Abstract
The leptin regulation of bone remodeling, which has been documented through studies of loss-of-function mutations of this hormone or of its receptor in mice and humans, still raised several unanswered questions. For instance, it has been assumed but not formally demonstrated that this regulation occurs through neuronal means. Likewise, it has not been possible until now to dissociate the influence leptin exerts on appetite and energy expenditure from this function. We show here through mouse genetic studies that a deletion of the leptin receptor in neurons results in an increase in bone formation and bone resorption, resulting in a high bone mass as seen in leptin-deficient mice. In contrast, the same deletion in osteoblasts only does not influence bone remodeling. Furthermore, through the use of l/l mice, a model of gain of function in leptin signaling harboring a Y985L substitution in the leptin receptor, we show that leptin signaling inhibits bone mass accrual by up-regulating sympathetic activity independently of any change in appetite or energy expenditure. This work establishes that in vivo leptin regulates bone mass accrual by acting through neuronal means and provides a direct demonstration that this function of leptin can occur independently of its regulation of energy metabolism.
Keywords: bone remodeling, energy expenditure, osteoblasts, osteoclasts
In the last 10 years, significant advances in our understanding of bone remodeling have come from the study of its endocrine regulation by leptin (1). Remarkably, many studies uncovered that the regulation of either arm of bone remodeling, bone formation and bone resorption, involves molecules not classically associated with bone physiology (1–4). For instance, it has been proposed that leptin regulation of bone mass occurs after its binding to its receptor presumably on hypothalamic neurons. This results in an activation of the sympathetic tone that then acts on osteoblasts to decrease bone formation and to increase osteoclast differentiation (4–8). In addition, leptin via another mediator, CART (cocaine and amphetamine-regulated transcript), inhibits bone resorption (4, 9). Thus, leptin, through the combined actions of these two mediators, prevents bone mass accrual (1).
Several questions surround leptin regulation of bone mass accrual. First, there has been no evidence to date that in vivo leptin regulates bone mass through neuronal means rather than through a direct effect on osteoblasts as initially proposed (10, 11). Another lingering point has been to rule out that leptin regulation of bone mass is merely a secondary consequence of regulation of energy metabolism by this hormone.
Leptin mediates its functions after its binding to a single receptor [LRb, the long form (b) of the leptin receptor, encoded by the Lepr gene] that is linked to the Jak2 tyrosine kinase (12–18). Leptin binding activates Jak2, resulting in the tyrosine phosphorylation of several residues on LRb, each of which possesses unique functions. Phosphorylated Tyr-1077 and Tyr-1138 on LRb recruit and activate the latent transcription factors STAT5 and STAT3, which mediate important aspects of leptin action (15–18). LRb is also phosphorylated on Tyr-985 (Y985), which binds SOCS3 to attenuate LRb signaling (16, 19, 20). An implication of these molecular studies is that inactivation of Y985 should result in increased leptin action/LRb signaling. Congruent with this hypothesis, homologous recombination-mediated mutation of LRb by a Y985L substitution (which abolishes phosphorylation and SOCS3 recruitment) in mice disinhibits LRb signaling to generate a mild gain-of-function model of leptin action, at least for some functions of this hormone (21). Thus, this mutant mouse strain, termed l/l, is an excellent model to ask further, in a gain-of-function model, whether leptin is a negative regulator of bone mass accrual.
Here we show, through cell-specific deletion of its receptor in mice, that leptin regulation of bone mass accrual occurs through neuronal means, not through a local effect on bone cells. We also show that l/l mice display a low-bone mass phenotype in the absence of any abnormality in appetite or energy expenditure. Together with observations already gathered with loss-of-function mutations, this work further establishes that leptin is a physiological regulator of bone remodeling acting in vivo through neuronal means and dissociates its effect on bone mass accrual from those on appetite and energy expenditure.
Results
Neuronal but Not Osteoblast-Specific Deletion of the Leptin Receptor Affects Bone Mass.
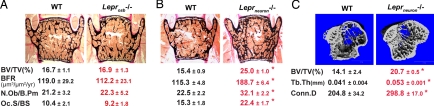
To determine the primary cell type affected by leptin during bone remodeling, we deleted Lepr either from osteoblasts, the bone-specific cells targeted by leptin signaling (4–6), or from all neurons [supporting information (SI) Fig. S1]. As shown in Fig. 1A, classical histomorphometry on nondemineralized bone sections showed that bone formation and bone resorption parameters were not affected by Lepr deletion specifically in osteoblasts [α1(I)Collagen-Cre;Leprfl/fl mice]. In contrast, mice lacking Lepr in neurons only (SynapsinI-Cre;Leprfl/fl mice) showed an increase in bone formation and bone resorption parameters resulting in a high-bone mass phenotype, mimicking the bone phenotype of ob/ob mice (Fig. 1B). Microcomputed tomography (μCT) analysis of long bones further confirmed the high-bone mass phenotype in SynapsinI-Cre;Leprfl/fl mice. Indeed, mutant mice displayed a higher bone volume, thicker trabeculae, and increased trabecular connectivity density (Conn.D) compared with wild-type (WT) littermates (Fig. 1C).
Fig. 1.
Neuronal mediation of leptin regulation of bone mass accrual. (A) Normal bone mass and normal bone-remodeling parameters in osteoblast-specific Lepr-deficient mice (Leprosb−/−, refer to α1(I)Collagen-Cre;Leprfl/fl mice) by histomorphometric analysis of vertebrae. (B) Higher bone volume (BV/TV, percentage), increased N.Ob/B.Pm (number of osteoblasts divided by bone perimeter), and BFR (μm3/μm2 per year), and increased Oc.S/BS (osteoclast surface divided by bone surface) in neuron-specific Lepr-deficient mice (Leprneuron−/−, refer to SynapsinI-Cre;Leprfl/fl mice). (C) Higher bone density in long bones of Leprneuron−/− mice by μCT analysis along with higher Tb.Th and increased Conn.D. WT refers to the littermate control groups, including Lepr+/+, Leprfl/fl, and specific-Cre;Lepr+/+, none of which had general and bone mass abnormality. Twelve-week-old female mice were used in these analyses (n = 6–10 per group for bone histomorphometry and n = 4 for μCT analysis; *, P < 0.05; error bars, SEM).
Normal Appetite and Energy Expenditure in l/l Mice.
Having established that leptin regulates bone mass through neuronal means, it was then important to determine directly whether it regulates bone mass independently of its influence on energy metabolism. To that end, we made use of the l/l mice that represent a gain-of-function model for leptin signaling (21). In these mice, the signaling form of the leptin receptor harbors a mutation abrogating binding of SOCS3, a negative regulator of its activity, that thereby leads to enhanced leptin signaling (19, 20). We analyzed female WT and l/l mice because it is in female l/l mice that the evidence of an increase in leptin signaling is the strongest. For instance, female l/l mice have a more significant decrease in circulating leptin level and smaller gonadal fat pads, two observations indicating an increase in leptin signaling, than male l/l mice (Table S1 and ref. 21).
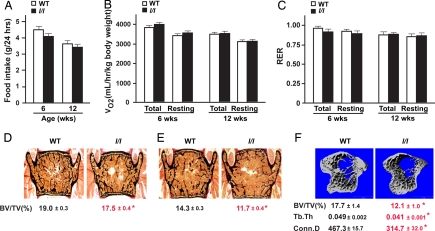
This increase in leptin signaling does not affect, however, all of the major functions of leptin. For instance, not only are l/l mice fertile as reported (21), but food intake and energy expenditure, two cardinal functions regulated by leptin, were also normal in 6- and 12-week-old l/l mice (Fig. 2 A–C). Indeed, whether we looked at oxygen consumption (VO2) or respiratory exchange ratio (RER) (Fig. 2 B and C), there was no difference between WT and l/l mice at 6 and 12 weeks of age. This absence of overt consequence of the Y985L mutation on appetite and energy expenditure makes the l/l mice an even more attractive model to determine whether the regulation of bone mass is a bona fide function of leptin.
Fig. 2.
Normal appetite and energy expenditure but low bone mass in l/l mice. (A) Normal appetite in l/l mice at 6 and 12 weeks of age measured by 24-h food intake. (B and C) Normal energy expenditure in 6- and 12-week-old l/l mice measured by VO2 (B) and RER (C), respectively. (D and E) Lower bone volume in 6- (D) and 12-week-old (E) l/l mice by histomorphometric analysis of vertebrae. (F) Lower bone density in long bones of 12-week-old mice l/l mice by μCT analysis. Also, 3D morphological parameters Tb.Th and Conn.D were abnormal in l/l mice (n = 6–10 per group for bone histomorphometry and n = 3 for μCT analysis; *, P < 0.05; error bars, SEM).
Low Bone Mass in l/l Mice.
As shown in Fig. 2 D and E, classical histomorphometry on nondemineralized vertebral sections showed that bone mass was significantly decreased in l/l mice at both 6 and 12 weeks of age. This osteopenia was not limited to the axial skeleton and could also be observed in long bones. Indeed, a detailed μCT analysis showed that 12-week-old l/l mice had not only a lower bone volume, but also thinner trabeculae (Fig. 2F). In addition, trabecular Conn.D was significantly decreased in l/l mice, indicating a decrease in intact trabecular bone network (Fig. 2F). Taken together, these results indicate that an activating mutation in the leptin receptor enhances the ability of this hormone to regulate bone mass without affecting appetite or energy expenditure.
Decreased Bone Formation and Increased Bone Resorption in l/l Mice.
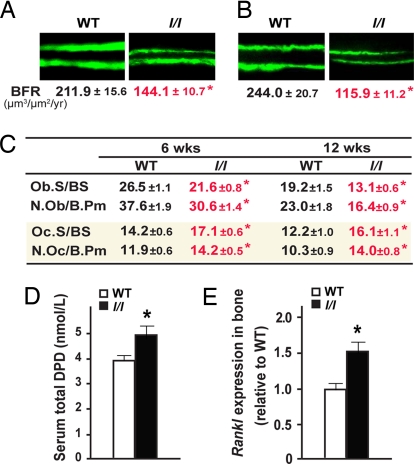
To determine the cellular bases of the osteopenia observed in l/l mice, we next performed static and dynamic histomorphometry. As shown in Fig. 3 A–C, there was at both 6 and 12 weeks of age a significant decrease in the number of osteoblasts per bone perimeter or in the osteoblast surface per bone surface and in the bone formation rate (BFR) in l/l mice, establishing that bone formation is decreased in these mutant mice. At the same time, we noticed in both 6- and 12-week-old l/l mice a significant increase in bone resorption as determined by an increase in the number of osteoclasts per bone perimeter and in the osteoclast surface per bone surface (Fig. 3C). In agreement with this observation, the serum level of total deoxypyridinoline (DPD), a byproduct of collagen degradation and a biomarker of osteoclast activity (22), and expression of Rankl, the major osteoclast differentiation factor (23, 24), were both significantly increased in l/l mice (Fig. 3 D and E). In summary, the low-bone mass phenotype observed in l/l mice mirrors the high-bone mass phenotype reported in ob/ob mice (5). This finding is consistent with the opposite nature of these two mutations.
Fig. 3.
Decreased bone formation and increased bone resorption in l/l mice. (A and B) Decrease BFR in l/l mice at both 6 (A) and 12 weeks of age (B). (C) Decreased osteoblast parameters, including Ob.S/BS and N.Ob/B.Pm, and increased osteoclast parameters, including Oc.S/BS and N.Oc/B.Pm in 6- and 12-week-old l/l mice. (D) Increased serum total DPD cross-links in 12-week-old l/l mice. (E) Increased Rankl expression in long bones of l/l mice (n = 6–10 per group; *, P < 0.05; error bars, SEM).
Increased Sympathetic Activity in l/l Mice.
The cellular basis of the l/l mice low-bone mass phenotype is the mirror image of what is observed in the case of mice lacking sympathetic signaling (4, 6). Therefore, we tested whether the bone phenotype of this model of mild gain of function in leptin signaling was caused by an increase in sympathetic tone.
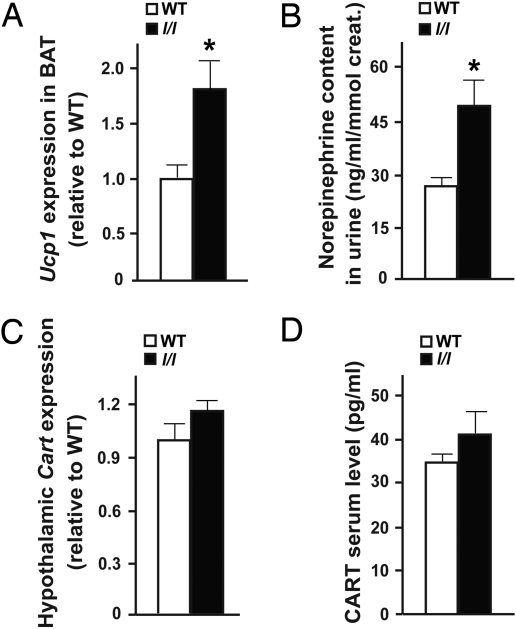
Sympathetic activity measured by Ucp1 expression in brown adipocyte tissue (BAT), which is decreased in ob/ob mice (6), was increased in l/l mice (Fig. 4A). Likewise, urinary elimination of norepinephrine was nearly doubled in l/l mice, indicating an increase in sympathetic activity (Fig. 4B). In contrast, we did not detect any abnormality in Cart expression in the hypothalamus or in circulating CART level in l/l mice (Fig. 4 C and D), indicating that the Y985L mutation does not affect Cart expression as a complete inactivation of leptin signaling does (4).
Fig. 4.
Increased sympathetic activity in l/l mice. (A) Increased Ucp1 expression in BAT in 6-week-old l/l mice. (B) Increased norepinephrine content in urine in l/l mice. (C) Normal Cart expression in hypothalamus in 12-week-old l/l mice. (D) Normal serum CART level in 12-week-old l/l mice (n = 5–8 per group; *, P < 0.05; error bars, SEM).
Discussion
Since the description in 2000 of the ability of leptin to inhibit bone mass accrual in vivo (5), one major issue has been to establish whether this regulation occurs through a neuronal pathway, as suggested by the results of leptin intracerebroventricular infusion in ob/ob mice (5), or through a direct effect on osteoblasts as initially thought (10, 11). The most rigorous way to address this question in vivo is through cell-specific deletion of its receptor in mice. We show here that the deletion of Lepr in neurons results in bone-remodeling abnormalities identical to those of ob/ob mice, whereas its deletion in osteoblasts only has no detectable effect on bone mass or bone-remodeling parameters. These results establish formally that in vivo leptin acts through a neuronal relay and not locally to regulate bone mass.
A second lingering question surrounding the regulation of bone mass by leptin has been to determine whether or not it was secondary to the regulation of energy metabolism by this hormone. The l/l mice were generated in an effort to obtain a model of increased leptin signaling, an expectation that was met to a limited extent if one looks at energy metabolism (21). Of particular interest for us was that appetite and energy expenditure were normal in these mutant mice when fed a regular chow. This was important because, despite the absence of an overt metabolic phenotype, l/l mice presented the expected bone phenotype, i.e., a low bone mass, secondary to a decrease in bone formation and to an increase in bone resorption parameters. Thus, analysis of this gain-of-function model of leptin signaling confirms that leptin regulates bone mass and, more importantly, provides in vivo evidence that this regulation occurs independently of leptin regulation of energy metabolism.
Molecular and biochemical analyses showed that of the two known mediators of leptin regulation of bone mass accrual, CART and the sympathetic tone, only the latter was increased. Assuming that leptin regulates bone mass through a hypothalamic relay (6), one molecular explanation for these observations could be that leptin regulates the sympathetic nervous system via Tyr-985-dependent pathways and CART through Tyr-985-independent pathways. Alternatively, it is possible that Cart expression was not affected because l/l mice display only a hypomorphic gain-of-function mutation in leptin signaling (21). Regardless of why energy metabolism is not affected in l/l mice, the elucidation of a bone phenotype in the absence of a metabolic phenotype in a leptin signaling gain-of-function mutant mouse model indicates that leptin regulation of bone mass is a true function of this hormone. This statement is further supported by the fact that sympathetic tone and CART regulate bone mass accrual but not appetite or energy expenditure, two other functions of leptin (4, 25).
The l/l mice harbor the mutation in the leptin receptor in all cells of the body. Given the dissociation that we and others have observed in these mice between the different functions of leptin, it will be important now to generate a floxed allele of this mutation to identify further the different populations of neurons required for each function of leptin.
Materials and Methods
Animals.
Leprfl/fl mice (C57BL/6J background) and SynapsinI-Cre transgenic mice (C57BL/6J background) used in these studies have been described (26, 27). α1(I)Collagen-Cre transgenic mice were generated on an FVB background in our laboratory and backcrossed for 9 generations on a C57BL/6J background (28). SynapsinI-Cre;Leprfl/fl mutant female mice and their littermate controls (Lepr+/+, SynapsinI-Cre;Lepr+/+, and Leprfl/fl) were generated by interbreeding of SynapsinI-Cre;Leprfl/+ mice with Leprfl/+ mice. A similar breeding strategy was used to generate α1(I)Collagen-Cre;Leprfl/fl mutant female mice and their littermate controls. l/l mice used in these studies have been described in ref. 21, and the breeders were backcrossed for >6 generations on the C57BL/6J genetic background. Female WT and l/l littermates produced by interbreeding of l/+ mice were used for all of the studies. Genotypes of the mice were determined by using tail DNA by PCR analysis as described (21, 26–28). All procedures were approved by the Columbia University Institutional Animal Care and Use Committee.
Physiological Measurements.
For food intake studies, mice were housed individually in metabolic cages (Nalge) and fed ad libitum. Food consumption amount was determined by weighing the chow before and after the 24-h measurement. VO2 and RER were measured by the indirect calorimetry method using a 6-chamber Oxymax system (Columbus Instruments). Mice were housed individually in the chamber and fed ad libitum. After 30-h acclimation to the apparatus, data for 24-h measurement were collected and analyzed.
Bone Histomorphometric Analyses.
Bone histomorphometry was performed as described (29–31). Briefly, lumbar vertebrae were dissected, fixed for 24 h in 10% formalin, dehydrated in graded ethanol series, and embedded in methyl methacrylate resin according to standard protocols (29). Von Kossa/von Gieson staining was performed by using 7-μm sections for bone volume over tissue volume (BV/TV) measurement. BFR was analyzed by the calcein double-labeling method (30). Calcein (Sigma) was dissolved in calcein buffer (0.15 M NaCl, 2% NaHCO3) and injected twice at 0.125 mg/g body weight on days 1 and 4, and then mice were killed on day 6. Unstained 4-μm sections were used for BFR measurements. For the analysis of parameters of osteoblast and osteoclast, 4-μm sections were stained with toluidine blue and tartrate-resistant acid phosphatase (TRAP), respectively (31, 32). Histomorphometric analyses were performed by using the Osteomeasure analysis system (Osteometrics).
μCT Analysis.
Trabecular bone architecture of distal tibia was assessed by using a μCT system (VivaCT 40; SCANCO Medical AG). Tibia bone specimen was stabilized with gauze in a 2-mL centrifuge tube filled with 70% ethanol and fastened in the specimen holder of the μCT scanner. One hundred μCT slices, corresponding to a 1.05-mm region distal from the growth plate, were acquired at an isotropic spatial resolution of 10.5 μm. A global thresholding technique was applied to binarize gray-scale μCT images where the minimum between the bone and bone marrow peaks in the voxel gray value histogram was chosen as the threshold value. The trabecular bone compartment was segmented by a semiautomatic contouring method and subjected to a model-independent morphological analysis (33) by the standard software provided by the manufacturer of the μCT scanner. Three-dimensional morphological parameters, including BV/TV, trabecular thickness (Tb.Th), and (Conn.D), were evaluated. The Conn.D is a quantitative description of the trabecular connection (34, 35).
Biochemistry.
Blood samples were collected from 3-month-old WT and l/l mice by cardiac puncture under isoflurane anesthesia, kept on ice for 5 min, and centrifuged at 1,000× g for 10 min at 4 °C. Serum samples were stored at −80 °C until hormonal analyses were performed. Serum level of CART was measured by RIA (RK-003-62; Phoenix Pharmaceuticals). The serum level of total DPD cross-links was measured by using the Merta tDPD kit (Quidel). Urinary elimination of norepinephrine was measured in acidified spot urine samples by EIA (Bi-CAT; Alpco), and creatinine (Merta creatinine kit; Quidel) was used to standardize between urine samples.
Real-Time Quantitative RT-PCR (qPCR).
Expression of the Cart gene in hypothalamus and Rankl gene in long bone was analyzed by real-time qPCR. Briefly, total RNA was extracted, DNase I-treated, and reverse-transcribed with random primers by using the SuperScript III first-strand cDNA synthesis kit (Invitrogen) to synthesize the first-strand cDNA. The cDNA samples were then used as templates for qPCR analysis, which was performed on an MX3000 instrument (Stratagene) by using primers from SABiosciences and the Taq SYBR Green supermix with ROX (Bio-Rad). Expression levels of the studied gene were normalized by using the β-actin expression levels as internal control for each sample.
Statistical Analyses.
Statistical significance was assessed by an unpaired Student's 2-tailed t test. Values were considered statistically significant at P < 0.05.
Supplementary Material
Acknowledgments.
We thank Dr. P. Ducy for critical reading of the manuscript and constructive comments, and Drs. S. Chua, Jr. (Albert Einstein College of Medicine, Bronx, NY), and T. Jessell (Columbia University, New York) for providing Leprfl/fl mice and SynapsinI-Cre transgenic mice, respectively. This work was supported by National Institutes of Health Grant R01-DK58883 (to G.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808701106/DCSupplemental.
References
- 1.Karsenty G. Convergence between bone and energy homeostases: Leptin regulation of bone mass. Cell Metab. 2006;4:341–348. doi: 10.1016/j.cmet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Baldock PA, et al. Hypothalamic Y2 receptors regulate bone formation. J Clin Invest. 2002;109:915–921. doi: 10.1172/JCI14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato S, et al. Central control of bone remodeling by neuromedin U. Nat Med. 2007;13:1234–1240. doi: 10.1038/nm1640. [DOI] [PubMed] [Google Scholar]
- 4.Elefteriou F, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 5.Ducy P, et al. Leptin inhibits bone formation through a hypothalamic relay: A central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 6.Takeda S, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 7.Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122:803–815. doi: 10.1016/j.cell.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 8.Elefteriou F, et al. Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci USA. 2004;101:3258–3263. doi: 10.1073/pnas.0308744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh MK, Elefteriou F, Karsenty G. Cocaine and amphetamine-regulated transcript may regulate bone remodeling as a circulating molecule. Endocrinology. 2008;149:3933–3941. doi: 10.1210/en.2008-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas T, et al. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140:1630–1638. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- 11.Khosla S. Leptin: Central or peripheral to the regulation of bone metabolism? Endocrinology. 2002;143:4161–4164. doi: 10.1210/en.2002-220843. [DOI] [PubMed] [Google Scholar]
- 12.Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- 13.Myers MG, Jr, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 14.Tartaglia LA, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 15.Baumann H, et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 17.White DW, Kuropatwinski KK, Devos R, Baumann H, Tartaglia LA. Leptin receptor (OB-R) signaling. J Biol Chem. 1997;272:4065–4071. doi: 10.1074/jbc.272.7.4065. [DOI] [PubMed] [Google Scholar]
- 18.Gong Y, et al. The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem. 2007;282:31019–31027. doi: 10.1074/jbc.M702838200. [DOI] [PubMed] [Google Scholar]
- 19.Bjørbaek C, et al. SOCS3 mediates feedback inhibition of the leptin receptor via Y985. J Biol Chem. 2000;275:40649–40657. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- 20.Bjørbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 21.Bjørnholm M, et al. Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest. 2007;117:1354–1360. doi: 10.1172/JCI30688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eyre DR, Dickson IR, Van Ness K. Collagen cross-linking in human bone and articular cartilage: Age-related changes in the content of mature hydroxypyridinium residues. Biochem J. 1988;252:495–500. doi: 10.1042/bj2520495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 24.Lacey DL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 25.Asnicar MA, et al. Absence of cocaine- and amphetamine-regulated transcript results in obesity in mice fed a high caloric diet. Endocrinology. 2001;142:4394–4400. doi: 10.1210/endo.142.10.8416. [DOI] [PubMed] [Google Scholar]
- 26.McMinn JE, et al. An allelic series for the leptin receptor gene generated by CRE and FLP recombinase. Mamm Genome. 2004;15:677–685. doi: 10.1007/s00335-004-2340-1. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Y, et al. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15:859–876. doi: 10.1101/gad.862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dacquin R, Starbuck M, Schinke T, Karsenty G. Mouse α(I)Collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev Dyn. 2002;224:245–251. doi: 10.1002/dvdy.10100. [DOI] [PubMed] [Google Scholar]
- 29.Chappard D, Palle S, Alexandre C, Vico L, Riffat G. Bone embedding in pure methyl methacrylate at low temperature preserves enzyme activities. Acta Histochem. 1987;81:183–190. doi: 10.1016/S0065-1281(87)80012-0. [DOI] [PubMed] [Google Scholar]
- 30.Parfitt AM, et al. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 31.Baron R VA, Neff L, Silverglate A, Maria AS. Processing of Undecalcified Bone Specimens for Bone Histomorphometry. Boca Raton, FL: CRC Press; 1983. [Google Scholar]
- 32.Suda T, Jimi E, Nakamura I, Takahashi N. Role of 1α,25-dihydroxyvitamin D3 in osteoclast differentiation and function. Methods Enzymol. 1997;282:223–235. doi: 10.1016/s0076-6879(97)82110-6. [DOI] [PubMed] [Google Scholar]
- 33.Hildebrand T, Laib A, Müller R, Dequeker J, Rüegsegger P. Direct three-dimensional morphometric analysis of human cancellous bone: Microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res. 1999;14:1167–1174. doi: 10.1359/jbmr.1999.14.7.1167. [DOI] [PubMed] [Google Scholar]
- 34.Feldkamp LA, Goldstein SA, Parfitt AM, Jesion G, Kleerekoper M. The direct examination of three-dimensional bone architecture in vitro by computed tomography. J Bone Miner Res. 1989;4:3–11. doi: 10.1002/jbmr.5650040103. [DOI] [PubMed] [Google Scholar]
- 35.Odgaard A, Gundersen HJ. Quantification of connectivity in cancellous bone, with special emphasis on 3D reconstructions. Bone. 1993;14:173–182. doi: 10.1016/8756-3282(93)90245-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.