Abstract
A conundrum of innate antiviral immunity is how nucleic acid-sensing Toll-like receptors (TLRs) and RIG-I/MDA5 receptors cooperate during virus infection. The conventional wisdom has been that the activation of these receptor pathways evokes type I IFN (IFN) responses. Here, we provide evidence for a critical role of a Toll-like receptor 3 (TLR3)-dependent type II IFN signaling pathway in antiviral innate immune response against Coxsackievirus group B serotype 3 (CVB3), a member of the positive-stranded RNA virus family picornaviridae and most prevalent virus associated with chronic dilated cardiomyopathy. TLR3-deficient mice show a vulnerability to CVB3, accompanied by acute myocarditis, whereas transgenic expression of TLR3 endows even type I IFN signal-deficient mice resistance to CVB3 and other types of viruses, provided that type II IFN signaling remains intact. Taken together, our results indicate a critical cooperation of the RIG-I/MDA5-type I IFN and the TLR3-type II IFN signaling axes for efficient innate antiviral immune responses.
Keywords: antiviral response, coxsackievirus, cytokine, IFN-γ, Toll-like receptor 3
The innate immune system uses an arsenal of two classes of nucleic acid-sensing receptor molecules against virus infections, namely, membrane-bound Toll-like receptors (TLRs) and cytosolic receptors that include retinoic acid-inducible protein I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5) (1). The hallmark of activation of these receptors is the induction of type I IFN (type I IFN or IFN-α/β), a family of cytokines vital to the frontline defense against most viruses (2–4). Indeed, mice deficient in type I IFN signaling succumb to various viruses, whether acutely or chronically infected, because of defects in mounting effective innate and/or adaptive immune responses (5, 6). In the context of type I IFN induction by nucleic acid-sensing receptors, it is well established that TLR7/8 sense single-stranded RNA and TLR9 senses hypomethylated DNA mainly within the endosome of plasmacytoid dendritic cells (2, 7). In contrast, the RIG-I and MDA5 cytosolic RNA sensing receptors function in many other cell types to induce type I IFN by means of recognition of double-stranded RNA (dsRNA) (4, 8).
DsRNA is a prominent activator of the innate immune system and is the transmittable genetic material of certain classes of virus or a product of most viruses as part of their life cycles (9). The cytosolic RIG-I and MDA5 receptors coordinate with each other to evoke robust type I IFN and other innate immune responses via recognition of different structural elements that are derived from viral dsRNA (10, 11). In the context of the activation of innate antiviral immunity by dsRNA, it is interesting that TLR3 is also a dsRNA-recognition receptor but is localized in the endosomal membrane (12, 13). Also, the expression levels of RIG-I/MDA5 and TLR3 are upregulated by type I IFN signaling, which points to a system of positive feedback regulation and mutual cooperation between these receptor classes (8, 14, 15). Thus, the immune system uses multiple strategies to sense virus-derived dsRNA to ensure the host's full-blown antiviral innate immune responses.
Interestingly, however, the precise function and virucidal properties of TLR3 are still controversial (16, 17). According to previous reports, TLR3 contributes to the elimination of specific RNA viruses such as the encephalomyocarditis virus (EMCV) (18), but its contribution to the clearance of the influenza virus and West Nile virus appears to be variable (19–22), possibly owing to the inoculation conditions and/or nature of the virus strain used in these studies. In addition, there is evidence that TLR3 is critical to the host defense against infection by certain DNA viruses (23, 24). In view of previous reports showing that there may be little, if any, contribution of TLR3 signaling to the induction of type I IFNs (10, 18), one may envisage that TLR3 signaling links to a pathway(s) other than those involving type I IFN in its cooperation with the RIG-I/MDA5-type I IFN pathway for effective antiviral innate immunity.
In this study, we use the CVB3 as a tool with which to gain insight into the functional interrelationship between TLR3 and RIG-I/MDA5 pathways. CVB3 is a member of the positive-stranded RNA virus family picornaviridae, is cytopathic for many mammalian cells, and causes severe myocarditis, pancreatitis, hepatitis, and meningitis (25, 26). In particular, CVB3-mediated myocarditis is associated with chronic dilated cardiomyopathy and is a major public health concern (27, 28); to date no effective prophylaxis or treatment exists for resolution of CVB3 infection. We show that mice deficient in Tlr3 are more vulnerable to CVB3 than wild-type mice in terms of higher mortality and more acute myocarditis. In contrast, the transgenic expression of TLR3 reduces the level of viremia during the early stages of infection and promotes survival of mice even when crossed onto a type I IFN signal-deficient background (Ifnar1−/−). We further show that the protective effect of TLR3 against CVB3 infection is abrogated by treatment with an antibody against IFN-γ (termed type II IFN hereafter for convenience), thus highlighting the importance of a TLR3-type II IFN signaling axis in the innate immune response against CVB3. Furthermore, we provide evidence that the TLR3-type II IFN signaling pathway also contributes to antiviral immune responses toward other types of RNA and DNA viruses.
Finally, we discuss the significance of our findings in a new context for how the two classically known classes of cytokines with antiviral activities, type I and II IFNs, functionally cooperate in relation to TLR3- and RIG-I/MDA5-mediated signaling networks during antiviral innate immune responses.
Results
A Critical Role for TLR3 Against CVB3 Infection.
To promote survival, many viruses have evolved strategies to evade the induction of the IFN genes and/or to inhibit IFN signaling pathway (6, 29). In this context, CVB3 also has a yet unclear but potent mechanism(s) to attenuate IFN gene induction: The induction of IFN-β mRNA in mouse peritoneal macrophages (PECs) or mouse embryonic fibroblasts (MEFs) upon CVB3 infection was almost undetectable, whereas an efficient IFN-β induction was observed in cells infected by vesicular stomatitis virus (VSV) or herpes simplex virus type I (HSV1) (Fig. S1A). On the other hand, the induction of other cytokines such as interleukin (IL)-1β and IL-12p35 was found to occur in CVB3-infected PECs as efficiently as in the PECs infected by other viruses (Fig. S1B), suggesting that the lack of the type I IFN gene induction is selective.
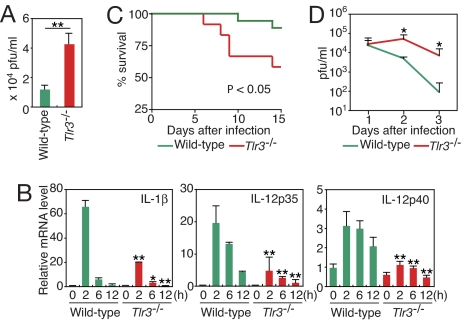
Notably, CVB3 replication was much more efficient in Tlr3-deficient (Tlr3−/−) splenocytes than in wild type, indicating an important contribution of the CVB3-mediated activation of TLR3 to an antiviral response (Fig. 1A). The activation of TLR3 by CVB3 was also evident by a severe impairment in the induction of the mRNAs for inflammatory cytokines, IL-1β, IL-12p35, and IL-12p40, in Tlr3−/− PECs (Fig. 1B). These results prompted us to ask whether TLR3 contributes to the host defense against CVB3 infection in vivo, which we tested by inoculating wild-type and Tlr3−/− mice with 5 × 104 plaque-forming units (pfu) of CVB3. As shown in Fig. 1C, Tlr3−/− mice were more susceptible to CVB3 infection than wild-type mice in terms of survival, and this higher mortality was accompanied by the development of more severe symptoms of illness such as rough hair coat and hunched posture during infection. Consistent with these data, Tlr3−/− mice exhibited significantly higher virus titers in sera 2 to 3 days after infection than wild-type mice (Fig. 1D), demonstrating the essential role of TLR3 in the innate immune response against CVB3 in vivo.
Fig. 1.
High levels of CVB3 replication and impaired inflammatory response in Tlr3−/− cells and mice. (A) CVB3 titers in splenocytes from wild-type or Tlr3−/− mice at 36 h after infection. Data represent mean ± SD of triplicate determinations. (B) PECs from wild-type or Tlr3−/− mice were infected for the indicated periods. Indicated cytokine mRNA levels were determined by real-time RT-PCR. Data are presented as mean ± SD of triplicate determinations. (C) Survival of CVB3-infected Tlr3−/− mice. Tlr3−/− (n = 12) and wild-type mice (n = 18) were inoculated i.p. with 5 × 104 pfu of CVB3, and survival was monitored daily. (D) Serum from each mouse was collected on the indicated days after CVB3 infection, and virus titers were determined. Data are presented as mean ± SD. All experiments were performed more than twice with similar results. **, P < 0.01; *, P < 0.05.
Development of Myocarditis in Tlr3−/− Mice Infected With CVB3.
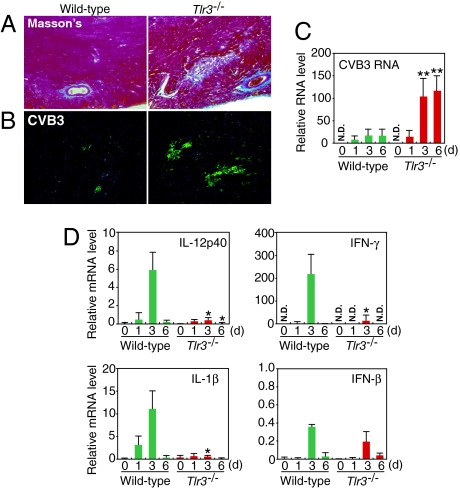
Because CVB3 is the most prevalent virus associated with dilated cardiomyopathy (27, 28), we monitored the course of histopathology and viral replication in the hearts of wild-type and Tlr3−/− mice during CVB3 infection. Mice were infected with 5 × 104 pfu of CVB3, and the hearts were collected and then examined by Masson's trichrome staining. Heart sections from wild-type mice showed small scattered foci of myocardial and perivascular inflammatory lesions at 12 days postinfection (p.i.) (Fig. 2A). In contrast, Tlr3−/− mice heart sections showed large and numerous myocarditic lesions (Fig. 2A). To compare the extent of viral spread in the hearts of these mice, we performed immunohistochemical analysis using anti-CVB3 antibody. Tlr3−/− hearts had much larger and more numerous CVB3-positive myocardial foci than the hearts of wild-type animals at 9 days p.i. (Fig. 2B). We also examined the expression of positive-stranded CVB3 genomic RNA in total heart tissue by real time RT-PCR analysis. Consistent with the immunostaining data for CVB3, we detected higher amounts of viral RNA in Tlr3−/− than in wild-type hearts, and the differences increased with time (Fig. 2C). These results indicate that TLR3 signaling is critical in resolving the virus infection from the heart.
Fig. 2.
Myocarditis in Tlr3−/− mice infected with CVB3. (A) Histopathologic results of hearts collected from Tlr3−/− and wild-type mice 12 days after CVB3 infection was evaluated with Masson's trichrome staining. Data represent eight mice per group. (B) Immunofluorescence of hearts from Tlr3−/− or wild-type mice 9 days after CVB3 infection followed by anti-CVB3 antibody staining. Data represent three mice per group. (C, D) Real-time RT-PCR analysis of the expression of positive-strand CVB3 RNA (C) or the indicated cytokine genes (D) in hearts of Tlr3−/− and wild-type mice on the indicated days after CVB3 infection. Data are presented as mean ± SD of triplicate determinations. All experiments were performed more than twice with similar results. **, P < 0.01; *, P < 0.05.
We next examined the expression levels of IL-12p40, IL-1β, IFN-γ, and IFN-β mRNAs in CVB3-infected hearts. As shown in Fig. 2D, in addition to IL-12p40 and IL-1β mRNAs, IFN-γ mRNA was also markedly induced in the CVB3-infected wild-type hearts, whereas all mRNA levels were impaired in the Tlr3−/− hearts (Fig. 2D). Consistent with the in vitro experiment described above, the expression of IFN-β was again low and not considerably different between the wild-type and Tlr3−/− hearts after CVB3 infection (Fig. 2D). These results suggest that the weak induction of type I IFN mRNA by CVB3 is mainly, if not entirely, mediated by the RIG-I/MDA5 pathway.
Induction of Type II IFN and Inflammatory Cytokines via TRIF.
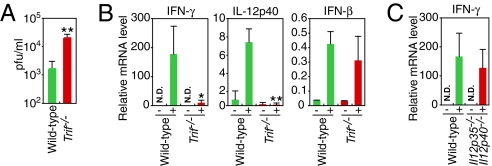
TRIF (toll/IL-1-receptor-domain-containing adaptor inducing IFN-β) is a critical adaptor molecule required for TLR3 signaling (2). We next examined the role of TRIF in the host defense against CVB3 infection using Trif−/− mice. Trif−/− mice showed about a 10-fold higher virus titer in the sera than wild-type mice after CVB3 infection (Fig. 3A). In the hearts of Trif−/− mice, induction of IFN-γ and IL-12p40 was ablated, whereas IFN-β mRNA induction was unchanged (Fig. 3B). These results indicate that the TLR3-TRIF pathway contributes to the host defense against CVB3 infection and, in this case, transmits signals that lead to the induction of type II IFN and inflammatory cytokines rather than type I IFN.
Fig. 3.
TRIF-dependent induction of inflammatory cytokine and IFN-γ genes in CVB3 infected mice heart. (A) Sera from Trif−/− (n = 5) or wild-type (n = 5) mice were collected 2 days after infection, and virus titers were determined by the plaque formation assay. (B) Real-time RT-PCR analysis of the indicated cytokine genes expression in hearts of Trif−/− and wild-type mice on 3 days after CVB3 infection. (C) Real-time RT-PCR analysis of IFN-γ mRNA expression in hearts of IL-12p35 and p40 doubly deficient and wild-type mice on 3 days after CVB3 infection. Data are presented as mean ± SD of triplicate determinations. All experiments were performed more than twice with similar results. **, P < 0.01; *, P < 0.05.
Because IL-12 is a well-established stimulator for type II IFN induction (30), we next investigated whether type II IFN induction in CVB3-infected hearts was an indirect effect of IL-12 signaling by analyzing mice doubly deficient in IL-12p35 and p40. We did not see any substantial decrease in the abundance of IFN-γ transcripts in hearts of the doubly deficient mice after CVB3 infection (Fig. 3C). Collectively, these results indicate the presence of a TLR3- and TRIF-dependent, but IL-12-independent, type II IFN induction pathway in CVB3-infected hearts.
Contribution of TLR3 Against CVB3 Infection in the Absence of Type I IFN Signaling.
The above results, together with previous reports (4, 8), suggest that the innate antiviral immune system consists of two major arms: a cytosolic, RIG-I/MDA5-activated type I IFN response and a TLR3-activated type II IFN/inflammatory cytokine response. These two arms of immunity presumably exert their functions by coupling with each other for a full-blown antiviral response. One may also envisage that, although the former pathway is generally involved in innate antiviral immune responses, the latter is more critical in cooperating with the former pathway to eliminate some viruses, such as CVB3, that have apparently evolved “stealth-type” strategies to evade the efficient induction of type I IFNs by the former pathway.
Can the TLR3 pathway per se cope with viruses when it is artificially amplified? To address this issue, we generated a transgenic C57BL/6 mouse line with TLR3 cDNA under the control of a chicken β-actin promoter (TLR3-Tg) (Fig. S2A). The TLR3 expressed by the transgene was fully functional in terms of hyper induction of IL-12p40 by a well-established agonist, poly(I:C) (Fig. S2D, S2E). This observation was made with two independent transgenic lines; hence, it is unlikely that this signal amplification is due to an accidental inactivation or activation of an unrelated gene locus by the integrated transgene (data not shown). As expected, the transgenic expression of TLR3 strongly sensitized the mice to poly(I:C)-mediated shock responses (Fig. S3). Interestingly, a DNA microarray analysis revealed that there is no significant increase in the level of the type I IFN signature mRNAs in the poly(I:C)-stimulated TLR3-Tg PECs, although a very strong increase in the expression of inflammatory cytokine mRNAs was observed (Fig. S4): These results further support the notion that the activation of the TLR3 pathway does not evoke a strong type I IFN response.
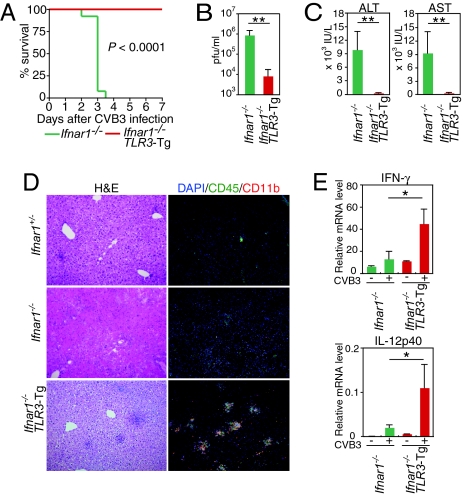
To examine the contribution of the TLR3 pathway further, we crossed TLR3-Tg mice with Ifnar1−/− mice (TLR3-Tg Ifnar1−/− mice), thereby allowing us to gain more insight into the TLR3-mediated antiviral immune responses in the absence of type I IFN signaling. As expected, the cells from TLR3-Tg Ifnar1−/− mice expressed very low levels of RIG-I, MDA5, and protein kinase R (PKR), but expressed high levels of TLR3 (Fig. S5). We then infected the Ifnar1−/− and TLR3-Tg Ifnar1−/− mice with 103 pfu of CVB3 and examined their responses. Consistent with previous reports (28, 31), Ifnar1−/− mice were highly vulnerable to CVB3 infection (Figs. 4A, 4B). These mice experienced massive liver damage, rather than damage to the heart, and showed markedly high levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in their sera (Fig. 4C). The major histopathological finding was massive necrosis of hepatocytes (Fig. 4D), which likely accounts for death at an early stage of infection and presumably before myocarditis could develop. Surprisingly, the CVB3 virus replication was strongly inhibited in TLR3-Tg Ifnar1−/− mice (Fig. 4B). Histological examination revealed only modest areas of necrosis and apoptosis in the livers of these mice, which was comparable to that observed in the Ifnar+/− mice (Fig. 4D). Consistent with the histological data and indicative of suppressed liver damage, TLR3-Tg Ifnar1−/− mice showed lower levels of ALT and AST in their sera than control mice (Fig. 4C). As one might expect, TLR3-Tg Ifnar1−/− mice did not exhibit any outward signs of morbidity and had lower mortality after CVB3 infection (Fig. 4A). These results indicate that, when amplified by TLR3 overexpression, the TLR3 pathway is sufficient to mount an effective innate immune response against CVB3 infection in the absence of type I IFN signaling.
Fig. 4.
Transgenic expression of TLR3 protects Ifnar1−/− mice from lethal CVB3 infection and hepatitis. Ifnar1−/− (n = 13) and TLR3-Tg Ifnar1−/− mice (n = 5) were infected with 1 × 103 pfu of CVB3. (A) The survival of these mice was monitored every 12 h. (B) Sera from each mouse were collected 2 days after infection, and virus titers in the sera were determined. Data are presented as mean ± SD. (C) The serum concentration of liver enzymes 2 days after CVB3 infection was determined. Data are presented as mean ± SD. (D) H&E staining and immunostaining with antibodies against CD45 (green), CD11b (red), and DNA (blue) of liver sections from the indicated mice were performed. (E) CD11b+ cells were sorted from the livers of CVB3-infected mice by MACS and analyzed for the expression of the indicated genes by real-time RT-PCR. Data are presented as mean ± SD of triplicate determinations. All experiments were performed more than twice with similar results. **, P < 0.01; *, P < 0.05.
Histological examination also revealed that CVB3 infection led to the formation of aggregations of CD11b+CD45+ hematopoietic cells in the livers of TLR3-Tg Ifnar1−/− mice (Fig. 4D). Interestingly, these CD11b+ cells expressed high levels of type II IFN and IL-12p40 (Fig. 4E). Because these phenomena were not observed in Ifnar1−/− mice, we infer that the rapid mobilization of CD11b+ cells into the liver and the production of type II IFN and other cytokines depend on TLR3 signaling and prevent liver damage. This notion is congruent with the fact that no sign of infiltration of T cells was observed in the liver of the CVB3-infected TLR3-Tg Ifnar1−/− and Ifnar1−/− mice (data not shown). Although further work is required to clarify the details of the nature and function of the CD11b+ cells, it is worth noting that a protective role for macrophages against infection by CVB3 and CVB4 has been reported (25, 32).
Type II IFN as the Integral Effector Molecule Downstream of TLR3.
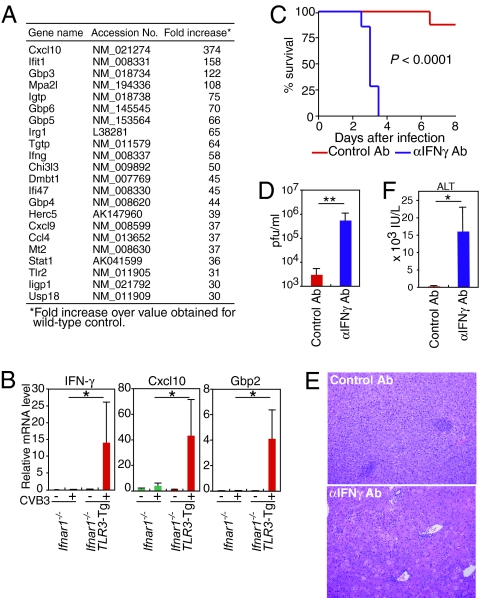
To further assess which genes are regulated by TLR3 signaling during CVB3 infection, we compared mRNAs from Ifnar1−/− versus TLR3-Tg Ifnar1−/− livers using DNA microarrays and real-time RT-PCR. The genes most highly up-regulated in the TLR3-Tg Ifnar1−/− liver were primarily type II IFN-inducible genes, including genes encoding IFN-γ itself, chemokines (Cxcl10, Cxcl9, and Ccl4), the 65-kDa guanylate-binding proteins (GBPs), and the p47 GTPase family members (Figs. 5A, 5B). These results suggest that type II IFN may be a critical downstream effector of TLR3 in the CVB3-infected liver. It is also possible that the chemokines revealed in our array are critical for antiviral responses by recruiting CD11b+ cells. The importance of type II IFN signaling in antiviral response has been appreciated for many years (5, 33, 34), although little is known about how the type II IFN response is elicited during viral infection. We observed that mice deficient in the IFN-γ receptor (Ifngr1−/−) showed an approximately 10-fold higher virus titer in the sera than wild-type mice 2 days after CVB3 infection (SI Text Fig. S6). These previous and current observations prompted us to investigate further whether the above anti-CVB3 innate immune response is mediated by the TLR3-type II IFN signaling axis. Accordingly, we treated the TLR3-Tg Ifnar1−/− mice with a blocking antibody against type II IFN during CVB3 infection. As shown in Fig. 5D, the TLR3-Tg Ifnar1−/− mice treated by anti-type II IFN blocking antibody exhibited a virus titer in the sera hundreds-fold higher than in the sera of mice treated by a control antibody. Furthermore, anti-type II IFN antibody-treated mice exhibited severe liver injury, accompanied by higher amounts of ALT in the sera (Figs. 5E, 5F). As expected, the anti-type II IFN antibody-treated mice succumbed to CVB3 (Fig. 5C), thereby establishing type II IFN as a critical effector molecule acting downstream of TLR3 in vivo.
Fig. 5.
TLR3 mounts IFN-γ response against CVB3 infection. (A) Comparative gene expression profiles of livers from Ifnar1−/− and TLR3-Tg Ifnar1−/− mice 2 days after CVB3 infection. Data represent genes expressed in TLR3-Tg Ifnar1−/− liver that showed greater than 30-fold changes relative to the liver from Ifnar1−/− mice. (B) Real-time RT-PCR analysis of the expression of the indicated genes in liver from Ifnar1−/− and TLR3-Tg Ifnar1−/− mice on pre (−) or 2 days post CVB3 infection (+). Data are presented as mean ± SD of triplicate determinations. (C-F) TLR3-Tg Ifnar1−/− mice were infected with 1 × 103 pfu of CVB3 on day 0 and injected i.p. with anti-type II IFN (IFN-γ) antibody or control rat IgG on days 0, 2, and 4. The survival of these mice was monitored every 12 h. (C). Sera from each mouse were collected 2 days after infection, and virus titers (D) and ALT levels (F) were determined. H&E staining of livers collected 2 days after infection are shown in (E). Representative results are shown from one of two (for C-F) or three (for B) independent experiments performed. **, P < 0.01; *, P < 0.05.
Contribution of the TLR3-Type II IFN Axis to the Immune Response to Other Viruses.
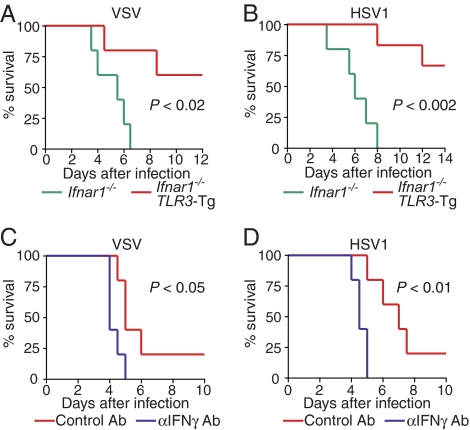
To examine whether the TLR3-type II IFN axis might be operational during infection by other viruses, we next infected TLR3-Tg Ifnar1−/− mice with VSV and HSV1 and monitored their survival. As shown in Fig. 6A and 6B, TLR3-Tg Ifnar1−/− mice were more resistant to infection by either VSV or HSV1 than Ifnar1−/− mice. Furthermore, the protective effect was again abrogated by the treatment with the anti-type II IFN blocking antibody (Fig. 6C, 6D). These results suggest that TLR3 is activated not only by CVB3 but also by other viruses, such as VSV or HSV1, and that the TLR3-type II IFN signaling axis contributes to the innate immune response to these viruses. Consistent with this notion, we adduced evidence that the clearance of VSV and HSV1 was impaired, albeit very modestly, in Tlr3−/− mice (Fig. S7A-D). In view of the fact that Ifnar1−/− mice succumb to VSV or HSV1, whereas Tlr3−/− mice do not (5, 16, 17; Fig. S7A-D), the type I IFN response elicited by the cytosolic RIG-I/MDA5 receptor and other TLRs contributes more to the overall immune response against VSV or HSV1 in these experimental settings. Nevertheless, the activation of the TLR3-type II IFN pathway by these viruses, as revealed by the analysis of infected TLR3-Tg Ifnar1−/− mice, also constitutes a critical mechanism of antiviral innate immune responses. Finally, these findings may offer a mechanistic explanation and insight into the connection between mutations of TLR3 and virus-induced pathogenesis in humans (24).
Fig. 6.
Role of TLR3-Type II IFN signaling in host defense against other viruses. (A, B) Ifnar1−/− mice and TLR3-Tg Ifnar1−/− mice were infected i.p. with 1 × 103 pfu of VSV (Ifnar1−/−; n = 5, TLR3-Tg Ifnar1−/−; n = 6) (A) or 1 × 103 pfu of HSV1 (Ifnar1−/−; n = 5, TLR3-Tg Ifnar1−/−; n = 5) (B). The survival of these mice was monitored every 12 h. (C, D) TLR3-Tg Ifnar1−/− mice were infected with VSV (C) or HSV1 (D) on day 0 and treated with anti-IFN-γ antibody or control rat IgG on days 0, 2, and 4 (n = 5 per group). All of the experiments were performed more than twice with similar results. Note that mice treated with control and anti-IFN-γ antibodies showed an increased mortality than without antibody treatment; the reason for this observation is currently unknown.
Discussion
Although TLR3 has previously been implicated in antiviral innate immune responses, precisely how TLR3 functions together with other nucleic acid-sensing receptors has remained elusive. In the present study, we used Tlr3−/− and TLR3-Tg mice to examine the contribution of TLR3 to the antiviral innate immune response. Our study revealed a critical role of the TLR3 signaling pathway in the host's protection against a picorna virus that causes chronic dilated cardiomyopathy, a major public health problem. In the TLR3 pathway, type II IFN rather than type I IFN functions critically in antiviral responses. Since it is clear that the RIG-I/MDA5-type I IFN pathway is a sine qua non for innate immune responses against viruses, we infer that the TLR3-type II IFN pathway has evolved to recognize and respond to classes of viruses, CVB3 for example, for which infection does not elicit robust type I IFN gene induction. In other words, the antiviral innate immune system may have evolved in such a way that the TLR3-type II IFN pathway serves to function as an “ace in the hole” in the sense that its contribution to antiviral immunity is more critical against certain “stealth-type” viruses that possess strategies to escape the robust operation of the RIG-I-MDA5-type I IFN pathway for efficient replication. Considering that TLR3 and RIG-I/MDA5 receptors are all type I IFN-inducible (8, 14, 15), it is evident that a type I IFN-dependent enhancement of both receptor pathways is also a critical element of the effective innate antiviral immunity. Collectively, our results may offer new mechanistic insight into how the dsRNA-sensing TLR3 and RIG-I/MDA5 cytosolic receptors cooperate during virus infection.
Our study also raises several other interesting issues. First, although TRIF is certainly involved, it is not known at present as to how the TLR3-TRIF signaling pathway results in the induction of type II IFN. Since the hearts from IL-12p35 and p40 doubly deficient mice and wild-type mice showed no significant difference in type II IFN expression after CVB3 infection, we infer that TLR3-TRIF pathway might directly, rather than indirectly, induce type II IFN transcription; although further investigation is needed. Another issue is the possibility that the operation of the TLR3-type II IFN pathway upon viral infection may show cellular or organ specificity. CVB3 infection causes Tlr3−/− mice to develop myocarditis, but not severe hepatitis as Ifnar1−/− mice do (the latter are devoid of both type I IFN and, due to the lack of TLR3 induction, TLR3-type II IFN pathways) (Fig. 2A and data not shown). Nevertheless, the transgenic expression of TLR3 prevented hepatitis in the absence of the type I IFN system (Fig. 4D). These results suggest that to mount an effective antiviral response against CVB3, either of the two pathways could be effective in the liver, whereas the weak induction of type I IFN pathway alone is insufficient in the heart. It is also worth noting that the in vitro induction of type II IFN mRNA by CVB3 infection was barely detectable in wild-type PECs, under the condition at which inflammatory cytokine mRNAs were efficiently induced (Fig. 1B and data not shown), yet was markedly induced in the heart by CVB3 infection (Fig. 2D). Thus, the activation as well as the contribution of the TLR3-type II IFN pathway may depend on the cell-context, the mechanism of which requires further study. We have shown that the TLR3-type II IFN pathway also contributes to antiviral immune responses against other types of viruses such as VSV and HSV1 (Fig. 6), suggesting that TLR3 might be activated, perhaps in response to dsRNA generated during infection and replication, by many classes of viruses. However, it has not been rigorously excluded that other molecular patterns present in the viruses may also activate TLR3. Whatever its mechanism of activation, our results may offer an explanation of a previous report showing that TLR3 mutations in human are associated with herpes virus-induced encephalitis (24); one may infer that the TLR3-type II IFN pathway is crucial to control the virus burden in neuronal cells.
These results bring the TLR3-type II IFN axis to the forefront of our understanding of the host's antiviral innate immune response. In our best hypothetical model, TLR3 mediates the production of type II IFN, which then functions in parallel with the type I IFN system that is elicited by RIG-I/MDA5 cytosolic receptors. The TLR3-type II IFN axis is sufficient to reduce CVB3 replication systemically and, at the same time, to prevent local tissue damage. Thus, these two arms of innate immunity presumably exert their functions by coupling with each other to mount a full-blown antiviral response. It must also be mentioned that, although our study places the TLR3-type II IFN axis in a new context in the antiviral innate immune response, it is highly likely that additional targets of TLR3 signaling also contribute to mounting the response, for example in the production of chemokine(s) to recruit CD11b+ cells (Fig. 4D). Finally, this study may offer new strategies for the treatment of virus-associated diseases.
Materials and Methods
Mice and Cells.
Tlr3−/− mice were generated as described previously (14), Trif−/− mice were kindly provided by S. Akira (Osaka University, Osaka, Japan), IL-12p35 and p40 doubly deficient mice were kindly provided by K. Bishop (University of Michigan, Ann Arbor, MI), and Ifnar1−/− mice were purchased from B & K Universal Group; these mice were backcrossed onto the C57BL/6 genetic background for more than 8 generations. PECs were prepared following a standard procedure. Liver CD11b+ cells were collected using CD11b MicroBeads and a MACS column (Miltenyi Biotec). For the in vivo blocking of IFN-γ, anti-IFN-γ (300 μg, R4–6A2, BD PharMingen) or control rat IgG (Sigma-Aldrich) was administered by i.p. injection.
Viral Infections.
CVB3 (Nancy strain), VSV, and HSV1 were prepared as described previously (35, 36). Virus titers were determined by standard plaque assay as described previously (36).
Serum Biochemistry.
Serum ALT and AST levels were measured at SRL, Inc.
Statistical Analysis.
Differences between control and experimental groups were evaluated using Student's t test. Survival curves were analyzed with the log-rank test. Statistical tests used PRISM software (Graphpad).
Supporting Information.
For additional details, see SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank David Savitsky, Tetsuro Suzuki, Mayumi Ueta and Takako Negishi for invaluable advice, Tatsuaki Mizutani, Shin-ichi Kano, Rie Takeda, Mizuho Kawasaki and Masashi Shishido for technical assistance, and Shizuo Akira for Trif−/− mice. This work was supported by Kakenhi (Grant-in-Aid for Scientific Research) on Priority Areas “Integrative Research Toward the Conquest of Cancer” and by Global COE Program “Integrative Life Science Based on the Study of Biosignaling Mechanisms” from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and grants from the Ichiro Kanehara Foundation, Sumitomo Foundation, Senri Life Science Foundation, and the Naito Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810372105/DCSupplemental.
References
- 1.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 2.Honda K, Taniguchi T. IRFs: Master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 3.Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 5.Van den Broek MF, et al. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol Rev. 1995;148:5–18. doi: 10.1111/j.1600-065x.1995.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 6.Katze MG, He Y, Gale M., Jr Viruses and interferon: A fight for supremacy. Nat Rev Immunol. 2002;2:675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 7.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: Sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 8.Yoneyama M, Fujita T. RIG-I family RNA helicases: Cytoplasmic sensor for antiviral innate immunity. Cytokine Growth Factor Rev. 2007;18:545–551. doi: 10.1016/j.cytogfr.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Ahlquist P. Parallels among positive-strand RNA viruses, reverse-transcribing viruses and double-stranded RNA viruses. Nat Rev Microbiol. 2006;4:371–382. doi: 10.1038/nrmicro1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gitlin L, et al. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci USA. 2006;103:8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoneyama M, Fujita T. Structural Mechanism of RNA Recognition by the RIG-I-like receptors. Immunity. 2008;29:178–181. doi: 10.1016/j.immuni.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto M, et al. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J Immunol. 2003;171:3154–3162. doi: 10.4049/jimmunol.171.6.3154. [DOI] [PubMed] [Google Scholar]
- 14.Honda K, et al. Selective contribution of IFN-α/β signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc Natl Acad Sci USA. 2003;100:10872–10877. doi: 10.1073/pnas.1934678100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miettinen M, Sareneva T, Julkunen I, Matikainen S. IFNs activate Toll-like receptor gene expression in viral infections. Genes Immun. 2001;2:349–355. doi: 10.1038/sj.gene.6363791. [DOI] [PubMed] [Google Scholar]
- 16.Edelmann KH, et al. Does Toll-like receptor 3 play a biological role in virus infections? Virology. 2004;322:231–238. doi: 10.1016/j.virol.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 17.Vercammen E, Staal J, Beyaert R. Sensing of viral infection and activation of innate immunity by Toll-like receptor 3. Clin Microbiol Rev. 2008;21:13–25. doi: 10.1128/CMR.00022-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardarson HS, et al. Toll-like receptor 3 is an essential component of the innate stress response in virus-induced cardiac injury. Am J Physiol Heart Circ Physiol. 2007;292:H251–H258. doi: 10.1152/ajpheart.00398.2006. [DOI] [PubMed] [Google Scholar]
- 19.Hidaka F, et al. A missense mutation of the Toll-like receptor 3 gene in a patient with influenza-associated encephalopathy. Clin Immunol. 2006;119:188–194. doi: 10.1016/j.clim.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Le Goffic R, et al. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2:e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang T, et al. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 22.Daffis S, et al. Toll-like receptor-3 has a protective role against West Nile virus infection. J Virol. 2008 doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabeta K, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci USA. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang SY, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 25.Horwitz MS, et al. Pancreatic expression of interferon-γ protects mice from lethal Coxsackievirus B3 infection and subsequent myocarditis. Nat Med. 2000;6:693–697. doi: 10.1038/76277. [DOI] [PubMed] [Google Scholar]
- 26.Huber S. Host immune responses to Coxsackievirus B3. Curr Top Microbiol Immunol. 2008;323:199–221. doi: 10.1007/978-3-540-75546-3_9. [DOI] [PubMed] [Google Scholar]
- 27.Baboonian C, Davies MJ, Booth JC, McKenna WJ. Coxsackie B viruses and human heart disease. Curr Top Microbiol Immunol. 1997;223:31–52. doi: 10.1007/978-3-642-60687-8_3. [DOI] [PubMed] [Google Scholar]
- 28.Knowlton KU. CVB infection and mechanisms of viral cardiomyopathy. Curr Top Microbiol Immunol. 2008;323:315–335. doi: 10.1007/978-3-540-75546-3_15. [DOI] [PubMed] [Google Scholar]
- 29.Haller O, Kochs G, Weber F. The interferon response circuit: Induction and suppression by pathogenic viruses. Virology. 2006;344:119–130. doi: 10.1016/j.virol.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frucht DM, et al. IFN-γ production by antigen-presenting cells: Mechanisms emerge. Trends Immunol. 2001;22:556–560. doi: 10.1016/s1471-4906(01)02005-1. [DOI] [PubMed] [Google Scholar]
- 31.Wessely R, Klingel K, Knowlton KU, Kandolf R. Cardioselective infection with Coxsackievirus B3 requires intact type I interferon signaling: Implications for mortality and early viral replication. Circulation. 2001;103:756–761. doi: 10.1161/01.cir.103.5.756. [DOI] [PubMed] [Google Scholar]
- 32.Horwitz MS, et al. Protection from lethal Coxsackievirus-induced pancreatitis by expression of gamma interferon. J Virol. 1999;73:1756–1766. doi: 10.1128/jvi.73.3.1756-1766.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang S, et al. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 34.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: An overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 35.Seko Y, et al. Effects of in vivo administration of anti-B7–1/B7–2 monoclonal antibodies on murine acute myocarditis caused by Coxsackievirus B3. Circ Res. 1998;82:613–618. doi: 10.1161/01.res.82.5.613. [DOI] [PubMed] [Google Scholar]
- 36.Honda K, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.