Abstract
Peripheral nerves show spontaneous regenerative responses, but recovery after injury or peripheral neuropathies (toxic, diabetic, or chronic inflammatory demyelinating polyneuropathy syndromes) is slow and often incomplete, and at present no efficient treatment is available. Using well-defined peripheral nerve lesion paradigms, we assessed the therapeutic usefulness of etifoxine, recently identified as a ligand of the translocator protein (18 kDa) (TSPO), to promote axonal regeneration, modulate inflammatory responses, and improve functional recovery. We found by histologic analysis that etifoxine therapy promoted the regeneration of axons in and downstream of the lesion after freeze injury and increased axonal growth into a silicone guide tube by a factor of 2 after nerve transection. Etifoxine also stimulated neurite outgrowth in PC12 cells, and the effect was even stronger than for specific TSPO ligands. Etifoxine treatment caused a marked reduction in the number of macrophages after cryolesion within the nerve stumps, which was rapid in the proximal and delayed in the distal nerve stumps. Functional tests revealed accelerated and improved recovery of locomotion, motor coordination, and sensory functions in response to etifoxine. This work demonstrates that etifoxine, a clinically approved drug already used for the treatment of anxiety disorders, is remarkably efficient in promoting acceleration of peripheral nerve regeneration and functional recovery. Its possible mechanism of action is discussed, with reference to the neurosteroid concept. This molecule, which easily enters nerve tissues and regulates multiple functions in a concerted manner, offers promise for the treatment of peripheral nerve injuries and axonal neuropathies.
Keywords: inflammation, macrophage, axonal growth, Translocator Protein (18 kDa)
The improvement of axonal regeneration and the modulation of inflammatory responses are two major objectives in the treatment of peripheral nerve injuries and neuropathies. To date, however, no drug is available to reliably enhance the rate or completeness of nerve regeneration. Peptide growth factors, such as NGF and BDNF, have been difficult to use in clinical practice because they are difficult to deliver and have serious side effects. On the other hand, molecules that easily diffuse into nerve tissues, such as ligands of the progesterone receptor, thyroid hormone receptors, and immunophilins, have beneficial influences on peripheral nerves in various experimental lesion and disease models, although investigations have often been limited to specific parameters describing regeneration (1–4). Other very promising molecules are drug ligands of the translocator protein (18 kDa) (TSPO), formerly known as the peripheral benzodiazepine receptor and homologous to the bacterial Tryptophan-rich Sensory Protein for Oxygen. This protein, mainly localized in the outer mitochondrial membrane, has multiple functions (5).
In the CNS, TSPO ligands have been shown to exert neuroprotective effects and to reduce neural inflammation (6–8). After peripheral nerve injury, TSPO expression is transiently induced in dorsal root ganglia (DRG) neurons, Schwann cells, and macrophages (9–11). Some of its drug ligands have indeed been found to protect motoneurons from degeneration after facial nerve injury, to reduce sensorimotor deficits following acrylamide intoxication, to prevent aging-associated peripheral nerve degeneration (12, 13), and to stimulate the outgrowth of DRG neurites (11). These experimental observations strongly suggest that TSPO ligands may be useful for stimulating axonal regeneration and for modulating macrophage activation in altered peripheral nerves. However, TSPO ligands experimentally shown to exert beneficial actions on the peripheral nervous system have not found clinical utility, and possible side effects are a concern for their clinical use. Recent experimental studies demonstrated that the drug etifoxine (2-ethylamino-6-chloro-4-methyl-4-phenyl-4H-3, 1-benzoxazine hydrochloride; Stresam, Biocodex) exerts its anxiolytic effects by targeting GABAA receptors but also TSPO (14, 15). Indeed, specific binding of the selective TSPO ligand PK11195 to membrane preparations of the rat brain is noncompetitively inhibited by etifoxine, which decreases both Bmax and Kd (15). It is already known that in rats ligand binding stimulates the cholesterol transfer function of TSPO. This mechanism, according to the concept of neurosteroids (16), is likely responsible for the increase of neurosteroid levels observed in the brain 0.5 h after administration of 50 mg/kg of etifoxine (15) and in peripheral nerves (C.G., M.S., and G.S.-G., unpublished data). We now report that etifoxine exerts remarkable beneficial effects on axonal regrowth and on macrophage responses after peripheral nerve injury. Moreover, its administration to the lesioned sciatic nerve not only increases the rate but also the extent of functional recovery.
Results
Axonal Regeneration After Cryolesion of the Rat Sciatic Nerve.
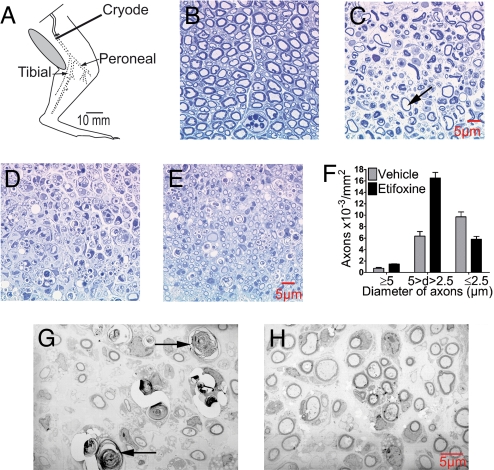
Local freeze injury of the sciatic nerve (Fig. 1A), like nerve crush, results in the rapid destruction of axons without disrupting connective tissues and basal lamina, thus allowing consistent spontaneous regeneration (17, 18). The loss of myelinated fibers was complete in both vehicle- and etifoxine-treated animals after 3 days (Fig. 1 B and C). At 7 and 15 days after injury, the regeneration of myelinated axons was more advanced in etifoxine- compared with vehicle-injected rats (Fig. 1 D and E). In etifoxine-treated animals, significantly more nerve fibers of medium size (diameter 2.5–5 μm) were present in nerves (Fig. 1F). In addition, the regenerating nerve fibers were of regular shape and surrounded by morphologically normal myelin (Fig. 1H), whereas degenerated axons could still be observed 15 days after cryolesion in vehicle-treated animals (Fig. 1G).
Fig. 1.
Cryolesion of the rat sciatic nerve resulted in the complete degeneration of nerve fibers. Treatment with etifoxine promoted nerve fiber regeneration at the lesion site. (A) Diagram illustrating cryolesion of the rat sciatic nerve. Dotted line indicates nerve undergoing Wallerian degeneration distal to the freeze lesion. Semithin sections (B–E) and ultrathin sections (G and H). (B) Intact rat sciatic nerve. (C) Complete destruction of myelinated axons 3 days after cryolesion. Arrow indicates remaining myelin sheath surrounding an empty space left by a degenerated axon. No spared fibers were observed. (D) Regenerated nerve fibers 15 days after cryolesion in rats treated with vehicle and (E) in rats treated with etifoxine. (F) Fifteen days after cryolesion, the number of regenerated medium-sized axons (diameter 2.5–5 μm) surrounded by myelin sheaths was increased by etifoxine. (G) Regenerated nerve fibers by electron microscopy in rats treated with vehicle (arrows indicate degenerated axons) and (H) in rats treated with etifoxine at 15 days.
To map the connectivity of the regenerated axons, diamidino yellow (DY) retrograde tracing was used. At 13 days after injury, etifoxine significantly increased the number of retrogradely labeled neurons: 68% ± 5% vs. 22% ± 3% in vehicle-treated rats (P < 0.001 by Student's t test) (all values are expressed as mean ± SEM).
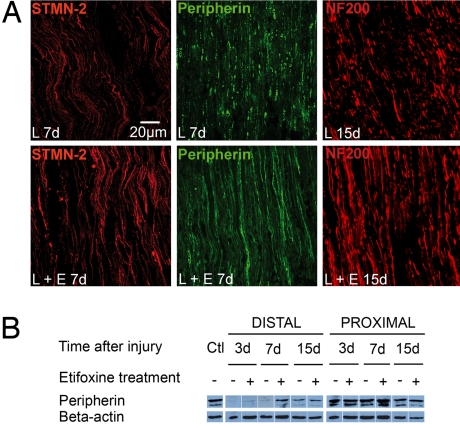
The effect of etifoxine was further investigated within the cryolesioned zone on axons expressing stathmin-like 2 protein (STMN-2), peripherin, or 200-kDa neurofilament (NF200). STMN-2, selectively expressed in regrowing axons and highly concentrated in growth cones, is a typical marker of axonal regeneration (19). STMN-2–immunoreactive fibers were observed as early as 3 days (not shown), and at 7 days a marked increase in the number of STMN-2-immunoreactive axons was observed in response to etifoxine treatment (Fig. 2A). The intermediary filament peripherin is present in all small axons and re-expressed during nerve regeneration (20, 21). At 7 days after injury, small degenerated axons with stained granules persisted in the nerves of vehicle-treated animals, although in etifoxine-treated animals a large number of linear peripherin-immunoreactive axons was observed at this stage (Fig. 2A). At 10 days the number of peripherin-immunoreactive axons distal to the lesion site was doubled in response to etifoxine (etifoxine: 108 ± 9 axons/0.1 mm2, vehicle: 54 ± 6 axons/0.1 mm2; P < 0.001 by Student's t test). Western blot results showed that in the distal nerve stump, peripherin protein underwent a dramatic decrease by 3 days, which was already reversed at 7 days by the administration of etifoxine (Fig. 2B). In the proximal nerve stump, peripherin protein persisted, and etifoxine caused a slight enhancement in its expression. By 10 days and afterward (Fig. 2A) the number of axons expressing NF200, a marker of more mature, large-diameter axons (22), was markedly increased by etifoxine treatment (distal stump at 10 days: etifoxine: 61 ± 2 axons/0.1 mm2, vehicle: 33 ± 3 axons/0.1 mm2; proximal stump at 10 days: etifoxine: 112 ± 4 axons/0.1 mm2, vehicle: 90 ± 12 axons/0.1 mm2; P < 0.001 by Student's t test for both comparisons). These immunohistologic observations show that etifoxine promotes the regeneration of lesioned axons.
Fig. 2.
Etifoxine treatment accelerated the regeneration of axons expressing either STMN-2, peripherin, or NF200 at the site of freeze injury within the distal stump. Immunoreactive fibers were observed by confocal microscopy. (A) STMN-2–immunoreactive axons (red) and peripherin-immunoreactive axons (green) 7 days after cryolesion (7d) and NF200-immunoreactive fibers 15 days after cryolesion (15d). Lesioned sciatic nerves were treated with vehicle (L) or etifoxine (L + E). (B) Western blot analysis of peripherin, normalized to β-actin. Ctl = unlesioned control.
Axonal Growth and Neurite Extension.
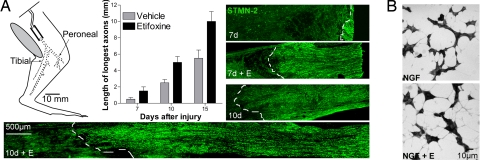
The influence of etifoxine on axonal growth into a silicone nerve guide tube after sciatic nerve transection (Fig. 3A) was followed by the extension of STMN-2–immunoreactive axons from the proximal stump into the tube. At all stages the maximal extension of axons doubled after etifoxine therapy compared with vehicle-treated rats (7 days: 1.5 ± 0.5 mm vs. 0.5 ± 0.2 mm; 10 days: 5 ± 0.7 mm vs. 2.5 ± 0.4 mm; 15 days: 10 ± 1.2 mm vs. 5.5 ± 0.9 mm; mean of triplicates) (Fig. 3A).
Fig. 3.
Etifoxine treatment promoted axonal extension after sciatic nerve transection and neurite outgrowth in PC12 cells. (A) Diagram illustrating transection of the rat sciatic nerve and installation of a silicone tube (dotted line indicates transected nerve undergoing Wallerian degeneration). STMN-2–immunoreactive axons (green) showed the increase of the elongation rate from the proximal stump into the tube, by more than 2-fold, 7 days (7d) and 10 days (10d) after surgery. The dotted white line delimits the extension of the regenerated axons. The maximal extension of nerve fibers into the tube was quantified at 7, 10, and 15 days after sciatic nerve transection. (B) Etifoxine (E) (20 μM) enhanced neurite extension in PC12 cells grown for 72 h in the presence of 10 ng/ml NGF.
The effects of etifoxine were also examined in PC12 cells, a model commonly used to study neurotrophic activities. Etifoxine robustly enhanced neurite extension, but only in PC12 cells showing a neuronal phenotype and grown in the presence of NGF. Maximum neurite length was 40 ± 1.1 μm for PC12 cells grown in the presence of NGF and etifoxine vs. 17.5 ± 0.5 μm for PC12 cells treated with NGF alone (P < 0.001 by Student's t test) (Fig. 3B). The neurotrophic effect of etifoxine could be mimicked by the selective TSPO ligands PK11195 and Ro5-4864, but their effect was weaker. In contrast, the GABAA receptor agonist muscimol or antagonist bicuculline were without effect [supporting information (SI) Table S1].
Modulation of Macrophage Responses to Sciatic Nerve Injury.
Within the CNS, TSPO is induced in activated microglia, and the protein has been identified as a potential target for the control of neural inflammation (6–8). Although TSPO immunoreactivity is associated with reactive macrophages in the injured rat sciatic nerve (9), it is unknown whether TSPO ligands influence inflammation in peripheral nerves.
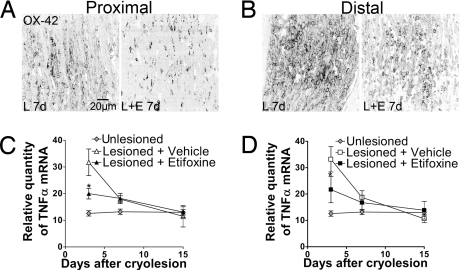
To determine whether etifoxine modulates inflammatory responses to peripheral nerve injury, the number of OX-42–immunoreactive macrophages was quantified in the regenerating sciatic nerve after cryolesion (Fig. 4 A and B and Fig. S1). At 3 days after injury, etifoxine treatment exerted a marked immunoprotective action on the proximal stump, with an important reduction (60%) in the number of reactive macrophages, but had little effect in the distal stump (proximal: etifoxine: 420 ± 40 cells/mm2, vehicle: 1,000 ± 120 cells/mm2; P < 0.001 by Tukey's test; distal: etifoxine: 1,800 ± 100 cells/mm2, vehicle: 2,000 ± 130 cells/mm2; not significant by Tukey's test; a significant difference between nerve stumps and treatment effect was revealed by two-way ANOVA: P < 0.05; n = 5). At later stages, 7 and 15 days after cryolesion, the number of macrophages was also significantly reduced by etifoxine within the distal stump (Fig. 4B and Fig. S1) (7 days, distal: etifoxine: 1,410 ± 210 cells/mm2, vehicle: 2,700 ± 200 cells/mm2; 15 days, distal: etifoxine: 880 ± 120 cells/mm2, vehicle: 1,920 ± 80 cells/mm2; n = 5; P < 0.001 by Tukey's tests). Within the proximal stump (Fig. 4A and Fig. S1), the number of macrophages had returned to very low levels at 15 days (7 days, proximal: etifoxine: 500 ± 80 cells/mm2, vehicle: 1,180 ± 90 cells/mm2; 15 days, proximal: etifoxine: 200 ± 70 cells/mm2, vehicle: 1,020 ± 50 cells/mm2; n = 5; P < 0.001 by Tukey's tests).
Fig. 4.
Influence of etifoxine (E) treatment on (A and B) the number of activated macrophages and (C and D) mRNA expression of the cytokine TNF-α in the regenerating sciatic nerve. The density of OX-42–immunoreactive macrophages is shown at 7 days (7d) after cryolesion (L) in the nerve endings (A) proximal and (B) distal to the lesion site. Relative mRNA levels were analyzed by real-time RT-PCR within the nerve stumps (C) proximal and (D) distal to the injury site (n = 5). *, P < 0.01 vs. vehicle-treated lesioned nerves by Tukey's tests after two-way ANOVA (treatment × time after cryolesion).
In agreement with the immunologic results, etifoxine maintained low OX-42 mRNA levels (only a 1.7-fold increase over control) in the proximal nerve stump, consistent with limited inflammation. Conversely, in the distal nerve stump, OX-42 mRNA expression was increased 3- to 4-fold 3 days after injury, and its levels progressively returned to control values by 15 days (not shown).
The strong influence of etifoxine on inflammation in the lesioned sciatic nerve was further documented by its marked effects on the expression of inflammatory cytokines. In both nerve stumps, the administration of etifoxine significantly blunted the injury-induced increase in TNF-α expression at 3 days (Fig. 4 C and D). Similarly, IL-1β mRNA levels were significantly upregulated by injury in both nerve stumps (not shown). As observed for OX-42, expression of IL-1β was maintained at low levels by etifoxine treatment in the proximal stump. In the distal stump, etifoxine slightly decreased IL-1β mRNA levels at 3 days. IL-6 mRNA expression increased in both nerve stumps in response to injury, and again, etifoxine limited its increase in the proximal stump. Conversely, in the distal nerve stump, etifoxine treatment caused a further increase in IL-6 mRNA expression at 3 and 7 days, its levels returning to control values only at 15 days (not shown).
Accelerated and Improved Functional Recovery.
The regeneration of sensory axons was evaluated by the nerve pinch test at 3, 7, and 15 days after cryolesion. As soon as 3 days after injury, when small STMN-2–immunoreactive fibers could already be observed in the distal nerve ending, the maximal distance of sensory axon elongation was greater in etifoxine-treated rats when compared with vehicle-treated rats. At 7 and 15 days the regrowth of sensory axons was also significantly improved by etifoxine (3 days: etifoxine: 3.0 ± 0.5 mm, vehicle: 1.0 ± 0.2 mm; 7 days: etifoxine: 9.0 ± 1.0 mm, vehicle: 3.0 ± 0.3 mm; 15 days: etifoxine: 31 ± 5 mm, vehicle: 21 ± 4 mm; P < 0.001 vs. respective controls by Tukey's tests). At 15 days the recovery of sensory functions was almost complete in etifoxine-treated rats. The two-way ANOVA performed before the paired comparisons revealed that the effects of both treatment and time as well as of their interactions were significant (P < 0.01).
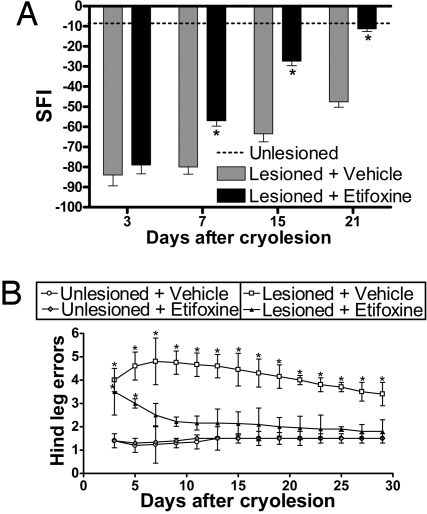
The recovery of locomotion was assessed by the walking track test (Fig. 5A). The daily analysis of the sciatic function index (SFI) revealed faster and better recovery of locomotion in lesioned rats treated with etifoxine. After 21 days their mean SFI was comparable to that of unlesioned controls, whereas walking patterns remained impaired in vehicle-treated rats. The recovery of fine motor coordination was assessed in the Locotronic device (Fig. 5B). As early as 5 days after cryolesion, motor coordination was significantly improved in response to etifoxine, and as early as 13 days lesioned animals treated with etifoxine became indistinguishable from unlesioned animals. Conversely, as late as 29 days after cryolesion, motor coordination remained poor in the vehicle-treated animals and was significantly different from the other groups.
Fig. 5.
Etifoxine treatment accelerated and improved functional recovery after cryolesion of the sciatic nerve. (A) Locomotion was assessed by the walking track test, and footprints were monitored at 3, 7, 15, and 21 days after cryolesion and in unlesioned control rats. Improved recovery of locomotion in etifoxine-treated animals was followed by SFI indexes, calculated according to the Dijkstra method (39). (B) Recovery of fine motor coordination was assessed in the Locotronic device by recording the number of hind footfalls. For the results of both walking track test and Locotronic device, two-way ANOVA with treatment and time after cryolesion as factors revealed both factors and their interaction to be significant at least at the 0.01 level. *, P < 0.01 vs. unlesioned rats by Tukey's tests after two-way ANOVA (n = 35; mean ± SEM).
Discussion
The primary aim of this study was to investigate whether etifoxine promotes the regeneration of peripheral axons in adults. This is a major therapeutic objective both for injured peripheral nerves and peripheral neuropathies, such as chronic inflammatory demyelinating polyneuropathy and chemotherapy-induced or diabetic neuropathies, the latter often being associated with diminished regeneration of nerve fibers (23). After cryolesion, etifoxine treatment accelerated the regeneration and increased the number of small axons immunoreactive for the growth-associated markers STMN-2 and peripherin, and of large axons expressing neurofilament NF200 or surrounded by myelin sheaths (thionine blue staining). Consistent with these findings, the number of retrogradely DY-labeled DRG neurons was also significantly enhanced. Moreover, etifoxine markedly accelerated the extension of regenerating STMN-2–expressing axons into a nerve guide tube after sciatic nerve transection. Taken together, these results demonstrate that etifoxine promotes axonal regeneration in the injured peripheral nerve.
Two studies have shown that, in response to sciatic nerve injury, TSPO is induced in neurons of the corresponding DRG (10, 11). That etifoxine exerts neurotrophic effects by acting directly on neurons was shown in cultures of PC12 cells, where the drug caused a striking outgrowth of neurites. PC12 cells contain functional TSPO binding sites but do not express functional GABAA receptors (24–27). In agreement, neurite extension in PC12 cells was sensitive to selective TSPO ligands but not to an agonist or an antagonist of GABAA receptors. We therefore conclude that neuronal TSPO sites rather than GABAA receptors are involved in neurite growth.
After lesion of peripheral nerves, TSPO expression is also strongly induced in macrophages (9). We report here that etifoxine has marked effects on the number of activated macrophages and their inflammatory products, and these actions are very likely to contribute to the protection and regeneration of lesioned nerve fibers. Indeed, although activated macrophages play a role in clearing away myelin debris, prolonged and strong inflammation can cause severe damage to neurons and nerve fibers (28, 29). It was thus an important finding that etifoxine therapy rapidly and strongly reduced macrophage activation and the production of inflammatory products within the nerve ending proximal to the lesion site, where inflammation can cause damage to neurons and may result in neuropathic pain (30). In contrast, within the distal nerve stump, where macrophages remove debris, the anti-inflammatory action of etifoxine was delayed. In this part, the accelerated regeneration of myelinated axons could have stimulated the efflux of macrophages (31).
A major finding was that etifoxine, in addition to its outstanding effects on axonal regeneration and macrophage responses, improved both the rate and quality of functional hind limb recovery after sciatic nerve injury. Speed is an important factor in successful nerve regeneration, because delayed muscle reinnervation results in poor recovery and permanent functional disability (32, 33). Functional tests revealed rapid and full recovery of locomotion, motor coordination, and sensory functions in animals receiving etifoxine, whereas neurologic recovery remained poor as late as 3 or 4 weeks after freeze injury in vehicle-treated animals.
As mentioned in the Introduction, etifoxine could stimulate neurosteroid production via its action on TSPO activity. Pregnenolone and progesterone were already shown to stimulate neuritic growth in PC12 cells (34) and myelinization in DRG cultures (18). Whether this mechanism of action is involved in or is part of the cascade of events observed after damage of peripheral neurons and their treatment with etifoxine will be the matter of further research.
In conclusion, acknowledging that the details of the mechanism of action of etifoxine are still incompletely understood, etifoxine fulfills the criteria of a drug with clinical potential for the treatment of altered peripheral axons: (i) easy diffusion into nerve tissues, (ii) 2-fold acceleration of axonal regeneration, and (iii) selective modulation of inflammatory responses to injury. Moreover, the drug is already efficiently used in long-duration (up to 3 months) treatment of adjustment disorders with anxiety, and it is devoid of many of the side effects linked to benzodiazepine anxiolytics (35–37). This is important, given that axonal regeneration after injury and in peripheral neuropathies is a slow process, and only molecules that can be administered over sufficient periods would be expected to have therapeutic benefits.
Methods
Surgical Procedures.
Male Sprague-Dawley rats (age 7 weeks) were purchased from CERJ. Animal procedures were performed in accordance with the guidelines of the International Association for the Study of Pain Committee for Research and Ethical Issues. Before surgery, rats were anesthetized with an i.p. injection of pentobarbital (60 mg/kg).
For cryolesion, the right sciatic nerve was exposed and frozen 15 mm above the separation between the peroneal and tibial branches (Fig. 1A). The nerve was submitted to 6 cycles of freezing–thawing using a copper cryode (1 mm in diameter) precooled in liquid nitrogen. The cryolesion site was marked at the proximal boundary with a single epineural suture (7-0) (Ethicon). Three series of rats were lesioned and killed 1, 3, 7, 10, 15, or 21 days after surgery. For the first series, the proximal (connected to the DRG at vertebral level L4/L6) and distal nerve stumps including the lesion site (10 mm each) were aseptically collected, frozen in liquid nitrogen, and stored at −80 °C until RNA and protein extraction (5 rats per condition per time). The second series was examined by immunohistochemistry (5 rats per condition per time) or electron microscopy (3 rats per condition per time). The third series was used for functional testing (35 rats per condition per time). For DY retrograde labeling, 1 μl of 3% DY solution (Sigma-Aldrich) in 1% DMSO was injected immediately after cryolesion into the distal stump of the injured sciatic nerve, 1 cm downstream of the cryolesioned area. Rats (5 rats per condition) were injected 13 days later with a lethal dose of pentobarbital and were then perfused with 4% paraformaldehyde (PFA, Merck).
For the section, the right sciatic nerve was cut 15 mm above the separation between the peroneal and tibial branches. The distal and proximal ends were secured into the ends of a 10-mm-long silicone tube (internal diameter of 1.6 mm and external diameter of 3.2 mm) (Fisher Scientific Bioblock). The transected nerve was held in place with epineural sutures (7-0) at each end, leaving a gap of 6 mm between the nerve stumps. The silicone tubes were collected 7, 10, and 15 days after the section and immediately fixed with 4% PFA for immunohistochemistry (3 rats per condition per time).
Drug Treatments.
Rats received daily i.p. injections of etifoxine (Batch 285; Biocodex) at the dose of 50 mg/kg, previously shown to exhibit anxiolytic-like effects in rats (15). The treatment was started 24 h after the surgery. Control animals received an equivalent volume (0.5 ml/100 g) of vehicle (1% Tween 80 in 0.9% NaCl solution).
Cell Cultures.
PC12 cells were cultured as previously described (34). Etifoxine dissolved in DMSO (20 μM final concentration), the TSPO ligands PK11195 and Ro5-4864 dissolved in ethanol (5 μM final concentration), and GABAA receptor ligands muscimol and bicuculline (respectively, 1 μM and 10 μM final concentration) dissolved in water or solvent alone were added to cultures grown in the presence or absence of NGF (10 ng/ml). After 72 h of treatment, cells were fixed with 2% PFA and colored with toluidine blue. The measurement of the longest neurite was performed on 600 cells per culture condition using Image J software (National Institutes of Health).
Histology of Peripheral Nerves.
Semithin cross-sections in the cryolesion site were stained with thionine blue for visualization of myelinated axons. Ultra-thin sections were stained with uranyl acetate and lead citrate for electron microscopy.
For immunohistochemistry, longitudinal nerve sections (30 μm) were stained with anti- STMN-2, anti-peripherin (Chemicon Europe), or anti-NF200 (Abcam). Analysis by confocal microscopy (System LSM 410; Carl Zeiss) allowed counting of all fluorescent fibers in the whole depth of the sections. Activated macrophages expressing the CD11b subunit of complement receptor were identified by OX-42 antibody (Serotec). For fiber or macrophage counting, the final values were the mean of 60 individual measurements for each location in the nerve. DY-labeled neurons of the entire DRG were counted on 20-μm sections at lumbar levels L4, L5, and L6 by using an Axioplan 2 microscope (Carl Zeiss).
Gene Expression Analysis.
Sciatic nerve samples were powdered in a glass–Teflon Potter-Elverheim homogenizer under liquid nitrogen and lysed with TRIzol (Invitrogen Life Technologies). Protein extracts were prepared according to the manufacturer's recommendations. Extraction of RNA was conducted using an RNeasy Extraction Kit (Qiagen).
For RNA analysis, coding strand cDNA synthesis was performed using SuperScript II RNase H reverse transcriptase (Invitrogen). Real-time PCR reactions were conducted using the ABI Prism Model 7000 Sequence Detection System (Applied Biosystems) and probes purchased from Applied Biosystems: the macrophage marker CD11b (OX-42) (Rn 00709342_m1), TNF-α (Rn99999017_m1), IL-1β (Rn01514149_g1), IL-6 (Rn99999011_m1), and 18S ribosomal RNA (Hs99999901_s1). mRNA levels were normalized against 18S rRNA in each sample.
Western blotting was performed as previously described (38). The staining intensity of protein bands was determined with Bio-Rad molecular analyst quantification software and normalized against β-actin protein levels.
Functional Tests of Neurologic Recovery.
Rats were always accustomed to handling and to the testing procedures during the week before an experiment. Functional tests were performed before the daily injection of etifoxine.
Nerve pinch test.
The rate of sensory axon regeneration was evaluated by the nerve pinch test in anesthetized animals. The right sciatic nerve was pinched with fine smooth forceps distally from the injury site. The extent of regeneration was determined by measuring the distance from the injury site to the most distal point of the nerve where a pinch produced a withdrawal reflex.
Walking tests.
For the walking track test, trained animals were submitted to conditioning trials in a 10 × 100-cm Plexiglas walking track with a dark box at one end. The hind feet were dipped in blue ink, and rats were allowed to walk down the track, which was covered with white paper, to the dark box. From the footprints, the SFI was calculated as described previously (39). For normal footprints, SFI is near zero, whereas an SFI value of approximately −100 reflects total impairment.
The second test was performed using the Locotronic device (Intellibio), an automated version of the grid-walk task assay allowing defects in fine motor coordination during walking to be revealed. The apparatus consists of a departure box, a flat ladder corridor, and an arrival box. Infrared sensors allow recording of the displacement of the rat and motor control defects. The number of hind footfalls was quantified.
Statistical Analysis.
All values are expressed as mean ± SEM. Comparisons between multiple groups were performed by either one-way or two-way ANOVA followed by Tukey's tests. Comparisons between two groups were performed by Student's t test. P values of <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments.
We thank Mrs. Josette Bacci for electron microscopy analysis, André Sobel for the generous gift of anti-STMN-2 antibody, and Krzysztof Rajkowski for a critical reading of the manuscript. This work was supported by Grants R04053LL/RAE04008LLA and R06042LL/RAE06011LLA from the Association Française contre les Myopathies and by a “Plan Pluri Formation” (“Peripheral and spinal axonal regeneration”) from the University Paris-Sud 11 (to D.A.). C.G. was supported by Biocodex. M.S. is the beneficiary of an “Interface Program” of the Institut National de la Santé et de la Recherche Médicale and the Assistance Publique–Hôpitaux de Paris (Department of Neurology, Kremlin-Bicêtre Hospital).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811201106/DCSupplemental.
References
- 1.Barakat-Walter I. Role of thyroid hormones and their receptors in peripheral nerve regeneration. J Neurobiol. 1999;40:541–559. doi: 10.1002/(sici)1097-4695(19990915)40:4<541::aid-neu10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 2.Schumacher M, et al. Progesterone synthesis and myelin formation in peripheral nerves. Brain Res Rev. 2001;37:343–359. doi: 10.1016/s0165-0173(01)00139-4. [DOI] [PubMed] [Google Scholar]
- 3.Gold BG, Udina E, Bourdette D, Navarro X. Neuroregenerative and neuroprotective actions of neuroimmunophilin compounds in traumatic and inflammatory neuropathies. Neurol Res. 2004;26:371–380. doi: 10.1179/016164104225013734. [DOI] [PubMed] [Google Scholar]
- 4.Melcangi RC, Garcia-Segura LM. Therapeutic approaches to peripheral neuropathy based on neuroactive steroids. Expert Rev Neurother. 2006;6:1121–1125. doi: 10.1586/14737175.6.8.1121. [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulos V, et al. Translocator protein (18kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Torres SR, et al. Anti-inflammatory effects of peripheral benzodiazepine receptor ligands in two mouse models of inflammation. Eur J Pharmacol. 2000;408:199–211. doi: 10.1016/s0014-2999(00)00760-3. [DOI] [PubMed] [Google Scholar]
- 7.Veiga S, Azcoitia I, Garcia-Segura LM. Ro5-4864, a peripheral benzodiazepine receptor ligand, reduces reactive gliosis and protects hippocampal hilar neurons from kainic acid excitotoxicity. J Neurosci Res. 2005;80:129–137. doi: 10.1002/jnr.20430. [DOI] [PubMed] [Google Scholar]
- 8.Ryu JK, Choi HB, McLarnon JG. Peripheral benzodiazepine receptor ligand PK11195 reduces microglial activation and neuronal death in quinolinic acid-injected rat striatum. Neurobiol Dis. 2005;20:550–561. doi: 10.1016/j.nbd.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Lacor P, Benavides J, Ferzaz B. Enhanced expression of the peripheral benzodiazepine receptor (PBR) and its endogenous ligand octadecaneuropeptide (ODN) in the regenerating adult rat sciatic nerve. Neurosci Lett. 1996;220:61–65. doi: 10.1016/s0304-3940(96)13187-6. [DOI] [PubMed] [Google Scholar]
- 10.Karchewski LA, Bloechlinger S, Woolf CJ. Axonal injury-dependent induction of the peripheral benzodiazepine receptor in small-diameter adult rat primary sensory neurons. Eur J Neurosci. 2004;20:671–683. doi: 10.1111/j.1460-9568.2004.03530.x. [DOI] [PubMed] [Google Scholar]
- 11.Mills CD, Bitler JL, Woolf CJ. Role of the peripheral benzodiazepine receptor in sensory neuron regeneration. Mol Cell Neurosci. 2005;30:228–237. doi: 10.1016/j.mcn.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Ferzaz B, et al. SSR180575 (7-chloro-N,N,5-trimethyl-4-oxo-3-phenyl-3,5-dihydro-4H- pyridazino[4,5-b]indole-1-acetamide), a peripheral benzodiazepine receptor ligand, promotes neuronal survival and repair. J Pharmacol Exp Ther. 2002;301:1067–1078. doi: 10.1124/jpet.301.3.1067. [DOI] [PubMed] [Google Scholar]
- 13.Leonelli E, et al. Ro5-4864, a synthetic ligand of peripheral benzodiazepine receptor, reduces aging-associated myelin degeneration in the sciatic nerve of male rats. Mech Ageing Dev. 2005;126:1159–1163. doi: 10.1016/j.mad.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Schlichter R, Rybalchenko V, Poisbeau P, Verleye M, Gillardin J. Modulation of GABAergic synaptic transmission by the non-benzodiazepine anxiolytic etifoxine. Neuropharmacology. 2000;39:1523–1535. doi: 10.1016/s0028-3908(99)00253-1. [DOI] [PubMed] [Google Scholar]
- 15.Verleye M, et al. The anxiolytic etifoxine activates the peripheral benzodiazepine receptor and increases the neurosteroid levels in rat brain. Pharmacol Biochem Behav. 2005;82:712–720. doi: 10.1016/j.pbb.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Baulieu EE. Neurosteroids: Of the nervous system, by the nervous system, for the nervous system. Recent Prog Horm Res. 1997;52:1–32. [PubMed] [Google Scholar]
- 17.Mira JC. Quantitative studies of the regeneration of rat myelinated nerve fibres: Variations in the number and size of regenerating fibres after repeated localized freezings. J Anat. 1979;129:77–93. [PMC free article] [PubMed] [Google Scholar]
- 18.Koenig HL, et al. Progesterone synthesis and myelin formation by Schwann cells. Science. 1995;268:1500–1503. doi: 10.1126/science.7770777. [DOI] [PubMed] [Google Scholar]
- 19.Manna T, Grenningloh G, Miller HP, Wilson L. Stathmin family protein SCG10 differentially regulates the plus and minus end dynamics of microtubules at steady state in vitro: implications for its role in neurite outgrowth. Biochemistry. 2007;46:3543–3552. doi: 10.1021/bi061819d. [DOI] [PubMed] [Google Scholar]
- 20.Oblinger MM, Wong J, Parysek LM. Axotomy-induced changes in the expression of a type III neuronal intermediate filament gene. J Neurosci. 1989;9:3766–3775. doi: 10.1523/JNEUROSCI.09-11-03766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chadan S, Moya KL, Portier MM, Filliatreau G. Identification of a peripherin dimer: Changes during axonal development and regeneration of the rat sciatic nerve. J Neurochem. 1994;62:1894–1905. doi: 10.1046/j.1471-4159.1994.62051894.x. [DOI] [PubMed] [Google Scholar]
- 22.Rao MV, et al. Neurofilament-dependent radial growth of motor axons and axonal organization of neurofilaments does not require the neurofilament heavy subunit (NF-H) or its phosphorylation. J Cell Biol. 1998;143:171–181. doi: 10.1083/jcb.143.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yasuda H, et al. Diabetic neuropathy and nerve regeneration. Prog Neurobiol. 2003;69:229–285. doi: 10.1016/s0301-0082(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 24.Miller LG, Tischler AS, Jumblatt JE, Greenblatt DJ. Benzodiazepine binding sites on PC12 cells: Modulation by nerve growth factor and forskolin. Neurosci Lett. 1988;89:342–348. doi: 10.1016/0304-3940(88)90550-2. [DOI] [PubMed] [Google Scholar]
- 25.Ohara-Imaizumi M, et al. Inhibitory action of peripheral-type benzodiazepines on dopamine release from PC12 pheochromocytoma cells. J Pharmacol Exp Ther. 1991;259:484–489. [PubMed] [Google Scholar]
- 26.Hales TG, Tyndale RF. Few cell lines with GABAA mRNAs have functional receptors. J Neurosci. 1994;14:5429–5436. doi: 10.1523/JNEUROSCI.14-09-05429.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peran M, Hicks BW, Peterson NL, Hooper H, Salas R. Lateral mobility and anchoring of recombinant GABAA receptors depend on subunit composition. Cell Motil Cytoskeleton. 2001;50:89–100. doi: 10.1002/cm.1043. [DOI] [PubMed] [Google Scholar]
- 28.Kiefer R, Kieseier BC, Stoll G, Hartung HP. The role of macrophages in immune-mediated damage to the peripheral nervous system. Prog Neurobiol. 2001;64:109–127. doi: 10.1016/s0301-0082(00)00060-5. [DOI] [PubMed] [Google Scholar]
- 29.Mueller M, et al. Macrophage response to peripheral nerve injury: The quantitative contribution of resident and hematogenous macrophages. Lab Invest. 2003;83:175–185. doi: 10.1097/01.lab.0000056993.28149.bf. [DOI] [PubMed] [Google Scholar]
- 30.Hu P, McLachlan EM. Distinct functional types of macrophage in dorsal root ganglia and spinal nerves proximal to sciatic and spinal nerve transections in the rat. Exp Neurol. 2003;184:590–605. doi: 10.1016/S0014-4886(03)00307-8. [DOI] [PubMed] [Google Scholar]
- 31.Fry EJ, Ho C, David S. A role for Nogo receptor in macrophage clearance from injured peripheral nerve. Neuron. 2007;53:649–662. doi: 10.1016/j.neuron.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci. 1995;15:3886–3895. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batt J, et al. Differential gene expression profiling of short and long term denervated muscle. FASEB J. 2006;20:115–117. doi: 10.1096/fj.04-3640fje. [DOI] [PubMed] [Google Scholar]
- 34.Fontaine-Lenoir V, et al. Microtubule-associated protein 2 (MAP2) is a neurosteroid receptor. Proc Natl Acad Sci USA. 2006;103:4711–4716. doi: 10.1073/pnas.0600113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Servant D, Graziani PL, Moyse D, Parquet PJ. Treatment of adjustment disorder with anxiety: Efficacy and tolerance of etifoxine in a double-blind controlled study. Encephale. 1998;24:569–574. [PubMed] [Google Scholar]
- 36.Micallef J, et al. A double blind parallel group placebo controlled comparison of sedative and amnesic effects of etifoxine and lorazepam in healthy subjects. Fundam Clin Pharmacol. 2001;15:209–216. doi: 10.1046/j.1472-8206.2001.00025.x. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen N, et al. Efficacy of etifoxine compared to lorazepam monotherapy in the treatment of patients with adjustment disorders with anxiety: A double-blind controlled study in general practice. Hum Psychopharmacol. 2006;21:139–149. doi: 10.1002/hup.757. [DOI] [PubMed] [Google Scholar]
- 38.Groyer G, et al. Expression and functional state of the corticosteroid receptors and 11beta-hydroxysteroid dehydrogenase type 2 in Schwann cells. Endocrinology. 2006;147:4339–4350. doi: 10.1210/en.2005-1625. [DOI] [PubMed] [Google Scholar]
- 39.Dijkstra JR, Meek MF, Robinson PH, Gramsbergen A. Methods to evaluate functional nerve recovery in adult rats: Walking track analysis, video analysis and the withdrawal reflex. J Neurosci Methods. 2000;96:89–96. doi: 10.1016/s0165-0270(99)00174-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.