BIOCHEMISTRY. For the article “Prolonged signaling at the parathyroid hormone receptor by peptide ligands targeted to a specific receptor conformation,” by Makoto Okazaki, Sebastien Ferrandon, Jean-Pierre Vilardaga, Mary L. Bouxsein, John T. Potts, Jr., and Thomas J. Gardella, which appeared in issue 43, October 28, 2008, of Proc Natl Acad Sci USA (105:16525–16530; first published October 22, 2008; 10.1073/pnas.0808750105), the authors note that due to a printer's error, in Fig. 1A, the data points were distorted, and the y-axis labels in C Inset and D Inset did not appear. Also, in Fig. 3A, the x-axis label did not appear, and Fig. 3D did not print at high resolution. In addition, the authors note that in Fig. 3C, the x-axis label “Time after injection (hr)” should instead appear as “Time after injection (min).” These errors do not affect the conclusions of the article. The corrected figures and their legends appear below.
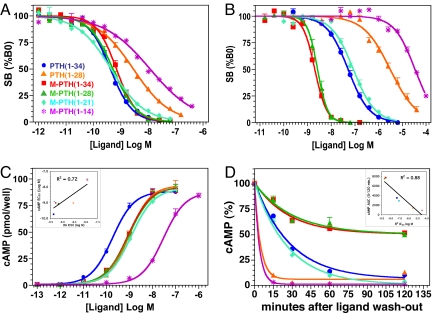
Fig. 1.
Binding and signaling properties of PTH analogs at the PTHR. (A) Ligand binding to the RG PTHR conformation was assessed in the presence of a high-affinity Gαs mutant using 125I-M-PTH(1–15) tracer radioligand; (B) binding to the R0 conformation was assessed in the presence of GTPγS (10−5 M) using 125IPTH(1–34) as a tracer radioligand. (C) Ligand-induced cAMP signaling was assessed in MC3T3-E1 mouse osteoblastic cells by dose–response and (D) ligand wash-out protocols. In the wash-out assays, IBMX was added for 5 min at times after ligand wash-out; cAMP levels are expressed as a percentage of the maximum cAMP observed in cells treated with ligand [10−7 M, or 10−6 M for M-PTH(1–14) analog] for 10 min in the presence of IBMX (range of maximum cAMP values 52–55 pmol per well; basal cAMP = 1.6 ± 0.5 pmol per well). In separate, more extended wash-out experiments, cAMP levels returned to, or near, basal levels by 24 h (data not shown). (C Inset) Correlation plot of the cAMP EC50 values and corresponding RG binding IC50 values. (D Inset) Correlation plot of the area-under-the-curve (AUC) values obtained from the cAMP wash-out assays and the corresponding R0 binding IC50 values. Binding and cAMP parameter values are reported in Table 1. Data are means (± SEM) of three to five experiments, each performed in duplicate.
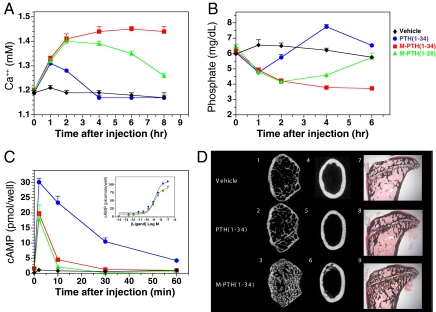
Fig. 3.
Actions of PTH ligands in vivo. Peptide ligands or vehicle were injected i.v. into C57/BL6 mice for (A–C) acute time course analyses or (D) 2-week bone metabolic studies. Acute studies were performed with hPTH(1–34), M-PTH(1–28), and M-PTH(1–34) (20 nmol/kg), and vehicle control. Blood withdrawn immediately prior to injection (t = 0) and at times thereafter was assessed for (A) ionized calcium, (B) inorganic phosphate, or (C) PTH analog content. Analog content was determined by treating HEK-293 cells transiently transfected with the hPTHR with 1 μl of plasma for 30 min in the presence of IBMX and then measuring intracellular cAMP. C Inset shows that the peptides, when measured directly, are approximately equipotent in the HEK-293/hPTHR cell assay; no cAMP response was detected for the plasma samples in mock-transfected HEK-293 cells (data not shown). The data show means (± SEM) of values from a single experiment in which (A) five, (B) six, or (C) three mice were used per group. For each analysis, similar results were obtained in at least two other experiments. (D) Two-week daily injection studies were performed with rPTH(1–34) and MPTH(1–34) (5 nmol/kg per day; n = 7 per group). At the 2-week end-point, femurs and tibiae were isolated and imaged by μCT (panes 1–6) or subjected to histology with von Kossa staining (panes 7–9). Shown are transverse views of the distal, metaphyseal (panes 1–3), and middiaphyseal (panes 4–6) regions of the femurs and sagittal views of proximal tibiae (panes 7–9). (Magnification: D, panes 7–9, 40×.)