Abstract
Modification of proteins by the addition of lysine (K)-63-linked polyubiquitin (polyUb) chains is suggested to play important roles in a variety of cellular events, including DNA repair, signal transduction, and receptor endocytosis. However, identifying such modifications in living cells is complex and cumbersome. We have generated a monoclonal antibody (mAb) that specifically recognizes K63-linked polyUb, but not any other isopeptide-linked (K6, K11, K27, K29, K33, or K48) polyUb or monoubiquitin. We demonstrate the sensitivity and specificity of this K63Ub-specific mAb to detect K63Ub-modified proteins in cell lysates by Western blotting and in cells by immunofluorescence, and K63Ub-modified TRAF6 and MEKK1 in vitro and ex vivo. This unique mAb will facilitate the analysis of K63-linked polyubiquitylation ex vivo and presents a strategy for the generation of similar reagents against other forms of polyUb.
Keywords: MAP kinase kinase kinase 1 (MEKK1), TNF receptor-associated factor 6 (TRAF6)
Posttranslational modification of proteins by ubiquitin (Ub) attachment plays an important role in diverse cellular events, including cell division, differentiation, signal transduction, protein trafficking, and quality control (1, 2). Ub can be attached to proteins by free lysine, cysteines, or N-terminal residues by specific E3 Ub ligases (3–5). Monoubiquitylation (the addition of a single Ub residue per targeting site) was shown to modulate protein endocytosis, intracellular transport, and DNA repair (6–8). Monoubiquitin can be further modified to generate polyubiquitin (polyUb) chains via an isopeptide linkage between the C-terminal glycine carboxyl group of the Ub moiety being added and the ε-amino group of a free lysine on the protein-attachedUb (9). Ubiquitin has 7 surface-exposed lysine residues (Fig. 1D), and elongation to generate polyUb chains can occur on any of these residues. Indeed, all 7 types of polyUb chains have been found in cells (10, 11) (Fig. 1D). K48-linked polyubiquitylation, associated with subsequent protein degradation by 26S proteasomes (3, 12), is perhaps the most frequently found Ub modification (10). However, other forms of polyUb are much more common than originally thought and are currently the subject of significant interest. K11-linked polyubiquitylation was recently suggested to mediate protein turnover (13), whereas K29-linked Ub chains formed by the E3 ligase ITCH have been implicated in the lysosomal degradation of proteins in cells (14). K63-linked polyubiquitylation is thought to modulate protein–protein interactions during DNA repair (6, 15, 16), signal transduction (17), receptor endocytosis (2), and other cellular events (18). Endogenous K6-, K27-, or K33-linked polyubiquitylation of protein substrates has been reported, but their functions are not clear (19–21). In addition to linear Ub chains with homogeneous linkages, mixed or forked Ub chains containing multiple linkages have been identified, although the function of these chains is also unclear (22–25).
Fig. 1.
PolyUb structure and immunogen design. (A–C) Structure of a single Ub. (A) Protein Data Bank (PDB) coordinates: N-terminal Ub in 2JF5. (B) K63-linked di-Ub (PDB ID code 2JF5). (C) K48-linked di-Ub (PDB ID code 1AAR). Light green, N-terminal Ub; dark green, C-terminal Ub; red, K63; pink, K48. Rendering was performed by using PyMol. (D) Sequence of Ub with K63 colored red and remaining 6 lysine residues colored blue. (E and F) Diagram representations of K63-linked (E) and K48-linked di-Ub (F). Coloring is as in A–C. The residues contained within the U63U immunogen are depicted in red, whereas the corresponding residues in the U48U control branched peptide are in pink.
Recently, several novel K63-linked polyUb-specific binding partners and modifying enzymes have emerged. NZF (Npl4 zinc finger) domains have been shown to bind specifically K63-polyUb (26), and tandem NZF domains have been used to purify K63-linked polyubiquitylated proteins (27). Proteins with NZF domains, such as TAB2/3 and Trabid, play important roles in Toll-like receptor (TLR) and CD40-induced NF-κB activation and in TCF-induced Wnt signaling (26, 27). Furthermore, some deubiquitylating enzymes specific for the K63 polyUb linkage, such as A20, CYLD, and Ataxin-3, have recently emerged as critically important to the regulation of various cellular pathways (28–31). All of these data suggest that K63-linked polyubiquitylation of protein substrates plays vital roles and is tightly regulated in diverse cellular events. However, it is not clear how K63-linked polyUb modification mediates these events. Furthermore, it is very difficult to identify K63-linked polyubiquitylation in living cells because of the absence of a simple method for directly determining the nature of polyUb isopeptide linkage type. Three methods are currently used. First, protein ubiquitylation reactions can be performed in vitro with the target protein, an E3 Ub ligase and Ub mutants that lack 1 or several lysine residues (e.g., K63R-Ub) to restrict the type of Ub chain extension (17). However, such reactions may not accurately reflect what occurs in cells and are likely to be promiscuous. Second, specific types of polyubiquitylation can be blocked by overexpression of Ub mutants in cells (32, 33). However, this approach is often restricted to cell lines because it is difficult to overexpress exogenous genes in many primary cells, such T and B cells. Furthermore, such an approach can lead to saturation and sequestration of rate-limiting E2 Ub-conjugating enzymes by the overexpressed Ub mutants, which allows modification to take place with Ub-loaded E2s present at high concentrations that are not physiologically representative. Third, mass spectrometry-based technology has been used to determine the type of ubiquitylation both in vitro and ex vivo (10). However, such procedures require high protein purity and quantity that prevent analysis of ubiquitylation in samples with either low cell number or low protein amounts. Given these limitations, new approaches and reagents need to be developed that can specifically identify different types of Ub modifications ex vivo without the need for ectopic expression or cellular modification.
Results and Discussion
Strategy for Generating K63-PolyUb-Specific Antibodies.
Monoclonal antibodies (mAbs) have the capacity to distinguish subtle chemical and structural differences in their ligands. This is well illustrated by the ability of phosphopeptide-specific mAbs to recognize their specific ligands, but not the unphosphorylated peptide or a different phosphorylated peptide (34). Our goal in this work was to apply a strategy similar to that used in developing phospho-specific antibodies to generate a mAb specific for K63-linked polyUb that did not cross-react with Ub or other types of Ub linkages. As an example, there are 2 clear differences between K63-linked and K48-linked polyUb. First, the conformation is quite different with K63-linked di-Ub having an elongated structure, whereas K48-linked di-Ub has a tightly packed, globular structure (Fig. 1) (35). Second, the linking isopeptide bond by definition targets a different lysine residue within the second Ub moiety, and thus the amino acid sequence motif proximal to the isopeptide bond is different. To mimic the unique microenvironment of the K63-Ub isopeptide bond, we generated a branched peptide, referred to as U63U, in which the COOH terminus of the Ub[71–76] peptide (RLRGG) was attached to the ε-amine of K63 in the Ub[58–66] peptide (DYNIQKEST) [Fig. 1E and supporting information (SI) Table S1]. Similarly, Ub[71–76] was linked to peptides containing the other Ub lysine residues to generate 6 branched peptide controls (Table S1 and Fig. S1).
The U63U branched peptide was conjugated to keyhole limpet hemocyanin (KLH) and used to immunize 5 BALB/c mice. After 3 immunizations, we collected sera and assessed reactivity to the immunogen and control peptides by ELISA. All 5 mice (termed HWA1–5) showed reactivity against the U63U branched peptide (Fig. S2 and Tables S2 and S3). Sera from mouse HWA4 exhibited high reactivity to the U63U immunogen, but had no detectable specificity against the U48U branched peptide and the component linear peptides (Ub[58–66] and Ub[68–76]), and this mouse was therefore selected for fusion. Of 253 hybridomas generated, 32 had positive reactivity against the U63U branched peptide by ELISA, with 8 having stable reactivity after subcloning by limiting dilution followed by single cell flow cytometric sorting.
High Specificity of the K63-PolyUb-Specific mAb.
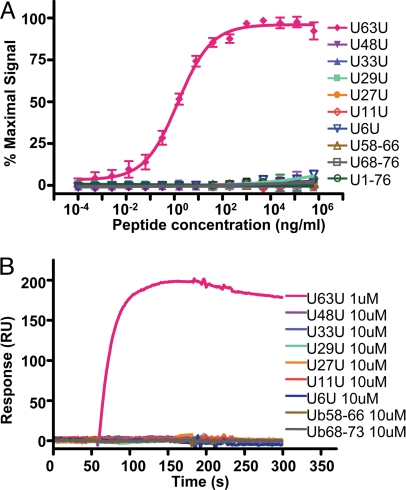
We further tested the reactivity of the mAb produced by these clones against Ub[58–66], Ub[68–76], and the U48U branched peptide. Clone HWA4C4 showed high selectivity for U63U, with no detectable reactivity against the related control Ub-derived peptides orUb protein and thus was selected for further analysis (Fig. 2A and Tables S2 and S3). These data suggested that the HWA4C4 mAb (IgG2a) may specifically recognize K63-linked polyUb. To test this possibility, we first checked by ELISA and Biacore analysis the binding activity of HWA4C4 with all 7 branched peptides mimicking the isopeptide linkages of the 7 possible polyUb chains (Fig. 2). Both assays demonstrated HWA4C4 to be highly specific for U63U and not to cross-react with any other Ub branched peptides. HWA4C4 also exhibited reasonable affinity against its immunogen (Kd = 8.8 nM) and may have a sensitivity comparable with that of P4D1, a commonly used mAb that recognizes all forms of Ub (Fig. S3).
Fig. 2.
The K63 isopeptide Ub linkage-specific mAb, HWA4C4. (A) Purified HWA4C4 (1 μg/mL) was tested by ELISA for reactivity against all 7 branched peptides, U[58–66] peptide, U[68–76] peptide, and Ub protein. (B) The HWA4C4 antibody was immobilized on CM5 biosensor chip and tested by Biacore analysis for binding ability to all 7 branched peptides, U[58–66] peptide, and U[68–76] peptide at the indicated concentrations.
To assess the specificity of HWA4C4 against protein targets, equal amounts of synthesized, recombinant K63-linked and K48-linked polyUb, and purified Ub were immunoblotted with purified HWA4C4. The P4D1 mAb, which recognizes all forms of Ub, was used as a control throughout. Our data clearly show that only K63-linked polyUb is recognized by HWA4C4 mAb (Figs. 3A and S4). Weak reactivity with free Ub is only observed at very high protein concentrations (30 ng of K63-linked polyUb vs. 10–30 μg of Ub, a 300- to 1,000-fold difference; Fig. S4A). Given that the K63-linked polyUb preparation contains multiple bands that constitute the total protein loaded, the actual ratio is likely to be much higher. No cross-reactivity is observed with K48-linked polyUb at any concentration (Fig. S4B). To exclude the possibility that HWA4C4 may recognize other polyUb chains, we assessed its reactivity to in vitro-generated K6-, K11-, K27-, K29-, K33-, K48-, and K63-linked polyubiquitylated substrate (Fig. S5 and Table S4). Although 3 different Ub-specific mAbs (P4D1, FK1, and FK2) and 1 Ub-specific polyclonal Ab (UG9510) all showed comparable recognition of the 7 polyubiquitylated proteins, the HWA4C4 mAb only recognized the K63-linked polyubiquitylated substrate (Figs. 3B and S5).
Fig. 3.
HWA4C4 is specific for K63-linked polyUb. (A) Equal amounts (300 ng) of free Ub, K63-linked polyUb, and K48-linked polyUb were separated by SDS/PAGE and immunoblotted with either Ub mAb (P4D1) or K63Ub mAb (HWA4C4). (B) In addition to K63-linked polyUb (300 ng), equal amounts of K6-, K11-, K27-, K29-, K33-, K48-, and K63-linked polyUb-modified substrates (1 μg), ([KX]Ub)n-substrate, were separated by SDS/PAGE and immunoblotted with either Ub mAb (P4D1) or K63Ub mAb (HWA4C4).
Two additional approaches were used to assess the specificity and sensitivity of HWA4C4 further. Ectopic expression of Ub mutants has been used to block/disrupt specific polyUb chain assemblies (16, 33). For example, the expression of K63R-Ub, in which lysine-63 is mutated to arginine, is able to compete with endogenous WT Ub for inclusion into K63-polyUb chains and block further chain extension. To test further the specificity of HWA4C4 ex vivo, we generated HEK293T cells expressing similar levels of WT, K48R, or K63R Ub (HA-tagged), as revealed by immunoblot analysis of lysates using HA mAb. Importantly, our K63Ub-specific mAb clearly detected K63-polyUb-conjugated proteins in whole, unmanipulated lysates from WT-Ub or K48R-Ub expressing HEK293T cells (Fig. 4A). In contrast, we observed a complete disappearance of high-molecular mass reactive bands with lysate from the K63R-Ub transfectants. The few lower-molecular mass bands still present in the K63R-Ub sample may represent nonsubstrate conjugated short K63-linked Ub chains that have not been terminated by the K63R Ub mutant. This almost complete inhibition of K63-linked polyUb chain formation in 293T cells expressing K63R-Ub provided an opportunity to assess the ability of HWA4C4 to work in immunofluorescence experiments. Although uniform staining with P4D1 was observed with both cell populations, a restricted pattern of staining with HWA4C4 was only observed with the WT Ub transfectants, but not the cells expressing K63R-Ub (Fig. S6). Given that the K63R mutation has been shown not to affect the assembly of other lysine linked polyUb chains, these data suggest that HWA4C4 only recognizes K63-linked polyUb, but not other lysine-linked polyUb, ex vivo.
Fig. 4.
HWA4C4 can specifically detect K63-linked polyUb-modified proteins in whole lysates. (A) HEK293T cells were transiently transfected with HA-tagged wild-type (WT), K48R, or K63R Ub. Cells were lysed 48 h after transfection. Cell lysates were gel-separated and immunoblotted with HA and K63Ub antibodies. (B) The lysates of stably transfected A20 cells expressing shRNA targeting Ubc13 (37) or an empty vector control were gel-separated and immunoblotted with Ub, K63Ub, Ubc13, and actin antibodies.
Ubc13 is a Ub-conjugating enzyme (E2) known to catalyze specifically K63-linked polyubiquitylation (15, 36), As a further demonstration of the selective utility of our K63Ub-specific mAb, we assessed the pattern of K63-linked polyubiquitylated proteins in a short hairpin RNA (shRNA)-targeted A20 B cell transductant that lacked Ubc13 (37). As expected, a selective loss/reduction of reactive bands was observed in lysate from Ubc13-deficient cells, demonstrating the sensitivity and specificity of HWA4C4 (Fig. 4B). The remaining HWA4C4-reactive bands in Ubc13-deficient cell lysate support the notion that other as yet unidentified E2s can catalyze K63-linked polyubiquitylation.
Collectively, these data suggest that HWA4C4 has high selectivity for the K63Ub isopeptide linkage and specifically recognizes K63-linked polyubiquitylated proteins both in vitro and ex vivo.
Sensitive Detection of K63-Polyubiquitylated TRAF6 in Vitro and ex Vivo.
To assess further the sensitivity and utility of our K63Ub mAb (HWA4C4) for ex vivo analysis, we examined 2 proteins known to be modified with K63-linked polyUb, TNF receptor-associated factor 6 (TRAF6) and mitogen-activated protein kinase kinase kinase 1 (MEKK1; also known as MAP3K1) (17, 38, 39). TRAF6 is an E3 Ub ligase that contains a RING domain that can mediate K63-linked polyubiquitylation in vitro after oligomerization (17). TRAF6 can both autoubiquitylate and mediate the ubiquitylation of other substrates. TRAF6 can also be ubiquitylated by other cellular E3 Ub ligases. We used a TRAF6-gyrase B stable cell line, in which a TAP (tandem affinity purification)-tagged TRAF6 was fused to a fragment of bacterial gyrase B such that oligomerization could be controlled by addition of coumermycin A (40). A ubiquitylated TRAF6 ladder was efficiently detected with both the Ub mAb (P4D1) and our K63Ub mAb (HWA4C4) after oligomerization (Fig. 5A).
Fig. 5.
Ex vivo analysis of TRAF6 polyubiquitylation. (A) TAP-tagged TRAF6-gyrase B in a stable HEK293T cell line was activated by oligomerization with coumermycin A. TRAF6 was TAP-purified from lysates of cells incubated with or without coumermycin A, gel-separated, and immunoblotted with P4D1 and HWA4C4. (B) WT and RING-mutated (MURING) TAP-tagged TRAF6 constructs were overexpressed in HEK293T cells, TAP-purified, and gel-separated. Blots were probed with P4D1 and HWA4C4. (C) (Upper) RAW264.7 cells were stimulated with LPS (1 μg/mL), CpG DNA (1 μM), and poly(I·C) (10 μg/mL) for 10 min. TRAF6 and TRAF2 were immunoprecipitated from cell lysates, gel-separated, and immunoblotted with P4D1 or HWA4C4. (Lower) Comparable loading was confirmed by reprobing with TRAF6 and TRAF2 antibodies.
Previous studies have shown that the overexpression of TRAF6 induces its K63-linked polyubiquitylation via its own RING domain (38, 41). We used this approach to assess further the specificity of our K63Ub mAb. Whereas overexpression of a RING domain mutant of TRAF6 resulted in reduced but detectable ubiquitylation compared with wild-type TRAF6, K63-linked polyubiquitylation was abolished in the RING mutant, and thus the K63Ub mAb (HWA4C4) only recognized ubiquitylated wild-type TRAF6 (Fig. 5B). These data suggest that HWA4C4 is capable of specifically detecting K63-linked polyubiquitylation ex vivo.
Next, we stimulated a mouse macrophage-like cell line (RAW 264.7) with a series of pathogen-associated molecular patterns that activate distinct TLRs. Activation by LPS, CpG DNA, and poly(I·C) resulted in rapid TRAF6 polyubiquitylation that was detected by both the Ub mAb (P4D1) and K63Ub mAb (HWA4C4) (Fig. 5C). The extent of TRAF6 K63-linked polyubiquitylation was higher in cells activated by TLR4 (LPS) or TLR9 (CpG) than TLR3 [poly(I·C)]. These results are consistent with the more substantial role played by TRAF3 in TLR3 signaling relative to TLR4 and TLR9 signaling that rely mainly on TRAF6 (40). Furthermore, under the same conditions no TRAF2 polyubiquitylation was observed in agreement with the lack of TRAF2 involvement in TLR signaling. However, TRAF2 K63-linked ubiquitylation was seen upon CD40 activation of B cells (37).
Sensitive Detection of K63-Polyubiquitylated MEKK1 in Vitro and ex Vivo.
MEKK1 also contains a RING finger-like domain (42), can autoubiquitylate, and in vitro can be modified with either K48- or K63-linked polyUb (39). Although the E3 Ub ligase activity of MEKK1 was suggested to play both positive and negative roles, leading to some MEKK1 degradation after its activation (43), we have not detected receptor-induced MEKK1 degradation in CD40-stimulated cells (39). This suggests that ex vivo, MEKK1 polyubiquitylation may be predominantly K63-linked because K48-linked polyubiquitylation may mediate proteasomal degradation. To determine the actual nature of MEKK1 modification, we first assessed the specificity of HWA4C4 toward ubiquitylated MEKK1 by performing an in vitro ubiquitylation assay with MEKK1 immunoprecipitates from anti-CD40-stimulated B cells, E1 and E2 Ub-conjugating enzymes, and either WT, K63R or K48R Ub mutants (Fig. 6A). The Ub mAb P4D1 was shown to recognize MEKK1 conjugated with all 3 recombinant Ub molecules, confirming that in vitro MEKK1 can undergo self-ubiquitylation through K63-linked, K48-linked, or other lysine-linked polyUb. As expected, our K63Ub mAb (HWA4C4) could only recognize the wild-type and K48R Ub-modified MEKK1. [The faint ladders in the K63R sample may be caused by MEKK1 being modified by endogenous Ub after B cell stimulation (Fig. 6B) (39)].
Fig. 6.
Ex vivo analysis of MEKK1 polyubiquitylation. (A) (Upper) Splenic CD43− B cells were stimulated with an agonistic CD40 antibody, and at the indicated times MEKK1 was immunoprecipitated and washed extensively. In vitro ubiquitylation assays were performed on the immunoprecipitated MEKK1 (which is free of TRAF2) with E1 and E2 Ub-conjugating enzymes plus WT Ub or Ub mutants (K63R or K48R). The reaction mixtures were separated by SDS/PAGE, and blots were probed with P4D1 or HWA4C4. (Lower) Comparable loading of MEKK1 was confirmed by reprobing with a MEKK1 Ab. (B) (Upper) Stably transfected A20 cells expressing shRNA targeting Ubc13, TRAF2, TRAF3, TRAF6, or an empty vector control were stimulated with CD40 Ab for the times indicated. The B cell lines 1.3E2 (Ikkγ−) and 70Z3 (Ikkγ+) were also stimulated with CD40 Ab. MEKK1 was immunoprecipitated, gel-separated, and immunoblotted with P4D1 or HWA4C4. (Lower) Comparable loading was confirmed by reprobing the same blots with MEKK1 antibody. (C) Appropriate knockdowns or absence of IKKγ in the cell lines examined above were confirmed by immunoblotting.
Finally, we assessed the ability of HWA4C4 to detect endogenous MEKK1 K63-linked ubiquitylation in CD40-stimulated B cells. Importantly, both the Ub mAb (P4D1) and K63Ub mAb (HWA4C4) revealed ubiquitylated MEKK1 only after cell stimulation (Fig. 6B). We used a variety of knockdown and mutant B cell lines to determine dependence of MEKK1 polyubiquitylation on other signaling molecules. We found that the K63-linked polyubiquitylation of MEKK1 was Ubc13-, TRAF2- and IKKγ-dependent (Fig. 6B). Notably, Ubc13 is a K63 Ub-specific E2 whose ablation in mice prevents the activation of MAP kinases by CD40 and other immunoreceptors (44). Knockdown of TRAF3 and TRAF6 had no effect. Interestingly, no MEKK1 ubiquitylation was detected with the Ub mAb (P4D1) in B cells lacking the K63-specific E2 Ub-conjugating enzyme Ubc13, suggesting that K48-linked ubiquitylation of MEKK1 does not occur in cells. These data not only emphasize the utility, sensitivity, and specificity of our K63Ub mAb, but also serve to illustrate that promiscuous polyubiquitylation of proteins can occur in in vitro assays that may not reflect events in cells. Furthermore, the absence of the K48-linked MEKK1 polyubiquitylation in CD40-engaged B cells is consistent with our inability to detect receptor-induced MEKK1 degradation in such cells for at least 2 h after CD40 engagement (Fig. S7). Although the results of these experiments indicate that MEKK1 K63-linked ubiquitylation is TRAF2-dependent, it should be noted that TRAF2 is required for the activation of MEKK1 protein kinase activity, which in turn is required for activation of its self-ubiquitylating activity (A.M., P.-H.T. and M.K., unpublished observations). Thus, it is not clear whether TRAF2 is directly or indirectly responsible for MEKK1 ubiquitylation. Activation of MEKK1 protein kinase activity also depends on the scaffold protein IKKγ/NEMO (37), which as shown above is also required for MEKK1 K63-linked polyubiquitylation (Fig. 6B).
Conclusions
The specificity of the HWA4C4 mAb demonstrates that a branched peptide is capable of mimicking the isopeptide linkage of K63-linked polyUb and can be used as an immunogen to generate a linkage-specific mAb. Specificity for polyUb but not monoUb is likely ensured by the unique structure of the isopeptide linkage between the COOH-terminal sequence of Ub and the lysine, whereas the amino acid residues around K63 make the mAb specific for the K63 polyUb chain. We demonstrate here that this antibody may be used for detection of synthesized, purified, or immunoprecipitated proteins and does not require high cell numbers or large-scale protein purification efforts. Furthermore, we also show that HWA4C4 can detect K63Ub-modified proteins in whole lysates, demonstrating that prior immunoprecipitation is not a prerequisite for its use. Although HWA4C4 does not appear to immunoprecipitate K63Ub presumably because of epitope accessibility, it can specifically detect K63Ub-modified proteins in cells by immunoprecipitation, further expanding the utility of this mAb. This represents a unique reagent/approach that can directly detect K63-linked polyubiquitylation ex vivo with minimal cellular manipulation, as has been recently demonstrated (37). The general strategy used here may be extended to generating mAbs specific for other polyUb linkages, such as K48Ub or K29Ub, or for Ub-modified substrates, where the target substrate lysine is known, for example, K21 and K22 of IκBα (45) or K855 of the NF-κB2 precursor p100 (46). Indeed and interestingly, a resent study has used a comparable strategy to develop an antibody specific for monoubiquitylated H2B by using a branched peptide as immunogen (47).
Materials and Methods
Cell Lines, Antibodies, and Ub Reagents.
1.3E2 (Ikkγ−) and the parental cells, 70Z3 cells (Ikkγ+), were kindly provided by Gilles Courtois, Institut National de la Santé et de la Recherche Médicale U697 (48). Cells were maintained at 37 °C, 5% CO2 in RPMI medium 1640 supplemented with FCS, glutamine, and antibiotics (Invitrogen). The following commercial antibodies were used: horseradish peroxidase (HRP)-conjugated anti-mouse IgG (Amersham) and anti-rabbit IgG (Jackson Laboratories); HRP-conjugated anti-goat IgG, MEKK1, IKKγ, TRAF2, TRAF3, TRAF6, and Ubc13 Ub (all from Santa Cruz Biotechnology), HA (12CA5, gift from Linda Hendershot, St. Jude Children's Research Hospital). K48- and K63-linked polyUb were purchased from Biomol, and free Ub was from Sigma. Ubiquitylation assay reagents were obtained from Boston Biochem. Polyubiquitylated substrates are available from Biomol (details of the approach for generation of these proteins are given in SI Materials and Methods).
Synthesis of Branched Peptides.
All peptides were synthesized by the Hartwell Center for Biotechnology and Bioinformatics at St. Jude Children's Research Hospital. The peptides were made by using Fmoc chemistry on a Rainin Symphony Multiplex 12-column synthesizer. To make the branched peptides, the orthogonally protected N-α-Fmoc-N-ε-1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)-3-methylbutyl-l-lysine [Fmoc-Lys(ivDde)-OH from NovaBiochem] was used. After the main peptides were synthesized and the N termini acetylated, the ivDde-protecting groups on the lysine ε-amino groups were selectively removed by using 3 mL of 2% hydrazine for 3 min. The short peptide branches were then synthesized. After synthesis, final deprotection, and cleavage, the final peptides were purified by reverse-phase HPLC on a Beckman Biosys 500 with a Vydac C-18 column. The immunogen U63U was conjugated to KLH via the N terminus of the Ub[71–76] peptide (RLRGG) by using glutaraldehyde.
Immunization of Mice and Generation of Hybridomas.
BALB/c mice (Jackson Laboratories) were immunized i.p. with KLH-conjugated U63U immunogen (20 μg) in complete Freund's adjuvant. Mice were boosted twice by injection of KLH-conjugated U63U in incomplete Freund's adjuvant at 3-week intervals. Mice were bled for screening purposes at every boost and killed 72 h after the last boost before splenectomy. The splenocytes and lymph node cells were collected, mixed with X63-Ag-8.653 mouse myeloma cells at a ratio of 5:1, pelleted, and incubated with 37 °C prewarmed polyethylene glycol 1500 (PEG 1500; Sigma). The cell mix was slowly and gently resuspended in 37 °C prewarmed HBSS buffer. After gentle agitation for 3 min, the cell mix was pelleted, resuspended in compete tumor medium (CTM) (49, 50) and plated in 96-well plates at 105 splenocytes per well. After a 24-h incubation, CTM containing Hypoxanthine Aminopterin Thymidine (HAT) (Invitrogen, 50×) was added to select hybrid clones. Plates were incubated at 37 °C in a tissue culture incubator until visible colonies were present in the wells. The supernatants were screened twice on two subsequent occasions by ELISA, and the positive wells were subcloned by limiting dilution and single-cell sorting. The mAb isotype was determined by ELISA and was purified by using protein G affinity chromatography (Amersham).
Oligomerization and Purification of TRAF6 from TRAF6-Gyrase B Stable Cell Lines.
The N terminus of TRAF6 was tagged with a tandem affinity tag consisting of a His6 element and a signature sequence for intracellular biotinylation (HBT) (21), whereas the C terminus of TRAF6 was fused with the N-terminal fragment of bacterial gyrase B. The HBT-TRAF6-gyrase B construct was transduced into HEK293 cells. To induce dimerization of TRAF6, cells were treated with coumermycin A, and cell extracts were prepared for purification as described in ref. 40. A tandem affinity tag-based 2-step purification under full denaturing conditions was used to purify the HBT-tagged TRAF6 (details of the purification are given in SI Materials and Methods) (21).
Splenic B Cell Isolation and in Vitro Ubiquitylation Assay.
Splenic B cells were isolated as described in ref. 39. Splenocyte suspensions were generated by grinding spleens between 2 cover slips. CD43− B cells were isolated with magnetic beads (MACS; Miltenyi) according to manufacturer's protocol. B cells or B cell lines (1 × 106 cells per mL) were stimulated with anti-CD40 (5 μg/mL; clone 3/23; PharMingen). Washed MEKK1 immunoprecipitated complexes from splenic B cell lysates were incubated with Ub, ATP, E1 and a mixture of E2 Ub conjugating enzymes: UbcH2, UbcH3, Ubc5a, Ubc5b, Ubc5c, UbcH6, UbcH7, and UbcH10. After 30 min, reaction mixtures were separated by SDS/PAGE and analyzed by immunoblotting with Ub, K63Ub, and MEKK1 antibodies.
ShRNA Constructs.
Oligonucleotides directed against the indicated mRNAs were cloned into the pLSLPw lentiviral plasmid [kindly provided by Peter M. Chumakov, Cleveland Clinic (51)]. The oligonucleotide sequences used for the shRNA constructs are as follows: m/h-Ubc13, 5′-AATCCAGATGATCCATTAGCA (52); m-TRAF2, 5′-CTAGACCAGGACAAGATTG; m-TRAF3, 5′-GCAAG A G AGAGATTCTGGC; m-TRAF6, 5′-CGTCCTTTCCAGAAGTGCC. Lentiviral packaging was performed as described, and details are provided in SI Materials and Methods (53).
The details on Biacore analysis, ELISA, immunoblotting, immunoprecipitation, and immunofluorescent staining are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank R. Cassell and P. Rodrigues for branched peptide synthesis; M. Zhuang for performing the structural rendering; D. Huang for valuable suggestions; J. Thomson and S. Howard for technical assistance in the preparation and characterization of the polyubiquitylated species; and R. Cross, J. Smith, and Y. He for FACS. This work was supported by National Institutes of Health Grants AI52199 (to D.A.A.V.) and AI043477 (to M.K.), Cancer Center Support (Core) Grant CA21765 (to D.A.A.V. and H.H.), and the American Lebanese Syrian Associated Charities (D.A.A.V. and H.H.). T.P.N. was supported by European Community Framework VI Program LSHG-CT-2005-018683. BIOMOL is a member of the RUBICON Network of Excellence.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810461105/DCSupplemental.
References
- 1.Ciechanover A. Intracellular protein degradation: From a vague idea, through the lysosome and the ubiquitin–proteasome system, and onto human diseases and drug targeting (Nobel lecture) Angew Chem Int Ed Engl. 2005;44:5944–5967. doi: 10.1002/anie.200501428. [DOI] [PubMed] [Google Scholar]
- 2.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 3.Glickman MH, Ciechanover A. The ubiquitin–proteasome proteolytic pathway: Destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 4.Bloom J, et al. Proteasome-mediated degradation of p21 via N-terminal ubiquitinylation. Cell. 2003;115:71–82. doi: 10.1016/s0092-8674(03)00755-4. [DOI] [PubMed] [Google Scholar]
- 5.Cadwell K, Coscoy L. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science. 2005;309:127–130. doi: 10.1126/science.1110340. [DOI] [PubMed] [Google Scholar]
- 6.Haglund K, Di Fiore PP, Dikic I. Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem Sci. 2003;28:598–603. doi: 10.1016/j.tibs.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Marmor MD, Yarden Y. Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene. 2004;23:2057–2070. doi: 10.1038/sj.onc.1207390. [DOI] [PubMed] [Google Scholar]
- 8.Stelter P, Ulrich HD. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature. 2003;425:188–191. doi: 10.1038/nature01965. [DOI] [PubMed] [Google Scholar]
- 9.Hochstrasser M. Lingering mysteries of ubiquitin-chain assembly. Cell. 2006;124:27–34. doi: 10.1016/j.cell.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 10.Peng J, et al. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol. 2003;21:921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 11.Xu P, Peng J. Characterization of polyubiquitin chain structure by middle-down mass spectrometry. Anal Chem. 2008;80:3438–3444. doi: 10.1021/ac800016w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 13.Jin L, et al. Mechanism of ubiquitin-chain formation by the human anaphase-promoting complex. Cell. 2008;133:653–665. doi: 10.1016/j.cell.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chastagner P, Israel A, Brou C. Itch/AIP4 mediates Deltex degradation through the formation of K29-linked polyubiquitin chains. EMBO Rep. 2006;7:1147–1153. doi: 10.1038/sj.embor.7400822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmann RM, Pickart CM. Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell. 1999;96:645–653. doi: 10.1016/s0092-8674(00)80575-9. [DOI] [PubMed] [Google Scholar]
- 16.Spence J, Sadis S, Haas AL, Finley D. A ubiquitin mutant with specific defects in DNA repair and multiubiquitination. Mol Cell Biol. 1995;15:1265–1273. doi: 10.1128/mcb.15.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C, et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 18.Spence J, et al. Cell cycle-regulated modification of the ribosome by a variant multiubiquitin chain. Cell. 2000;102:67–76. doi: 10.1016/s0092-8674(00)00011-8. [DOI] [PubMed] [Google Scholar]
- 19.Wu-Baer F, Lagrazon K, Yuan W, Baer R. The BRCA1/BARD1 heterodimer assembles polyubiquitin chains through an unconventional linkage involving lysine residue K6 of ubiquitin. J Biol Chem. 2003;278:34743–34746. doi: 10.1074/jbc.C300249200. [DOI] [PubMed] [Google Scholar]
- 20.Baboshina OV, Haas AL. Novel multiubiquitin chain linkages catalyzed by the conjugating enzymes E2EPF and RAD6 are recognized by 26S proteasome subunit 5. J Biol Chem. 1996;271:2823–2831. doi: 10.1074/jbc.271.5.2823. [DOI] [PubMed] [Google Scholar]
- 21.Tagwerker C, et al. A tandem affinity tag for two-step purification under fully denaturing conditions: Application in ubiquitin profiling and protein complex identification combined with in vivo cross-linking. Mol Cell Proteomics. 2006;5:737–748. doi: 10.1074/mcp.M500368-MCP200. [DOI] [PubMed] [Google Scholar]
- 22.Kirkpatrick DS, et al. Quantitative analysis of in vitro ubiquitinated cyclin B1 reveals complex chain topology. Nat Cell Biol. 2006;8:700–710. doi: 10.1038/ncb1436. [DOI] [PubMed] [Google Scholar]
- 23.Kim HT, et al. Certain pairs of ubiquitin-conjugating enzymes (E2s) and ubiquitin-protein ligases (E3s) synthesize nondegradable forked ubiquitin chains containing all possible isopeptide linkages. J Biol Chem. 2007;282:17375–17386. doi: 10.1074/jbc.M609659200. [DOI] [PubMed] [Google Scholar]
- 24.Al-Hakim AK, et al. Control of AMPK-related kinases by USP9X and atypical Lys(29)/Lys(33)-linked polyubiquitin chains. Biochem J. 2008;411:249–260. doi: 10.1042/BJ20080067. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda F, Dikic I. Atypical ubiquitin chains: New molecular signals. Protein modifications: Beyond the usual suspects review series. EMBO Rep. 2008;9:536–542. doi: 10.1038/embor.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanayama A, et al. TAB2 and TAB3 activate the NF-κB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15:535–548. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Tran H, Hamada F, Schwarz-Romond T, Bienz M. Trabid, a new positive regulator of Wnt-induced transcription with preference for binding and cleaving K63-linked ubiquitin chains. Genes Dev. 2008;22:528–542. doi: 10.1101/gad.463208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winborn BJ, et al. The deubiquitinating enzyme ataxin-3, a polyglutamine disease protein, edits K63-linkages in mixed linkage ubiquitin chains. J Biol Chem. 2008;283:26436–26443. doi: 10.1074/jbc.M803692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wertz IE, et al. Deubiquitination and ubiquitin ligase domains of A20 down-regulate NF-κB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 30.Kovalenko A, et al. The tumour suppressor CYLD negatively regulates NF-κB signalling by deubiquitination. Nature. 2003;424:801–805. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]
- 31.Komander D, et al. The structure of the CYLD USP domain explains its specificity for Lys-63-linked polyubiquitin and reveals a B box module. Mol Cell. 2008;29:451–464. doi: 10.1016/j.molcel.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 32.Fujimuro M, Nishiya T, Nomura Y, Yokosawa H. Involvement of polyubiquitin chains via specific chain linkages in stress response in mammalian cells. Biol Pharm Bull. 2005;28:2315–2318. doi: 10.1248/bpb.28.2315. [DOI] [PubMed] [Google Scholar]
- 33.Chiu RK, et al. Lysine-63 polyubiquitination guards against translesion synthesis-induced mutations. PLoS Genet. 2006;2:e116. doi: 10.1371/journal.pgen.0020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun T, Arlinghaus RB. Preparation and application of polyclonal and monoclonal sequence-specific anti-phosphoamino acid antibodies. Curr Protoc Protein Sci. 2004;13:13.6. doi: 10.1002/0471140864.ps1306s34. [DOI] [PubMed] [Google Scholar]
- 35.Varadan R, et al. Solution conformation of Lys-63-linked diubiquitin chain provides clues to functional diversity of polyubiquitin signaling. J Biol Chem. 2004;279:7055–7063. doi: 10.1074/jbc.M309184200. [DOI] [PubMed] [Google Scholar]
- 36.Eddins MJ, et al. Mms2-Ubc13 covalently bound to ubiquitin reveals the structural basis of linkage-specific polyubiquitin chain formation. Nat Struct Mol Biol. 2006;13:915–920. doi: 10.1038/nsmb1148. [DOI] [PubMed] [Google Scholar]
- 37.Matsuzawa A, et al. Essential cytoplasmic translocation of a cytokine receptor-assembled signaling complex. Science. 2008;321:663–668. doi: 10.1126/science.1157340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamothe B, et al. Site-specific Lys-63-linked tumor necrosis factor receptor-associated factor 6 autoubiquitination is a critical determinant of IκB kinase activation. J Biol Chem. 2007;282:4102–4112. doi: 10.1074/jbc.M609503200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallagher E, et al. Kinase MEKK1 is required for CD40-dependent activation of the kinases Jnk and p38, germinal center formation, B cell proliferation, and antibody production. Nat Immunol. 2007;8:57–63. doi: 10.1038/ni1421. [DOI] [PubMed] [Google Scholar]
- 40.Hacker H, et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 41.Trompouki E, et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-κB activation by TNFR family members. Nature. 2003;424:793–796. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- 42.Lu Z, et al. The PHD domain of MEKK1 acts as an E3 ubiquitin ligase and mediates ubiquitination and degradation of ERK1/2. Mol Cell. 2002;9:945–956. doi: 10.1016/s1097-2765(02)00519-1. [DOI] [PubMed] [Google Scholar]
- 43.Witowsky JA, Johnson GL. Ubiquitylation of MEKK1 inhibits its phosphorylation of MKK1 and MKK4 and activation of the ERK1/2 and JNK pathways. J Biol Chem. 2003;278:1403–1406. doi: 10.1074/jbc.C200616200. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto M, et al. Key function for the Ubc13 E2 ubiquitin-conjugating enzyme in immune receptor signaling. Nat Immunol. 2006;7:962–970. doi: 10.1038/ni1367. [DOI] [PubMed] [Google Scholar]
- 45.DiDonato J, et al. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amir RE, Haecker H, Karin M, Ciechanover A. Mechanism of processing of the NF-κB2 p100 precursor: Identification of the specific polyubiquitin chain-anchoring lysine residue and analysis of the role of NEDD8-modification on the SCF(β-TrCP) ubiquitin ligase. Oncogene. 2004;23:2540–2547. doi: 10.1038/sj.onc.1207366. [DOI] [PubMed] [Google Scholar]
- 47.Minsky N, et al. Monoubiquitinated H2B is associated with the transcribed region of highly expressed genes in human cells. Nat Cell Biol. 2008;10:483–488. doi: 10.1038/ncb1712. [DOI] [PubMed] [Google Scholar]
- 48.Courtois G, Whiteside ST, Sibley CH, Israel A. Characterization of a mutant cell line that does not activate NF-κB in response to multiple stimuli. Mol Cell Biol. 1997;17:1441–1449. doi: 10.1128/mcb.17.3.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kappler JW, Skidmore B, White J, Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas: Lack of independent antigen and H-2 recognition. J Exp Med. 1981;153:1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodland D, Happ MP, Bill J, Palmer E. Requirement for cotolerogenic gene products in the clonal deletion of I-E reactive T cells. Science. 1990;247:964–967. doi: 10.1126/science.1968289. [DOI] [PubMed] [Google Scholar]
- 51.Budanov AV, et al. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 52.Andersen PL, et al. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. J Cell Biol. 2005;170:745–755. doi: 10.1083/jcb.200502113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gurova KV, et al. p53 pathway in renal cell carcinoma is repressed by a dominant mechanism. Cancer Res. 2004;64:1951–1958. doi: 10.1158/0008-5472.can-03-1541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.