Abstract
The factors controlling memory T (Tm)-cell longevity are still poorly defined, and their identification is pivotal to the design of a vaccine conferring long-term protection against infection. Tm cells have the ability to survive in the absence of the T-cell receptor (TCR)–MHC interaction. This does not exclude a possible role for TCR-intrinsic ligand-independent constitutive signaling in Tm-cell homeostasis. Using a unique TCR tetracycline-inducible expression system, we show that the ablation of TCR expression, which abrogates any possible signaling via the TCR, did not influence the survival and self-renewal of antigen-specific CD8+ Tm cells even when they have to compete with endogenous T cells for survival factors. Moreover, CD8+ Tm-cell functionality was not altered even on prolonged maintenance in the absence of TCR-MHC interactions. Furthermore, our results show that a subset of CD4+ Tm cells can survive in the absence of TCR expression in nonlymphopenic hosts.
Keywords: cytokine, T-cell homeostasis, vaccine
Memory T (Tm) cells are crucial for the long-term maintenance of protective immunity against reinfection. The factors controlling Tm cell longevity are still poorly defined, and their identification is pivotal to the design of a vaccine conferring long-term protection against infection. Like naive T cells, the survival of Tm cells is regulated by cytokines. IL-7 regulates the survival of both CD4+- and CD8+-naive and Tm cells, whereas IL-15 controls the homeostasis of naive CD8+ T cells, CD4+ Tm cells, and CD8+ Tm cells (1–4). Therefore, naive and Tm cells have to compete for the same cytokines for their maintenance in the T-cell pool. This suggests that other homeostatic signal(s) may contribute to the independent and noncompetitive maintenance of naive and Tm cells.
It is generally believed that T-cell receptor (TCR)–MHC interactions are not needed for the survival of Tm cells (5–7). However, there are numerous reports showing that antigen dependency (8–11) or TCR-MHC interactions (12, 13) are required for the maintenance of Tm cells. For example, the survival of HY TCR transgenic Tm cells depends on MHC class I expression (12, 13), although a nonrestricting allele was sufficient (12). Moreover, CD4+ Tm cells maintained in the absence of TCR-MHC class II interactions have impaired functionality (14, 15). However, the studies reporting MHC-independent survival of Tm cells were done in a situation in which Tm cells did not have to compete for survival factors with endogenous T cells (5–7). Therefore, these observations suggest the possibility that TCR-MHC interaction may contribute to Tm-cell survival and functionality in a normal environment. Alternatively, we propose that intrinsic constitutive signals from the TCR might allow for MHC-independent survival of Tm cells. Accordingly, abrogation of TCR signaling by turning off the tyrosine kinases lck and fyn caused a significant drop in the number of CD4+CD44hi Tm cells (16). Because of possible competition for cytokines between naive and Tm lymphocytes, it is plausible that Tm cells use constitutive TCR signaling to receive survival signal allowing them to be maintained in a different homeostatic niche than naive T cells.
To evaluate if constitutive TCR signaling contributes to the survival of functional Tm cells, we used a transgenic tetracycline (tet)-inducible expression system in which we can abrogate TCR expression on T cells (17). Our results show that the ablation of TCR expression, which abrogates any possible TCR signaling, did not influence the survival and self-renewal of antigen-specific CD8+ Tm cells even when they have to compete with endogenous T cells. Moreover, CD8+ Tm cell functionality was not altered on prolonged maintenance in the absence of TCR-MHC interactions. Furthermore, our results show that a subset of CD4+ Tm cells can survive without TCR expression even if they have to compete with TCR-positive endogenous CD4+ Tm lymphocytes.
Results
Antigen-Specific CD8+ Tm Cells Survive in the Absence of TCR Expression.
The ability of Tm cells to survive in the absence of TCR-MHC interactions (5–7) does not exclude a role for intrinsic constitutive TCR signaling (independent of MHC) in Tm-cell homeostasis. To determine if TCR expression is necessary for CD8+ Tm-cell survival, we used a transgenic mouse model, Vβ5LTAOCα−/−, that expresses the ovalbumin (OVA)-specific OT-1 TCR whose expression is dependent on tet (17). In this model, the TCRVβ5 chain is expressed constitutively, whereas the expression of the TCRVα2 chain is inducible by tet. Tet administration to Vβ5LTAOCα−/− mice blocks TCR expression by inhibiting the TetR-VP16 transactivator (tTA)-dependent transcription of the TCRVα2 transgene (17). We have previously extensively characterized this model and have shown complete ablation of TCR expression following tet treatment (17). To generate CD8+ Tm cells expressing a tet-inducible TCR, we adoptively transferred 106 Vβ5LTAOCα−/− (CD45.2+) T cells into congenic B6.SJL (CD45.1+) mice. Forty-eight h later, recipients were immunized with 5 × 105 bone marrow–derived mature dendritic cells (DCs) pulsed with OVA257–264 peptide. We transferred female T cells into female hosts and immunized them with male DCs to provide the T-cell help necessary to generate functional and long-lived CD8+ Tm cells (18). Long-lived CD8+ central Tm cells with the ability to make recall response were generated [supporting information (SI) Fig. S1] and were present in similar numbers in the different superficial lymph nodes (LNs) (Fig. S2). Before stopping TCR expression, we removed a LN by surgery to evaluate the number of Tm cells 40 days after immunization. As shown in Fig. 1A, Vβ5LTAOCα−/− Tm cells (CD8+CD45.2+Ly6Chi) were detected in the inguinal LN. Mice were then administered tet (400 μg/ml) to stop TCR expression completely (17) in OVA-specific CD8+ Tm cells (Fig. S3 and Fig. S4). Superficial LNs were removed surgically at different days after treatment to follow the survival of Vβ5LTAOCα−/− Tm cells (CD45.2+Ly6Chi). OVA-specific CD8+ Tm cells were still present in similar numbers after 35 and 65 days of tet treatment (Fig. 1A, 75 and 105 days postimmunization). Similar results were obtained 190 days postimmunization in the mesenteric LNs and spleen (Fig. 1B). Because the maintenance of functional CD4+ Tm cells requires TCR-MHC interactions (14, 15), we evaluated if CD8+ Tm cells maintained in the peripheral pool without the TCR were functional. Because of their lack of TCR expression, cells were stimulated in vitro for 4 h with phorbol 12-myristate 13-acetate (PMA) and ionomycin to measure IFN-γ production. CD8+ Tm cells denuded of the TCR were comparable to their TCR-positive counterpart for IFN-γ production (Fig. 1C). Altogether, these results suggest that the survival of CD8+ Tm cells and the maintenance of their functionality do not require TCR expression.
Fig. 1.
TCR expression is not required for the survival of OVA-specific CD8+ Tm cells. (A) Survival of OVA-specific CD8+ Tm cells. Forty days after immunization, a LN was collected surgically to evaluate the number of OVA-specific CD8+ Tm cells (Left). The same mouse was then treated or not treated with tet (400 μg/ml) to stop TCR expression, and the survival of Tm cells was evaluated over time by removal of superficial LNs by surgery. Percentages and numbers of OVA-specific Tm cells (CD8+CD45.2+) present in one LN are indicated. Survival of Vβ5LTAOCα−/− CD8+ Tm cells in the absence of tet treatment (Upper) and maintenance of Tm cells in the absence of TCR expression (tet-treated) (Lower). The histogram in the lower right corner of the dot plots shows Ly6C expression by OVA-specific CD8+ Tm cells. (B and C) Long-term maintenance of functional OVA-specific CD8+ Tm cells in the absence of TCR expression. (B) Percentages and numbers of OVA-specific CD8+ Tm cells (CD8+CD45.2+) in mesenteric LN (MLN) and spleen 190 days after immunization are shown. (C) Splenocytes (190 days after immunization) were stimulated for 4 h in vitro with phorbol 12-myristate 13-acetate (PMA) and ionomycin, followed by intracellular IFN-γ staining. Histograms show IFN-γ production by OVA-specific Tm cells (CD8+CD45.2+) and endogenous T cells (CD8+CD45.2−) in the absence (Bottom) or presence (Top) of TCR expression.
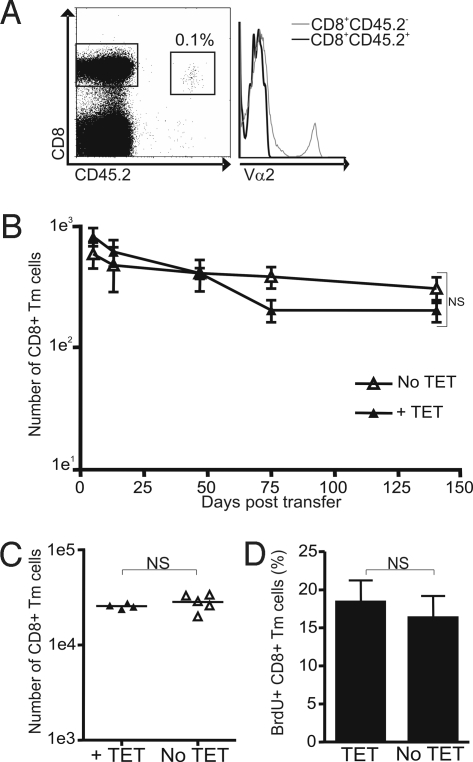
To confirm the lack of TCR dependency for CD8+ Tm-cell maintenance, we generated OVA-specific CD8+ Tm cells in vitro (19) and transferred them in naive mice. At the end of the culture, the cells had acquired a central memory phenotype (Fig. S4). A total of 3 × 106 in vitro–generated OVA-specific CD8+ Tm cells (CD45.2+) were adoptively transferred into B6.SJL recipients (CD45.1+). Seven days after transfer, half of the mice were treated with tet, whereas the other half were kept untreated. Tet treatment abrogated TCR expression (Fig. 2A and Fig. S3) but did not affect the maintenance of the OVA-specific CD8+ Tm cells in the LNs (Fig. 2B) and spleen (Fig. 2C). Moreover, self-renewal of the transferred Vβ5LTAOCα−/− CD8+ Tm cells was similar in cells expressing or not expressing the TCR as measured by BrdU incorporation (Fig. 2D). These results show that CD8+ Tm cells survive and self-renew in the absence of TCR expression, which indicates that TCR signals are not necessary for the long-term maintenance of antigen-specific CD8+ Tm cells. Furthermore, these results show that CD8+ Tm cells denuded of the TCR can be maintained even when they have to compete for survival factors with endogenous T cells.
Fig. 2.
TCR expression is not required for the survival and self-renewal of in vitro–generated OVA-specific CD8+ Tm cells. (A) Ablation of TCR expression on OVA-specific CD8+ Tm cells. The dot plot shows the population of transferred OVA-specific Tm cells (CD8+CD45.2+). The histogram shows TCR expression, detected with an anti-TCRVα2 Ab, for antigen-specific CD8+ Tm cells (CD8+CD45.2+) and endogenous T cells (CD8+CD45.2−) treated with tet for 50 days. (B and C) Similar in vivo survival of OVA-specific CD8+ Tm cells lacking or not lacking TCR expression. A total of 3 × 106 in vitro–generated Vβ5LTAOCα−/− Tm cells (CD8+CD45.2+) were adoptively transferred into B6.SJL recipients (CD45.1+). A LN was removed by surgery at days 5, 13, 47, and 75 to quantify the number of OVA-specific CD8+ Tm cells (B) expressing (no tet) or not expressing (treated with tet from day 7 after transfer) the TCR. At day 140, mice were killed and the number of CD8+ Tm cells was evaluated in the mesenteric LN (B) and spleen (C). The relative number of OVA-specific CD8+ Tm cells is shown in B and was calculated as the number of CD8+CD45.2+ cells in 106 cells to correct for variation in LN size. For the spleen, the total number of OVA-specific CD8+ Tm cells is shown for the different mice. (D) Self-renewal of OVA-specific CD8+ Tm cells is not affected by TCR ablation. Mice were treated with BrdU for a week before they were killed (day 140 posttransfer). The percentage of BrdU-positive OVA-specific Tm cells (CD8+CD45.2+) is shown for mice treated or not treated with tet.
A Subset of CD4+ Tm Cells Lacking TCR Expression Can Survive in Lymphoreplete Hosts.
The unimpaired survival of CD8+ Tm cells following TCR ablation does not exclude a role for TCR expression in the maintenance of CD4+ Tm cells. Moreover, abrogation of TCR signaling by turning off the tyrosine kinases lck and fyn was shown to cause a significant drop in the number of CD4+CD44hi Tm cells (16). This suggests that intrinsic constitutive signals from the TCR might contribute to the MHC-independent survival of CD4+ Tm cells. To evaluate the role of TCR expression for the maintenance of CD4+ Tm cells, we used our tet-inducible TCR expression system in the absence of the transgenic TCRβ chain (LTAO mice) (17). In these mice, the tet-regulatable TCR Vα2+ cells are selected into the CD4 lineage (17). Furthermore, LTAO mice possess a polyclonal repertoire of T cells (Fig. S5), and their encounter with antigens from the environment leads to the development of a pool of CD4+ Tm cells with a CD44hiCD62LloCD45RBlo phenotype (20, 21). At first, we treated LTAO mice on a TCR Cα-deficient background (LTAOCα−/−) with tet to abrogate TCR expression (Fig. S6). Tet treatment led to the rapid loss of both naive and CD4+ Tm cells (data not shown). However, this loss of all T cells is accompanied by an important decrease in total LN cellularity with a loss of the non-T-cell population (data not shown), suggesting that T cells regulate secondary lymphoid organ homeostasis (Hardy M.-P., manuscript in preparation). The perturbation of secondary lymphoid organ homeostasis that occurs in the LTAOCα−/− model could lead to the disappearance of a cell type secreting or expressing a factor controlling CD4+ Tm-cell survival. Therefore, to address the role of TCR expression for the maintenance of CD4+ Tm cells definitively, we used LTAO mice on a TCRα-sufficient background (LTAOCα+/−). In these mice, only 10 to 20% of CD4+ T cells express the tet-regulatable TCRVα2 chain (17). Therefore, T cells expressing endogenous TCRα chain would not be affected by tet, and they should allow for the normal homeostasis of secondary lymphoid organs. LTAO mice were treated with tet to follow the survival of CD4+ Tm cells lacking TCR expression (CD4+CD3−CD44hi). LN cellularity was not affected by tet administration, indicating normal secondary lymphoid organ homeostasis (Fig. 3A). In previous work, we have shown that TCR-negative cells can be distinguished from TCR-positive cells after 7 days of tet treatment (17); thus, this time point was used to evaluate the initial percentage of CD4+CD44hi Tm cells expressing a tet-inducible TCR (Fig. 3B). About half of the CD4+ Tm cells stripped of their TCRs (Fig. S6) decay rapidly over tet treatment (Fig. 3B), whereas the other half remain detectable for over 100 days (Fig. 3B). Furthermore, tet treatment did not affect the survival of TCR-positive CD4+ Tm cells (Fig. 3C). These results suggest that a subset of CD4+ Tm cells need TCR expression to survive in the T-cell pool, whereas some CD4+ Tm cells can be maintained in the absence of TCR expression even when they have to compete with TCR-positive CD4+ Tm cells.
Fig. 3.
TCR expression is not required for the survival of a subset of CD4+ Tm cells. (A) No reduction of LN cellularity was detected in LTAOCα+/− mice treated with tet. LNs were sequentially removed by surgery to follow cellularity over the time of tet treatment (400 μg/ml). (B and C) Survival of a subset of CD4+ Tm cells denuded of TCR when secondary lymphoid organ homeostasis is not impaired. The survival of CD3− (B) and CD3+ (C) CD4+ Tm cells (CD44hi) was followed in tet-treated LTAOCα+/− mice. LNs were removed by surgery, and the percentage of CD3− and CD3+ CD4+ Tm cells was followed over time in the same mouse. Day 7 represents the number of CD4+ Tm cells expressing a tet-regulatable TCR in LTAOCα+/− mice.
TCR Expression Is Not Needed for IL-7 Responsiveness and Bim Down-Regulation.
The defective survival of a subset of CD4+ Tm cells in the absence of TCR expression could result from their unresponsiveness to the survival factor IL-7. Therefore, we evaluated the expression level of the IL-7 receptor α-chain (CD127) on T cells lacking TCR expression for 14 days (Fig. 4). CD127 expression was identical on CD4+ Tm cells expressing or not expressing the TCR (Fig. 4). Because Bcl-2 is regulated by IL-7 signals (22, 23), we evaluated its expression in CD4+ LTAOCα+/− T cells expressing or not expressing the TCR after 14 days of tet treatment. There was no difference in Bcl-2 expression in T cells expressing or not expressing the TCR (Fig. 4). Another pathway controlled by IL-7 is glucose uptake and regulation of cell surface expression of the glucose transporter, Glut1 (24, 25). As shown in Fig. 4, cell surface expression of Glut1 was similar in CD4+ LTAOCα+/− T cells expressing or not expressing the TCR. Moreover, T cells lacking TCR expression did not up-regulate the expression of the proapoptotic molecule Bim (Fig. 4). Similar results were obtained for CD127, Bcl-2, Glut1, and Bim expression after a longer tet treatment of 56 days (not shown). These results suggest that TCR signals do not control IL-7 responsiveness and Bim expression in CD4+ Tm cells.
Fig. 4.
CD4+ Tm cells denuded of TCR molecules express a normal level of CD127, Bcl-2, Glut1, and Bim. The overlays show expression of the different molecules by CD4+CD44hi Tm cells lacking or not lacking TCR expression. The staining was done on LTAOCα+/− T cells after 14 days of tet treatment.
Preferential Loss of Rapidly Dividing CD4+ Tm Cells in the Absence of TCR Expression After Transfer into Lymphopenic Hosts.
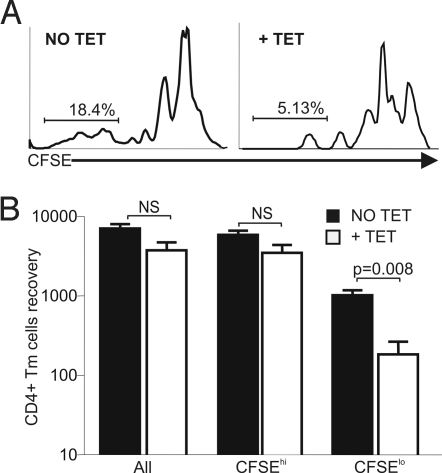
The pool of CD4+CD44hi Tm cells in WT mice contains two subsets of Tm cells (2, 26). One subset requires interaction with self-MHC class II molecules for its maintenance and undergoes rapid homeostatic division when transferred into lymphopenic hosts, which also depends on MHC class II expression (2, 26). The other subset of CD4+ Tm cells is maintained independent of MHC class II interaction and divides slowly when transferred into lymphopenic recipients (2, 26). Therefore, we tested if the subset of CD4+ Tm cells lost following TCR ablation (Fig. 3B) depends on interaction with MHC class II molecules for its survival and homeostatic proliferation. If this is the case, we would expect a selective loss of rapidly dividing CD4+ Tm cells after their transfer into lymphopenic recipients in the absence of TCR expression. To test that, we sorted carboxyfluorescein succinimidyl ester (CFSE)-labeled CD4+CD44hi Tm cells lacking (+TET) or not lacking (No TET) TCR expression from LTAOCα−/− mice (treated or not treated with tet for 7 days) and adoptively transferred these cells into lymphopenic hosts that were treated or not treated with tet. As shown in Fig. 5, a selective loss of rapidly dividing CD4+CD44hi Tm cells occurs in the spleen when T cells lack TCR expression (+TET). This suggests that the loss of CD4+ Tm cells we have seen after TET treatment of LTAOCα+/− mice (Fig. 3B) is attributable to the disappearance of the subset of CD4+ Tm cells that is dependent on MHC class II expression. Furthermore, the subset of CD4+ Tm cells that survives in the absence of TCR expression in LTAOCα+/− mice most likely represents the pool of antigen-specific CD4+ Tm cells with slow homeostatic proliferation potential.
Fig. 5.
Preferential loss of rapidly dividing CD4+ Tm cells lacking TCR expression after transfer into lymphopenic hosts. (A) carboxyfluorescein succinimidyl ester (CFSE) profile of LTAOCα−/− CD4+ Tm cells 5 days after adoptive transfer into irradiated B6.SJL congenic recipients treated or not treated with tet. The proliferation of CD4+CD44hi Tm cells expressing (Left) or not expressing (Right) the TCR is shown. (B) Recovery of TCR-positive (NO TET) and TCR-negative (+TET) CD4+ Tm cells in the spleen 5 days after adoptive transfer in recipients that were untreated or treated with tet, respectively. The results were obtained from a pool of three mice in two independent experiments.
Discussion
Using a tet-inducible expression system of the TCR in mice, we showed that TCR expression is not required for the survival and self-renewal of CD8+ Tm cells. These observations are in agreement with the lack of requirement for TCR-MHC interactions for the survival of CD8+ Tm cells (7). Furthermore, our data show that TCR-less CD8+ Tm cells are efficiently maintained in the T-cell pool even if they had to compete for survival factors with naive and Tm cells that are present in nonlymphopenic mice. Therefore, the proper survival of antigen-specific CD8+ Tm cells in the absence of MHC interactions that was reported by others (7) is not attributable to the lymphopenic state of the mice used in these experiments. Our results clearly show that CD8+ Tm cells use neither TCR-MHC interactions nor ligand-independent TCR signaling for their maintenance in the Tm-cell pool. Our results are in contrast with those of Polic et al. (27), who have reported a decay of CD8+CD44hi Tm cells after TCR ablation. This difference is probably attributable to the fact that they did not follow the survival of antigen-specific CD8+ Tm cells generated following immunization but rather followed the survival of CD8+CD44hi T cells, which contain naturally occurring Tm cells and activated T cells. Another explanation for the loss of CD8+CD44hi memory-like T cells observed by Polic et al. (27) is the concomitant loss of the other T-cell subsets, leading to a lymphopenic environment that might be associated with the loss of a cell type producing a survival factor for CD8+CD44hi Tm cells. Our observation that T-cell loss, and therefore lymphopenia, leads to a concomitant decrease in the non-T-cell compartment supports this possibility. Moreover, unlike CD4+ Tm cells, which need TCR-MHC interactions to maintain functionality (14, 15), antigen-specific CD8+ Tm cells are still competent to produce IFN-γ even after prolonged maintenance in the absence of TCR expression. Therefore, TCR signaling does not contribute to the maintenance of a functional pool of CD8+ Tm cells, suggesting that cytokine signals alone are sufficient.
A different picture emerges for the survival of CD4+ Tm cells. A subset of CD4+ Tm cells requires TCR expression for their maintenance in the T-cell pool, whereas the other subset of CD4+ Tm cells could survive in the absence of TCR expression. The subset of CD4+ Tm cells that can survive in the T-cell pool without TCR expression is probably bona fide antigen-specific CD4+ Tm cells. Therefore, constitutive TCR signaling does not contribute to the homeostasis of both antigen-specific CD4+ and CD8+ Tm cells. On the other hand, we believe that the subset of CD4+ Tm cells that needs TCR expression to be maintained in the T-cell pool includes CD4+ T cells with a memory-like phenotype, which develop spontaneously in mice in response to self-MHC class II molecules (4, 28, 29). Moreover, it was shown that the spontaneously arising CD4+ memory-like T-cell subset needs TCR-MHC interaction to undergo rapid homeostatic proliferation in lymphopenic hosts (2). This is in agreement with our observation that some CD4+ memory phenotype T cells need TCR expression to divide rapidly in lymphopenic hosts, suggesting that these cells are lost in our model because of a lack of interaction with MHC class II molecules rather than a lack of constitutive TCR signaling. We believe that the ablation of TCR expression, and probably of MHC interaction, is the direct event that leads to the loss of this subset of CD4+ Tm cells because they normally express CD127 in the absence of TCR expression. Furthermore, the abrogation of TCR expression did not influence expression of Bcl-2 and Glut1, which are downstream targets of IL-7 receptor signaling. Moreover, it was shown that the survival of human central CD4+ Tm cells was dependent on the down-regulation of the proapoptotic molecule Bim via TCR and cytokine signaling (30), but we did not observe any up-regulation of Bim expression following TCR ablation. This suggests that the loss of TCR signals in presence of homeostatic cytokine is not sufficient to induce Bim expression in CD4+ Tm cells or that there is a differential regulation of Bim in mouse and human T cells.
We cannot exclude that very low residual expression of the TCR, undetectable by flow cytometry, contributes to the survival of Tm cells after tet treatment. This is unlikely, however, because very low residual expression of the TCR should only be able to transmit a very low-grade TCR signal. Therefore, these Tm cells should be unable to compete with Tm cells expressing normal levels of the TCR, which receive a stronger TCR signal.
Our results also highlight an important issue when studying Tm cell homeostasis. In our initial experiments with LTAOCα−/− mice, we thought that all CD4+ Tm cells needed TCR expression to survive in the T-cell pool. Further experiments clearly showed that the loss of all CD4+ Tm cells was attributable to the concomitant perturbation of secondary lymphoid organ homeostasis in these mice. This suggests that the disappearance of the other cellular subsets constituting secondary lymphoid organs affects the availability of CD4+ Tm-cell survival factor(s). For example, the small size of LNs might reduce the availability of IL-7 and IL-15.
In conclusion, antigen-specific CD4+ and CD8+ Tm cells do not use constitutive TCR signaling for their maintenance in the T-cell pool. Future work is necessary to address how naive and Tm cells can be maintained in different niches, although they compete for the same survival factors, IL-7 and IL-15.
Materials and Methods
Mice.
B6.SJL and C57BL/6 mice were bred under specific pathogen-free conditions. Vβ5LTAO mice expressing a tet-inducible TCR specific for the OVA257–264 peptide in the context of Kb (OT-1 TCR) (17) were bred to Cα-deficient mice (Vβ5LTAOCα−/−). LTAO mice expressing an OT-1 TCR α-chain inducible by tet were used on a Cα-sufficient background (LTAOCα+/−) (17). Mouse experiments were approved by the Animal Care Committee of the Maisonneuve-Rosemont Research Center. To stop TCR expression, 400 μg/ml tet or 1 mg/ml tet (Sigma) was administered in the drinking water supplemented with 2% (weight/vol) sucrose.
Abs and Cell Surface Staining.
The following Abs were used: anti-CD4 (L3/T4 or CT-CD4), anti-CD62L (MEL-14), anti-Vα2 (B20.1), anti-IFN-γ (clone XMG1.2), anti-CD8α (53–6.7), anti-CD45RB (C363.16A), anti-CD3 (145–2C11), anti-CD44 (IM7), anti-Ly6C (AL-21), anti-CD45.2 (104), and anti-CD127 (A7R34). Abs and streptavidins were from Caltag, Cedarlane Laboratories, BD Biosciences, or eBioscience. To detect Glut1 expression, we used a polyclonal Ab directed against the extracellular portion (Santa Cruz Biotechnology, Inc). Staining was performed as described previously (31).
Generation of Bone Marrow–Derived DCs.
DCs were obtained by culturing bone marrow cells for 7 days with GM-CSF and IL-4 as described previously (31). To induce DC maturation, LPS (1 μg/ml; Sigma) was added on day 6. On day 7, cells were pulsed for 4 h with OVA257–264 peptide (2 μg/ml).
Adoptive Transfer and Immunization.
A total of 106 LN cells from female Vβ5LTAOCα−/− (CD45.2+) mice were injected i.v. in female B6.SJL (CD45.1+) hosts. Recipients were immunized 2 days later by i.v. injection of 5 × 105 male C57BL/6 mature DCs pulsed or not pulsed with OVA257–264 peptide (2 μg/ml) (31). At different times after immunization, brachial or inguinal LNs were removed surgically (17) and sequentially to follow the T-cell response in the same mouse.
In Vitro CD8+ Tm Cell Generation and Adoptive Transfer.
CD8+ Tm cells were generated as described (19). Briefly, splenocytes from Vβ5LTAOCα−/− mice were stimulated for 16 h with OVA257–264 peptide (10 μg/ml) in complete RPMI. Cells were washed to remove peptides and cultured for another 24 h. Nonadherent cells were transferred to a new flask and cultured for 24 h. Viable cells were recovered using Ficoll and cultured with human recombinant IL-15 (20 ng/ml; R&D Systems) for 6 days. The medium containing IL-15 was changed every 2 days. A total of 3 × 106 in vitro–generated Tm cells were transferred into B6.SJL (CD45.1+) hosts. The number of OVA-specific CD8+ Tm cells (CD45.2+) was evaluated in the same mouse by sequential surgical removal of brachial or inguinal LNs. One week before they were killed, mice were injected i.p. with 0.8 mg/ml BrdU and treated for a week with 1 mg/ml BrdU in the drinking water. BrdU staining was performed as previously described (17).
IFN-γ Production.
Splenocytes were stimulated with PMA and ionomycin for 4 h. Brefeldin A (Sigma) was added for the last 2 h. Cells were fixed and stained as described elsewhere (31).
Bcl-2 and Bim Expression.
Bcl-2 staining was performed after saponin permeabilization, as described previously (32). Intracellular detection of Bim was done using a monoclonal anti-Bim Ab (14A8; Alexis Biochemicals) after fixation in 2% (vol/vol) formaldehyde followed by methanol (90% vol/vol) permeabilization.
Homeostatic Proliferation of CD4+ Tm Cells in Lymphopenic Hosts.
CD4+CD44hiCD25−NK1.1− memory-phenotype T cells from LTAOCα−/− mice were sorted and labeled with CFSE, as described elsewhere (2). A total of 2 × 105 CD4+ Tm cells (CD45.2+) were injected i.v. into sublethally irradiated (600 rad) congenic B6.SJL mice (CD45.1+). Five days later, spleens were collected to measure homeostatic proliferation and T-cell recovery.
Statistical Analysis.
Statistics were done using a Student's t test.
Supplementary Material
Acknowledgments.
We thank N. Henley for FACS technical assistance and L. Sabbagh and T. Watts for sharing the protocol for in vitro Tm-cell generation and Bim staining. We acknowledge the critical review of the manuscript by C. Perreault, S. Lesage, S. Fournier, and E. B. Affar. This work was supported by operating grants from the Canadian Institutes of Health Research (CIHR) to N.L. The FACS facility was supported by a multiuser maintenance grant of the CIHR. N.L. was supported by a New Investigator award of the CIHR and is currently supported by a Senior Scholarship from the Fonds de la recherche en santé du Québec. J. L. is the recipient of a studentship from the Department of Microbiology and Immunology of the University of Montreal.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806289106/DCSupplemental.
References
- 1.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 2.Purton JF, et al. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sprent J, Cho JH, Boyman O, Surh CD. T cell homeostasis. Immunol Cell Biol. 2008;86:312–319. doi: 10.1038/icb.2008.12. [DOI] [PubMed] [Google Scholar]
- 4.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 5.Garcia S, DiSanto J, Stockinger B. Following the development of a CD4 T cell response in vivo: From activation to memory formation. Immunity. 1999;11:163–171. doi: 10.1016/s1074-7613(00)80091-6. [DOI] [PubMed] [Google Scholar]
- 6.Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science. 1999;286:1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 7.Murali-Krishna K, et al. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 8.Gray D, Matzinger P. T cell memory is short-lived in the absence of antigen. J Exp Med. 1991;174:969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen P, Andersen AB, Sorensen AL, Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995;154:3359–3372. [PubMed] [Google Scholar]
- 10.Ochsenbein AF, et al. A comparison of T-cell memory against the same antigen induced by virus versus intracellular bacteria. Proc Natl Acad Sci USA. 1999;96:9293–9298. doi: 10.1073/pnas.96.16.9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oehen S, Waldner H, Kundig TM, Hengartner H, Zinkernagel RM. Antivirally protective cytotoxic T cell memory to lymphocytic choriomeningitis virus is governed by persisting antigen. J Exp Med. 1992;176:1273–1281. doi: 10.1084/jem.176.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 13.Markiewicz MA, et al. Long-term T-cell memory requires the surface expression of self-peptide/major histocompatibility complex molecules. Proc Natl Acad Sci USA. 1998;95:3065–3070. doi: 10.1073/pnas.95.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kassiotis G, Garcia S, Simpson E, Stockinger B. Impairment of immunological memory in the absence of MHC despite survival of memory T cells. Nat Immunol. 2002;3:244–250. doi: 10.1038/ni766. [DOI] [PubMed] [Google Scholar]
- 15.De Riva A, Bourgeois C, Kassiotis G, Stockinger B. Noncognate interaction with MHC class II molecules is essential for maintenance of T cell metabolism to establish optimal memory CD4 T cell function. J Immunol. 2007;178:5488–5495. doi: 10.4049/jimmunol.178.9.5488. [DOI] [PubMed] [Google Scholar]
- 16.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 17.Labrecque N, et al. How much TCR does a T cell need? Immunity. 2001;15:71–82. doi: 10.1016/s1074-7613(01)00170-4. [DOI] [PubMed] [Google Scholar]
- 18.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 19.Manjunath N, et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 21.Blander JM, et al. A pool of central memory-like CD4 T cells contains effector memory precursors. J Immunol. 2003;170:2940–2948. doi: 10.4049/jimmunol.170.6.2940. [DOI] [PubMed] [Google Scholar]
- 22.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- 23.Kondrack RM, et al. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T cell survival. Blood. 2007;111:2101–2111. doi: 10.1182/blood-2007-06-096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barata JT, et al. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J Exp Med. 2004;200:659–669. doi: 10.1084/jem.20040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson JM, MacLeod M, Marsden VS, Kappler JW, Marrack P. Not all CD4+ memory T cells are long lived. Immunol Rev. 2006;211:49–57. doi: 10.1111/j.0105-2896.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 27.Polic B, Kunkel D, Scheffold A, Rajewsky K. How alpha beta T cells deal with induced TCR alpha ablation. Proc Natl Acad Sci USA. 2001;98:8744–8749. doi: 10.1073/pnas.141218898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobber R, Hertogh-Huijbregts A, Rozing J, Bottomly K, Nagelkerken L. The involvement of the intestinal microflora in the expansion of CD4+ T cells with a naive phenotype in the periphery. Dev Immunol. 1992;2:141–150. doi: 10.1155/1992/57057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vos Q, Jones LA, Kruisbeek AM. Mice deprived of exogenous antigenic stimulation develop a normal repertoire of functional T cells. J Immunol. 1992;149:1204–1210. [PubMed] [Google Scholar]
- 30.Riou C, et al. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J Exp Med. 2007;204:79–91. doi: 10.1084/jem.20061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lacombe MH, Hardy MP, Rooney J, Labrecque N. IL-7 receptor expression levels do not identify CD8+ memory T lymphocyte precursors following peptide immunization. J Immunol. 2005;175:4400–4407. doi: 10.4049/jimmunol.175.7.4400. [DOI] [PubMed] [Google Scholar]
- 32.Ostiguy V, Allard EL, Marquis M, Leignadier J, Labrecque N. IL-21 promotes T lymphocyte survival by activating the phosphatidylinositol-3 kinase signaling cascade. J Leukocyte Biol. 2007;82:645–656. doi: 10.1189/jlb.0806494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.