitude modulation and learning scores (Fig. S9). Our dataset was inappropriate to analyze fully the relationship between the modulation index and the percentage correct performance of all of the rats (see Fig. S9 legend). However, this initial analysis suggests that the phase–amplitude coupling we detected may be related to learning state as well as to active on-line behavioral state.
Discussion
Our findings were unequivocal in suggesting that phase–amplitude coupling is a prominent feature of the oscillatory LFP activity both in the striatum and in the hippocampus under conditions of active, goal-oriented behavior. These dynamic phase–amplitude modulations were distinct for different high-frequency bands modulated by theta phase and for different subbands within the theta range and thus suggested previously undescribed, behaviorally relevant frequency ranges for both striatal and hippocampal oscillations. Moreover, the phase of striatal theta could modulate high-frequency oscillations not only in the striatum but also in the hippocampus, and hippocampal–striatal modulation also was present. Adjustments in phase–amplitude coupling thus occurred not only within but also across striatal and hippocampal circuits during active behavior. The strongest phase–amplitude coupling tended to occur during behavioral epochs involving decision and behavioral choice, suggesting that the couplings relate, at least in part, to ongoing cognitive demands. These findings suggest that dynamic, frequency-specific phase–amplitude coupling may be a key feature coordinating the activity of striatal and hippocampal learning circuits during sequential voluntary behavior.
Multiple High-Frequency Bands Are Modulated by Theta Phase.
The striatal high-frequency range for which we found amplitude modulation by striatal theta phase was ≈80–120 Hz. It is striking that we did not observe phase–amplitude coupling for the low-gamma (30–60 Hz) range, which is the main gamma band so far analyzed in LFP recording experiments in the rodent striatum (14–17, 32, 33). Furthermore, the theta frequencies for which we found the modulation tended to be in the low theta range (≈3–8 Hz), despite the cooccurrence of strong theta oscillations at higher frequencies within the theta band (≈8–12 Hz). Oscillatory activity in the striatal LFPs likely reflect rhythms in striatal inputs both from distant sources such as the thalamus and neocortex and from local sources, particularly the pallidum, itself part of a subthalamo–pallidal oscillatory circuit (34, 35). Interneurons in the striatum have also been found to exhibit oscillatory activity, and although some of their oscillatory activity has been linked to that of the neocortex, some may be intrinsic or be driven by intrastriatal networks (16, 36, 37). For most of these potential sources, the presence of phase–amplitude coupling has not been examined. However, our finding that the modulated high-frequency striatal LFP oscillations tend to occur at the trough of striatal theta, in the pattern of neocortical theta–gamma modulations, suggests that the striatal couplings are part of a broader network of oscillatory activity coupling that includes the neocortex. Cross-frequency coupling between 8 and 12 Hz and gamma oscillations has been reported for the human nucleus accumbens (38). Moreover, the phases of maximal gamma amplitude were reported to vary between trials in which subjects won or lost. These findings suggest a relationship to reward circuits and, thus, like our own findings, point to a potential function for cross-frequency coupling effects in basal ganglia-based circuits.
In contrast to this striatal phase–amplitude coupling, theta–gamma modulation in the hippocampus is well-known. Hippocampal theta modulates 30- to 100-Hz frequencies as rats engage in exploratory behavior (3). In addition, high-frequency ripples (140–200 Hz) are modulated by lower-frequency sharp-wave events during periods of rest and sleep (2, 29). Here, we demonstrate simultaneous modulation of both traditional gamma-frequency oscillations (30–100 Hz) and higher-frequency rhythms (>100 Hz) by ongoing hippocampal theta as rats perform a T-maze task. Our results suggest that within the traditional gamma frequency interval (2–4, 9, 10, 23–28), there are 3 distinct bands for hippocampal LFP oscillations modulated by theta rhythms: LG, HG, and HFO.
The LG and HG hippocampal oscillations varied differently in power during different parts of the maze runs and also had different phase–amplitude modulation patterns. Overall, theta phase tended to modulate HG activity more than LG activity in the CA1 region (see Fig. S3). The LG and HG activity thus may represent independent physiological processes, a conclusion in accord with findings for gamma rhythms recorded in the olfactory bulb (39). The HG and HFO oscillations were also distinct. Their theta modulations were strongest at different times during the maze runs (Figs. 2 C and D, 4D Right, and Fig. S8C), were modulated to different degrees by theta phase (Figs. 2 C and D, 4D, and Fig. S8C), peaked at different phases of theta (Fig. 4 A–C Right), and were mutually independent (Fig. S7b). These findings may relate to the proposal that, in the human neocortex, “low gamma” (30–60 Hz) and “high gamma” (≈60–250 Hz but typically focused on 80–150 Hz) can be distinguished (27) and may result from independent physiological mechanisms with different functions (26–28). Given the heterogeneity in nomenclature, we reserve the term “gamma” to denote the lower high-frequency ranges that have been characterized as inhibition-based (40).
The HFO that we identified in the phase–amplitude analyses probably are distinct from the hippocampal ripple oscillations, because the latter are usually accompanied by sharp waves and are not related to the theta rhythm (2, 29, 31). They are also likely not the hippocampal “very fast oscillations” (VFO) described as depending on axonal gap junctions (41), because these have been most prominently observed in the stratum oriens of the CA1 region and were shown to be modulated by the gamma rhythm (42), in contrast to the HFO described here. One possibility is that the HFO are the remnants of the spiking activity detected by the tetrodes. Another possibility is that the HFO result from entorhinal synaptic input to CA1 (43) and HG from CA3 input. If so, cross-frequency analyses could be used to study entorhinal and CA3 influences on CA1 activity and their functions in relation to memory and navigation.
Cross-Coupling of Low- and High-Frequency Oscillations Occurs Across Striatal and Hippocampal Circuits.
In addition to prominent within-structure phase–amplitude coupling in the striatum and hippocampus, we found cross-structure coupling. This coupling was particularly evident for striatal theta phase modulation of hippocampal fast oscillations. The relative weakness of hippocampal–striatal phase–amplitude coupling that we detected raises the possibility that these coupling interactions are asymmetric. Like the within-structure phase–amplitude modulation, the cross-structure modulation was strongest during midrun, the period in which coherence between the theta rhythms in the striatum and hippocampus is maximal (14). The cross-structure couplings were highly frequency-dependent, however, suggesting that they represent specific coupling of striatal–hippocampal rhythms rather than simply a general synchronization of the LFP activity in these 2 structures. The striatum and hippocampus have largely been considered as parallel, and even competing, learning and memory systems engaged differentially in procedural and episodic memory function (44–47). Our findings raise the possibility that these 2 circuitries can interact at multiple levels, including not only by coherence within their theta bands (14), but also by modulation of their high-frequency oscillations by low-frequency oscillations in the other structure.
The existence of such dynamic cross-coupling presents a challenge to the view that activity in single frequency bands satisfactorily captures the oscillatory activity related to particular brain states. Considered together with previous observations (3, 4, 9, 10, 31, 48), our findings suggest, instead, that multiple coexisting patterns of cross-frequency coupling occur and may actually better characterize different states. Our observation of cross-structure striatal–hippocampal coupling is a striking demonstration of this possibility. We observed prominent phase–amplitude coupling for intrastriatal rhythms at low theta-band striatal LFP frequencies (≈3–8 Hz), and cooccurring striatal–hippocampal phase–amplitude coupling for higher-frequency (≈8–12 Hz) striatal LFP oscillations. This finding highlights the possibility that simultaneously activated oscillations, even within a single “band” such as theta, may characterize different behavioral and cognitive states.
It has been proposed that “phase coded” information is used in the hippocampus for representing locations in space and items held in working memory (25). Cross-frequency coupling such as we report here may also serve as a form of phase coding, in which, in addition to single spikes relative to phase, bursts of oscillatory activity would gate or convey information for neural computation.
Cross-Coupling Is Strongest During Decision-Making Epoch.
Within the hippocampus, phase–amplitude modulation peaked during the decision period of the task, but was weak before locomotion onset and during the approach to goal. This timing could not be accounted for solely by running speed. This pattern raises the possibility that the modulation of gamma-band activity and HFO in the hippocampus occurs especially when the rat is actively accessing sequential information during locomotion or navigation, but is weak or absent during other periods of the task, despite the presence of prominent theta activity. In the striatum, phase–amplitude modulation also was strongest during the tone-turn decision period and, again, could not be accounted for by running speed. We also found that the modulation could be high early in the trial (periods of low velocity) and that it could be low during turning (high-velocity periods). This large spread of the modulation across the different events within the runs suggests that phase–amplitude modulation in the striatum could be related to the active recall or throughput of sequential information related to task performance. Notably, the tone-turn period is the period in which the lowest density of spiking by striatal projection neurons occurs in rats that have acquired the task (49). The cross-coupled oscillatory patterns could thus especially reflect the accessing of inputs by striatal circuitry. Interestingly, in the one rat in which fully adequate records were available at all sessions, there was a clear learning-related increase in the phase–amplitude coupling both within the striatum and within the hippocampus.
The finding that cross-couplings were most prominent as the rats neared the choice point of the maze raises the possibility that cognitive processes related to decision, choice, and selection influenced the appearance of the phase–amplitude couplings both within the striatum and the hippocampus and across these forebrain regions. If so, this decision phase may be one that particularly calls for multiple frequency-band coordination of striatal and hippocampal activity. This behavioral epoch also appears to be the time at which network coherence within the theta band is enhanced across hippocampal, prefrontal, and striatal circuits (14, 50). These findings suggest that phase–amplitude coupling may reflect the engagement across different time scales of network activity related to active cognitive processing.
Materials and Methods
Behavioral training and electrophysiology recording methods were approved by the Committee on Animal Care of Massachusetts Institute of Technology and are described in detail in refs. 14 and 15. Briefly, 6 male Sprague–Dawley rats were implanted with head stages containing 12 tetrodes, with 6 tetrodes targeting the dorsomedial caudoputamen (AP: +1.7 mm, ML: 1.8 mm, DV: 3.6–4.6 mm) and 6 tetrodes targeting the dorsal CA1 region of the hippocampus (AP: −3.3 mm, ML: 2.2 mm, DV: 2.4–2.8 mm). Tetrodes were lowered to their target depths during a 1-week postsurgical recovery period. Rats then received daily training sessions (usually 40 trials) on an auditory tone-cued T-maze task. Rats were trained to turn right or left at the choice point of the maze as instructed by 1- and 8-kHz tone cues. A click warning cue preceded the opening of a start gate. Rats were rewarded with chocolate sprinkles if the baited goal was correctly approached. Throughout training, LFPs were amplified (gain: 1,000), filtered (1–475 Hz) and continuously sampled at 1 kHz by using a Cheetah recording system (Neuralynx). In the hippocampus, the definition of “deep” and “superficial” CA1 layers of the dorsal hippocampus was performed based on the phase reversal of the theta wave combined with daily records of tetrode depth. All analyses were done with MATLAB 7.5 software (MathWorks). Details are given in SI Text.
Supplementary Material
Acknowledgments.
We thank Miles Whittington and Roger Traub for comments on a preliminary version of this manuscript. This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil (A.B.L.T.), the Burroughs Wellcome Fund (A.B.L.T., M.A.K., and N.J.K.), a National Science Foundation Research Training Grant (to A.B.L.T., M.A.K., and N.J.K.), a Friends of the McGovern Institute for Brain Research Graduate Student Fellowship (to C.T.), National Institutes of Health Grant MH60379 (to C.T., D.J.G., Y.K., and A.M.G.), and Office of Naval Research Grant N00014-04-1-0208 (to C.T., D.J.G., Y.K., and A.M.G.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810524105/DCSupplemental.
Roughly, “superficial” and “deep” CA1 layers correspond to stratum pyramidale and stratum lacunosum-moleculare, respectively; see SI Text.
References
- 1.Buzsaki G. Rhythms of the Brain. Oxford: Oxford Univ Press; 2006. [Google Scholar]
- 2.Buzsaki G, et al. Hippocampal network patterns of activity in the mouse. Neuroscience. 2003;116:201–211. doi: 10.1016/s0306-4522(02)00669-3. [DOI] [PubMed] [Google Scholar]
- 3.Bragin A, et al. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hentschke H, Perkins MG, Pearce RA, Banks MI. Muscarinic blockade weakens interaction of gamma with theta rhythms in mouse hippocampus. Eur J Neurosci. 2007;26:1642–1656. doi: 10.1111/j.1460-9568.2007.05779.x. [DOI] [PubMed] [Google Scholar]
- 5.Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature. 1999;399:781–784. doi: 10.1038/21645. [DOI] [PubMed] [Google Scholar]
- 6.O'Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 7.Hasselmo ME, Bodelon C, Wyble BP. A proposed function for hippocampal theta rhythm: Separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 2002;14:793–817. doi: 10.1162/089976602317318965. [DOI] [PubMed] [Google Scholar]
- 8.Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- 9.Lakatos P, et al. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol. 2005;94:1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- 10.Canolty RT, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickson CT, Magistretti J, Shalinsky M, Hamam B, Alonso A. Oscillatory activity in entorhinal neurons and circuits. Mechanisms and function. Ann NY Acad Sci. 2000;911:127–150. doi: 10.1111/j.1749-6632.2000.tb06723.x. [DOI] [PubMed] [Google Scholar]
- 12.Magill PJ, Sharott A, Bolam JP, Brown P. Delayed synchronization of activity in cortex and subthalamic nucleus following cortical stimulation in the rat. J Physiol. 2006;574:929–946. doi: 10.1113/jphysiol.2006.110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pare D, Collins DR, Pelletier JG. Amygdala oscillations and the consolidation of emotional memories. Trends Cognit Sci. 2002;6:306–314. doi: 10.1016/s1364-6613(02)01924-1. [DOI] [PubMed] [Google Scholar]
- 14.DeCoteau WE, et al. Learning-related coordination of striatal and hippocampal theta rhythms during acquisition of a procedural maze task. Proc Natl Acad Sci USA. 2007;104:5644–5649. doi: 10.1073/pnas.0700818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeCoteau WE, et al. Oscillations of local field potentials in the rat dorsal striatum during spontaneous and instructed behaviors. J Neurophysiol. 2007;97:3800–3805. doi: 10.1152/jn.00108.2007. [DOI] [PubMed] [Google Scholar]
- 16.Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron. 2004;43:883–896. doi: 10.1016/j.neuron.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 17.Boraud T, Brown P, Goldberg JA, Graybiel AM, Magill PJ. Oscillations in the basal ganglia: The good, the bad, and the unexpected. In: Bolam JP, Ingham CA, Magill PJ, editors. The Basal Ganglia VIII. New York: Springer Science and Business Media; 2005. pp. 3–24. [Google Scholar]
- 18.Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- 19.Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 20.Borgers C, Epstein S, Kopell NJ. Background gamma rhythmicity and attention in cortical local circuits: A computational study. Proc Natl Acad Sci USA. 2005;102:7002–7007. doi: 10.1073/pnas.0502366102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Harris KD, Csicsvari J, Hirase H, Dragoi G, Buzsaki G. Organization of cell assemblies in the hippocampus. Nature. 2003;424:552–556. doi: 10.1038/nature01834. [DOI] [PubMed] [Google Scholar]
- 23.Montgomery SM, Buzsaki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc Natl Acad Sci USA. 2007;104:14495–14500. doi: 10.1073/pnas.0701826104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Driver JE, et al. Impairment of hippocampal gamma-frequency oscillations in vitro in mice overexpressing human amyloid precursor protein (APP) Eur J Neurosci. 2007;26:1280–1288. doi: 10.1111/j.1460-9568.2007.05705.x. [DOI] [PubMed] [Google Scholar]
- 25.Lisman J. The theta/gamma discrete phase code occurring during the hippocampal phase precession may be a more general brain coding scheme. Hippocampus. 2005;15:913–922. doi: 10.1002/hipo.20121. [DOI] [PubMed] [Google Scholar]
- 26.Crone NE, Boatman D, Gordon B, Hao L. Induced electrocorticographic gamma activity during auditory perception. Brazier Award-winning article, 2001. Clin Neurophysiol. 2001;112:565–582. doi: 10.1016/s1388-2457(00)00545-9. [DOI] [PubMed] [Google Scholar]
- 27.Edwards E, Soltani M, Deouell LY, Berger MS, Knight RT. High gamma activity in response to deviant auditory stimuli recorded directly from human cortex. J Neurophysiol. 2005;94:4269–4280. doi: 10.1152/jn.00324.2005. [DOI] [PubMed] [Google Scholar]
- 28.Ray S, Niebur E, Hsiao SS, Sinai A, Crone NE. High-frequency gamma activity (80–150Hz) is increased in human cortex during selective attention. Clin Neurophysiol. 2008;119:116–133. doi: 10.1016/j.clinph.2007.09.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- 30.Jensen O, Colgin LL. Cross-frequency coupling between neuronal oscillations. Trends Cognit Sci. 2007;11:267–269. doi: 10.1016/j.tics.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Chrobak JJ, Lorincz A, Buzsaki G. Physiological patterns in the hippocampo-entorhinal cortex system. Hippocampus. 2000;10:457–465. doi: 10.1002/1098-1063(2000)10:4<457::AID-HIPO12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 32.Masimore B, Schmitzer-Torbert NC, Kakalios J, Redish AD. Transient striatal gamma local field potentials signal movement initiation in rats. NeuroReport. 2005;16:2021–2024. doi: 10.1097/00001756-200512190-00010. [DOI] [PubMed] [Google Scholar]
- 33.Brown P, et al. Oscillatory local field potentials recorded from the subthalamic nucleus of the alert rat. Exp Neurol. 2002;177:581–585. doi: 10.1006/exnr.2002.7984. [DOI] [PubMed] [Google Scholar]
- 34.Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ. Move to the rhythm: Oscillations in the subthalamic nucleus–external globus pallidus network. Trends Neurosci. 2002;25:525–531. doi: 10.1016/s0166-2236(02)02235-x. [DOI] [PubMed] [Google Scholar]
- 35.Magill PJ, Sharott A, Bolam JP, Brown P. Brain state-dependency of coherent oscillatory activity in the cerebral cortex and basal ganglia of the rat. J Neurophysiol. 2004;92:2122–2136. doi: 10.1152/jn.00333.2004. [DOI] [PubMed] [Google Scholar]
- 36.Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Dejean C, Gross CE, Bioulac B, Boraud T. Dynamic changes in the cortex–basal ganglia network after dopamine depletion in the rat. J Neurophysiol. 2008;100:385–396. doi: 10.1152/jn.90466.2008. [DOI] [PubMed] [Google Scholar]
- 38.Cohen MX, et al. Good vibrations: Cross-frequency coupling in the human nucleus accumbens during reward processing. J Cognit Neurosci. 2008 doi: 10.1162/jocn.2009.21062. in press. [DOI] [PubMed] [Google Scholar]
- 39.Kay LM. Two species of gamma oscillations in the olfactory bulb: Dependence on behavioral state and synaptic interactions. J Integr Neurosci. 2003;2:31–44. doi: 10.1142/s0219635203000196. [DOI] [PubMed] [Google Scholar]
- 40.Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: Experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- 41.Traub RD, et al. Axonal gap junctions between principal neurons: A novel source of network oscillations, and perhaps epileptogenesis. Rev Neurosci. 2002;13:1–30. doi: 10.1515/revneuro.2002.13.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Traub RD, et al. GABA-enhanced collective behavior in neuronal axons underlies persistent gamma-frequency oscillations. Proc Natl Acad Sci USA. 2003;100:11047–11052. doi: 10.1073/pnas.1934854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cunningham MO, et al. Coexistence of gamma and high-frequency oscillations in rat medial entorhinal cortex in vitro. J Physiol. 2004;559:347–353. doi: 10.1113/jphysiol.2004.068973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeCoteau WE, Kesner RP. A double dissociation between the rat hippocampus and medial caudoputamen in processing two forms of knowledge. Behav Neurosci. 2000;114:1096–1108. doi: 10.1037//0735-7044.114.6.1096. [DOI] [PubMed] [Google Scholar]
- 45.White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- 46.Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 47.Foerde K, Knowlton BJ, Poldrack RA. Modulation of competing memory systems by distraction. Proc Natl Acad Sci USA. 2006;103:11778–11783. doi: 10.1073/pnas.0602659103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mormann F, et al. Phase/amplitude reset and theta-gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus. 2005;15:890–900. doi: 10.1002/hipo.20117. [DOI] [PubMed] [Google Scholar]
- 49.Barnes T, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- 50.Jones MW, Wilson MA. Phase precession of medial prefrontal cortical activity relative to the hippocampal theta rhythm. Hippocampus. 2005;15:867–873. doi: 10.1002/hipo.20119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.