Abstract
Biological invasions are often closely associated with human impacts and it is difficult to determine whether either or both are responsible for the negative impacts on native communities. Here, we show that human activity, not biological invasion, is the primary driver of negative effects on native communities and of the process of invasion itself. In a large-scale experiment, we combined additions of the exotic fire ant, Solenopsis invicta, with 2 disturbance treatments, mowing and plowing, in a fully crossed factorial design. Results indicate that plowing, in the absence of fire ants, greatly diminished total native ant abundance and diversity, whereas fire ants, even in the absence of disturbance, diminished some, but not all, native ant abundance and diversity. Transplanted fire ant colonies were favored by disturbance. In the absence of disturbance and on their own, fire ants do not invade the forest habitats of native ants. Our results demonstrate that fire ants are “passengers” rather than “drivers” of ecological change. We propose that fire ants may be representative of other invasive species that would be better described as disturbance specialists. Current pest management and conservation strategies should be reassessed to better account for the central role of human impacts in the process of biological invasion.
Keywords: community organization, competition, disturbance, exotic species, pest management
Habitat degradation and biological invasion are the 2 greatest threats to global biodiversity (1), and are closely linked in 3 important ways. First, invasive species most often invade and become abundant in human-altered habitats (2). Second, community assembly is shaped by disturbance (3). Third, the simplification of native ecosystems by habitat degradation and the onslaught of species invasions are human-driven and can potentially be mitigated or at least better predicted (4–6). The correlation between human-caused disturbance and species invasions has long been recognized (7), and there is now evidence (4–6) that human activity (4, 8), as opposed to natural processes (9, 10), is the primary driver of the vast and growing number of species invasions. Despite increased awareness that human activity is a primary driver of biological invasion, the idea has only rarely been tested with experiments, and then primarily with invasive plants (11–13). Given the paucity of experimental evidence and the lack of tests using invasive animals, there is growing recognition that we lack even a basic understanding of how the interaction of species invasions, human impacts, and natural processes will affect biodiversity and ecosystem function in the future (1, 13, 14). Understanding the relationship between disturbance and invasion is now critically important, because human activity affects a large and growing portion of the terrestrial and aquatic biomes on earth and the number and impact of invasions are closely correlated with the intensity of human activity (4–6, 15).
Community assembly and biological invasions are presumably controlled by the same mechanisms (3). An important mechanism that has been proposed to explain the success and dynamics of invasive species is their ability to compete with native species (11, 12, 16). Invasive species may be competitively superior to native species for a number of reasons, including unique physical or physiological characteristics, superior ability to survive or grow under resource limitation, or because they have escaped the burden of many natural enemies (16). Alternatively, invasive species may benefit from the conditions created by anthropogenic disturbance, because they recruit to and survive better than native species in disturbed habitats (11, 12). Under the latter scenario, where invasive species are the “passengers” rather than the “drivers” of ecological change, the primary factor governing the success and abundance of the invasive species is simply the availability of disturbed habitat, whereas the competitive abilities of both native and exotic species are much less important (11, 12). If disturbance alone is the primary factor affecting the success of many invasive species, this scenario would provide the basis for reassessing our conceptual understanding of how many dominant biological invaders have succeeded and provide a scientific basis for rethinking many chemical and biological pest management strategies.
We used the invasive fire ant, Solenopsis invicta Buren, to test whether disturbance or superior competitive ability is largely responsible for the success of this species. S. invicta is largely restricted to human-modified habitats throughout its introduced and native ranges and achieves its greatest abundance in these areas (17, 18). Thus, S. invicta is an excellent invasive ant for testing whether competitive superiority, habitat disturbance, or their interaction largely structures ant communities. Invasive fire ants are absent from undisturbed pine flatwoods ecosystems in northern Florida, but colonize this habitat after disturbance (17), making this habitat suitable for testing the effects of fire ants and disturbance, separately and together on native ant communities. A native of South America, S. invicta is now a globally distributed pest species. Listed among the 100 worst invasive species in the world, S. invicta is perhaps the most well known invasive ant, because it is both a significant pest affecting human interests and has been described as a significant threat to numerous native communities (19, 20). The negative impact of S. invicta on native ants has been hypothesized to be so great (20) in part because ant communities are thought to be highly interactive. Behaviorally dominant species with large colonies are thought to be better able to gain access to limiting resources and thereby to suppress or exclude subordinates (21). Thus, the success of S. invicta and many other invasive ant species is also thought to result primarily from behavioral dominance and release from natural enemies that allows them to attain numerical superiority and competitively suppress co-occurring species (20, 22, 23). However, experimental demonstration of competition in ant communities is uncommon, especially for invasive species, and very few manipulative studies exist (24–26); to our knowledge, only King and Tschinkel (27) have previously manipulated entire populations of ants over multiple years.
Results
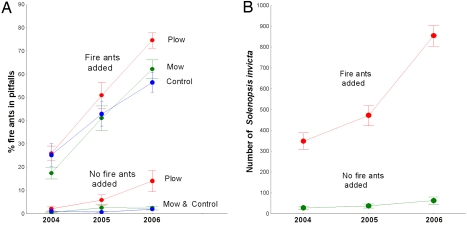
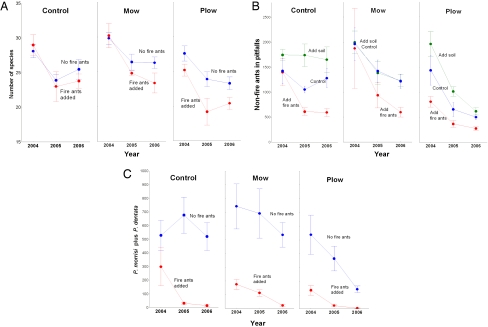
Fire ants persisted in plots where we added them in significantly higher numbers than control plots (Fig. 1), but were also favored by disturbance, surviving better and reaching higher abundance in disturbed plots. Both disturbance treatments, even in the absence of fire ants, had a negative effect on the abundance and diversity of co-occurring ants (Fig. 2 A and B). These data showed that plowing by itself greatly diminished native ant abundance and diversity (Fig. 2 A and B). Fire ants by themselves also greatly diminished native ant abundance and, less so, diversity (Fig. 2 A and B). The effect of disturbance by itself was sufficient to significantly reduce the entire ant fauna, whereas the combined effect of disturbance and fire ants was greater than either disturbance or fire ants alone (Fig. 2 A and B). However, the effect of plowing was not significantly different from the combined effect of plowing and fire ants [see Table S1, Table S2, and Table S3 in supporting information (SI) Appendix]. Also, by adding mature colonies to undisturbed natural areas, we did reduce some but not all of the native ants. The significant effect was largely a result of the reduction of 2 of the most abundant native species, Pheidole morrisi Forel, and Pheidole dentata Mayr (Fig. 2C).
Fig. 1.
Fire ants populations persisted wherever we established them in significantly higher numbers than in control plots. This pattern is reflected in both the average percentage of fire ants, out of all ants (ANOVA, P < 0.0001) (A), and the average total number of fire ants in addition and control plots captured in pitfall traps over 3 years (ANOVA, P < 0.0001) (B). For both representations, error bars represent SEM. Note that “no-fire-ants-added” plots include both control (nothing added) and sham control (soil plugs added), and results are identical when controls are considered separately (see Tables S1 and S2 in SI Appendix). Fire ants appearing in the no-fire-ants-added plots are naturally-founded, or naturally occurring, colonies. Naturally-founded colonies, because they were small at the time of sampling, produced workers that were distinctly smaller than those from large colonies (either transplanted, or moved in).
Fig. 2.
Disturbance and fire ants had a significantly negative effect on co-occurring ant populations separately and in combination over 3 years. (A) For total ant diversity, the negative effect of fire ants, mowing and plowing were all significant (ANOVA, P < 0.0001), and the combined effect of fire ants and plowing was greatest. Rarefaction analyses showed the same patterns. (B) For total abundance of ants, plowing had the greatest negative effect as a single treatment and, again, the combined effect of fire ants and plowing was greatest (ANOVA, P < 0.0001). (C) For the total abundance of Pheidole, the most abundant native genus, fire ants additions alone had a strongly negative impact, an effect that contributed to the pattern seen among all ants (in B), although the combined effect of disturbance and fire ants was strongly negative (ANOVA, P < 0.0001). For all representations, error bars represent SEM. All effects were tested by using mixed-model ANOVAs and Tukey's HSD test for multiple comparisons (see Table S1 and Table S2 in SI Appendix).
Plowing also caused a significant increase in a few native species known to specialize on disturbed habitats [e.g., Dorymyrmex bureni (Trager)], although the increase in the abundance of fire ants was much greater than any other species (Fig. 1). The tendency for fire ants to recruit to and establish in the plowed plots was so great that by the third year, our best efforts to remove colonies could not eliminate them completely from plowed plots that were supposed to be fire ant free (Fig. 1B; workers from naturally-founded colonies were distinguished by their smaller size, because at the time of sampling, they came from smaller colonies). This observation of natural founding in plowed plots, combined with the lack of fire ant recruitment to undisturbed plots (Fig. 1), suggests that the critical link between these experimental results and natural patterns of invasive ant distribution lies in the recruitment patterns we observed in self-founding colonies (Fig. 1). Fire ants clearly become established and persist in much higher numbers in highly disturbed habitats (Fig. 1).
Discussion
These results demonstrate that human activity is the primary force that drives fire ant invasions and suggests that disturbance, not interspecific competition, has the greatest impact on structuring these ant communities. This outcome has broad implications for our understanding of ant community assembly, because it suggests that for ants, the processes of queen dispersal and colony founding potentially holds the key to understanding community assembly and why the success of fire ants, and many other invasive exotic ants, is so closely tied to disturbed habitats. Thus, S. invicta, and perhaps most invasive ants are passengers rather than drivers of ecological change. To date and without experiments, it has been difficult to separate the impacts of, sometimes subtle, disturbances (e.g. edge effects) from the impact of an invasive species that rapidly exploits disturbed habitats. Thus, many “high profile” invasive ants, such as fire ants and Argentine ants [Linepithema humile (Mayr)], have been solely credited with disrupting native ant communities in correlational studies (22, 23) without regard to the central role that abiotic factors (25) and even mild disturbances can have in structuring these same ant communities (17, 18, 28, 29). Further community-scale experiments conducted in different ecosystems and at different latitudes are necessary to validate these hypotheses.
Although these results demonstrate a negative effect of fire ants, it is important to dispel a potential misunderstanding. By moving entire colonies, we were able to establish populations of fire ants in undisturbed habitats at ≈60–70% of the high forager and colony densities they achieve under natural conditions in highly disturbed habitats, such as pasture (27). However, we cannot emphasize enough that the suppression of native ants by fire ants in the undisturbed plots of our experiment occurred only because we planted hundreds of mature colonies into native habitat, a habitat that they neither recruit into nor persist in on their own. In contrast, fire ants clearly naturally recruit to and become established in much higher numbers in highly disturbed habitats that we created (Fig. 1). This pattern is an experimental result that suggests that habitat selection by queens may be the mechanism that explains why, throughout the native and introduced range of fire ants, areas that are largely free of disturbance are also free of fire ants. These results may also suggest that this is general mechanism in ant community assembly, at least in disturbed habitats. Native disturbance specialists (e.g., D. bureni) showed similar patterns of recruitment into disturbed areas (Fig. 1), and were absent from undisturbed habitats, whereas differences in life history (colony size, production of sexuals) explain their differences in relative abundance (Fig. 1).
Pine flatwoods ecosystems in Florida contain a diverse natural ant community where, absent disturbance, exotic species typically comprise <7% of the total diversity and <1% of the total abundance of ants on average (28). Under natural conditions, fire ants simply do not occur in this habitat; therefore, normally have little or no effect on the natural ant community (17, 28). Previous manipulative experiments have also revealed that dominant ant species may lack widespread competitive effects, instead only negatively impacting ecological equivalents (24, 26, 27). In this system, native species such as P. morrisi and P. dentata have similar-sized workers, similar diets, large colonies, and high abundance (28), and can thus be described as ecologically similar to S. invicta. Therefore, in the absence of disturbance, these Pheidole species are suppressed by an artificial invasion of an ecological equivalent. However, these species and most other native species are greatly and directly diminished by disturbance (Fig. 2), which clearly must occur before fire ants invade in significant numbers (Fig. 1). In support of this conclusion, King and Tschinkel (27) demonstrated that removing fire ants from pasture plots did not increase the recruitment or abundance of native species, including P. morrisi and P. dentata, into those highly-disturbed habitats. In such species-poor, man-made habitats, exotic ants clearly benefit from disturbance as they may comprise as much as 25% of the diversity and 90% of the abundance of ants (27). Like plants (11, 12), recruitment and persistence of native ant species is limited by disturbance, whereas many dominant invasive species are better at exploiting disturbed habitat.
In this first explicit test of MacDougall and Turkington's (11) passenger-driver hypothesis using animals, we have provided a starting point for better understanding the biology of invasive ants and other terrestrial animals. This outcome has direct implications for the applied biology of invasive ants, because it calls into question the wisdom of chemical and biological control or suppression programs that have assumed competitive superiority of invasive species as a conceptual basis for the ongoing search for and introduction of biological control agents and widespread chemical control of S. invicta in the United States and in newly infested areas in Australia, China, Southeast Asia, and the Caribbean. These management approaches do not consider the role of habitat disturbance but do have known negative effects (e.g., widespread effects of pesticides on nontarget organisms) and unknown ecological consequences (17). If these management programs are to be based on the biology of the organism, they must address the critical role that disturbance has in assuring the establishment and spread of a species that specializes in disturbance and functions in a community that is, in effect, free from native species as well as many natural enemies. This conclusion also applies directly to the polygyne or multiple queen form of fire ants as this social form co-occurs with the monogyne form in disturbed habitats throughout its introduced range (17), and is spread almost entirely by human transport (8).
Methods
We combined additions of entire colonies of S. invicta with 2 disturbance treatments, mowing and plowing, in a full factorial design over 3 years (Table 1) to test effects on the diversity and abundance of native ants. Plowing and mowing were selected as landscape disturbance treatments because they approximate the first steps in the process of land use change (i.e., vegetation clearing and soil excavation) associated with human activity. These land use changes are necessary steps in the process of real estate development, which has been shown to be an effective measure of the human activities that are positively correlated with the establishment, dispersal, and spread of invasive species (4). Three years is sufficient time, given the high annual reproductive output and variable modes of colony reproduction of most native and exotic species in this region, to determine whether species were lost from or recruited into plots (17, 27).
Table 1.
Design of the experiments carried out in moderately shrubby flatwoods habitat in the Apalachicola National Forest in northern Florida, showing the number of replicates
| Disturbance treatment | Ant treatment |
||
|---|---|---|---|
| Add mature colonies in soil | Add soil without ants | Add nothing | |
| Mowing | 5 | 5 | 5 |
| Plowing | 5 | 5 | 5 |
| Control | 5 | 5 | 5 |
The study was conducted in the pine flatwoods of Apalachicola National Forest in Florida. This forest is the largest remaining intact longleaf pine forest in the world and a recognized biodiversity hotspot ranking among the highest floral diversity of any temperate zone plant community (30). These forests are associated with flat topography, and poorly drained, acidic, sandy soil. They are structurally characterized by an open overstory of pines (Pinus palustris Mill. and Pinus elliottii Engelm.), no understory, and a dense ground cover layer [the dominant species include Serenoa repens (W. Bartram) Small, Ilex glabra (L.) A. Gray, Lyonia lucida (Lam.) K. Koch, Aristida beyrichiana Trin. and Rupr., and other herbs] (30).
Replicate plots were 40 × 40 m that included a 7.5 m buffer that was mowed and plowed, but not sampled with pitfall traps. All plots were separated by 40 m from one another and at least 40 m from ecotones and roadsides. To control vegetation, all disturbance plots were mowed twice per year to a height of 15 cm, once in early spring (April) and once in the fall (October), by using a 2-m wide mowing deck pulled behind a medium-sized tractor. All plow plots were plowed to a depth of ≈0.3 m once per year in the spring (April) by using a 2-m wide, 16 disk-blade harrow pulled behind a medium-sized tractor, but were only mowed in the fall (October). Monogyne (the single-queen social form), S. invicta colonies were visually counted 3 times per year on all plots. In 2004, 25 colonies were added to each addition plot during the spring (April) and again in the fall. After the first year, additions were based on the census, and 5 to 30 mature colony fragments (depending on how many colonies were established with brood) were added twice annually from 2005 to 2006 to all fire ant addition plots. Colony fragments were collected by using a shovel to take most of the above-ground soil mound and all of the ants and brood and putting them into a heavy plastic bag. Colonies could then be moved and “planted” into plots by digging a small hole and dumping the bag contents into them. Colonies were collected along roadsides during cool winter months (December to February) during sunny mornings to increase the likelihood of capturing queenright colony fragments. A similar number of soil “plugs” (without any ants) was added to soil control plots, functioning as a sham control for the fire ant additions. Self-founding colonies that appeared in nonaddition plots were killed by using hot water (27, 31), whenever they were discovered during the census.
We operated 36 pitfall traps arrayed in a 6 × 6 grid with 5-m spacing within the central 25 × 25 m of each plot once per year in July and early August during the peak of annual ant activity to assess the diversity and abundance of ants. A 7.5-m unsampled buffer was left between the plot margin and the sampling grid. Pitfall traps are the best method for estimating the presence and relative abundance of ground-dwelling species in this region and habitat type (32). Pitfall traps were 85-mm long plastic vials with 30-mm internal diameter. Traps were filled to a depth of ≈15 mm with propylene-glycol antifreeze, inserted flush with the surface of the ground, and operated for 7 days. Traps were installed with a hand-held, battery powered drill by using an auger bit and then covered with a clear plastic rain shield suspended ≈10 cm above the ground surface.
The primary data consisted of the identity and abundance of all ant species collected by pitfall trapping. All data were square root plus 0.5 transformed throughout to satisfy normality assumptions. Data were analyzed in SAS version 8 (SAS Institute, Cary, NC) by using a mixed-model, repeated measures factorial design with year and site assigned as random effects and disturbance and fire ant treatments as fixed effects. Sites were locations within 5 different forest stands within the Apalachicola National Forest where we placed 1 replicate set of all of the possible 9 treatment plots (Table 1). Species richness was also analyzed by using rarefaction curves. Rarefaction curves were compared across treatments by ANOVA and showed the same pattern as those seen with average total number of species, thus rarefaction data are not shown.
Supplementary Material
Acknowledgments.
We thank the United States National Forest Service for land access; Clayton (Sandy) Heath for help with construction of kilns; Kevin Haight, Chris Smith, Jon Seal, Dacia Drury, Josh Gold, and Daniel Peterson for help with sampling, additions, and removals; and Adrian Smith, Josh Gold, and Emily Owens for assistance with sorting specimens. Rarefaction curves were created with R. K. Colwell's program EstimateS (http://viceroy.eeb.uconn.edu/estimates). The authors identified all species, and voucher specimens have been deposited at the Museum of Comparative Zoology, Harvard University and Archbold Biological Station. This work was supported by United States Department of Agriculture-National Research Initiative Grant 2003-01453.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809423105/DCSupplemental.
References
- 1.Sala OE, et al. Global Biodiversity Scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- 2.Orians GH. In: Ecology of Biological Invasions of North America and Hawaii. Mooney HA, Drake JA, editors. New York: Springer; 1986. pp. 133–148. [Google Scholar]
- 3.Tillman D. Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. Proc Natl Acad Sci USA. 2004;101:10854–10861. doi: 10.1073/pnas.0403458101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor BW, Irwin RE. Linking economic activities to the distribution of exotic plants. Proc Natl Acad Sci USA. 2004;101:17725–17730. doi: 10.1073/pnas.0405176101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halpern BS, et al. A global map of human impact on marine ecosystems. Science. 2008;319:948–952. doi: 10.1126/science.1149345. [DOI] [PubMed] [Google Scholar]
- 6.Leprieur F, Beauchard O, Blanchet S, Oberdorff T, Brosse S. Fish invasions in the world's river systems: When natural processes are blurred by human activities. PLOS Biology. 2008;6:e28. doi: 10.1371/journal.pbio.0060028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elton CS. The Ecology of Invasions by Animals and Plants. London: Methuen; 1958. [Google Scholar]
- 8.King JR, Tschinkel WR, Ross KG. A case study of human exacerbation of the invasive species problem: Transport and establishment of polygyne fire ants in Tallahassee, Florida. Biol Inv. 2008 doi: 10.1007/s10530–008-9254-x. [DOI] [Google Scholar]
- 9.Fridley JD, et al. The invasion paradox: Reconciling pattern and process in species invasions. Ecology. 2007;88:3–17. doi: 10.1890/0012-9658(2007)88[3:tiprpa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Levine JM. Species diversity and biological invasions: Relating local process to community pattern. Science. 2000;288:852–854. doi: 10.1126/science.288.5467.852. [DOI] [PubMed] [Google Scholar]
- 11.MacDougall AS, Turkington R. Are invasive species the drivers or passengers of change in degraded ecosystems? Ecology. 2005;86:42–55. [Google Scholar]
- 12.Seabloom EW, Harpole WS, Reichman OJ, Tilman D. Invasion, competitive dominance, and resource use by exotic and native California grassland species. Proc Natl Acad Sci USA. 2003;100:13384–13389. doi: 10.1073/pnas.1835728100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Didham RK, Tylianakis JM, Gemmell NJ, Rand TA, Ewers RM. Interactive effects of habitat modification and species invasion on native species decline. TREE. 2007;22:489–496. doi: 10.1016/j.tree.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Puth LM, Post DM. Studying invasion: Have we missed the boat? Ecol Lett. 2005;8:715–721. [Google Scholar]
- 15.Watts RD, et al. Roadless space of the conterminous United States. Science. 2007;316:736–738. doi: 10.1126/science.1138141. [DOI] [PubMed] [Google Scholar]
- 16.Bruno JF, Fridley JD, Bromberg KD, Bertness MD. In: Species Invasions: Insights into Ecology, Evolution, and Biogeography. Sax DF, Stachowicz JJ, Gaines SD, editors. Sunderland, MA: Sinaur Associates; 2005. pp. 9–40. [Google Scholar]
- 17.Tschinkel WR. The Fire Ants. Cambridge, MA: Harvard Univ Press; 2006. [Google Scholar]
- 18.Deyrup M, Davis L, Cover S. Exotic ants in Florida. Trans Am Entomol Soc. 2000;126:293–326. [Google Scholar]
- 19.Lowe S, Browne M, Boudjelas S, De Poorter M. 100 of the World's Worst Invasive Alien Species: A Selection from the Global Invasive Species Database. Gland, Switzerland: Invasive Species Specialist Group; 2004. [Google Scholar]
- 20.Holway DA, Lach L, Suarez AV, Tsutsui ND, Case TJ. The causes and consequences of ant invasions. Ann Rev Ecol Syst. 2002;33:181–233. [Google Scholar]
- 21.Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard Univ Press; 1990. [Google Scholar]
- 22.Porter SD, Savignano DA. Invasion of polygyne fire ants decimates native ants and disrupts arthropod community. Ecology. 1990;71:2095–2106. [Google Scholar]
- 23.Sanders NJ, Gotelli NJ, Heller NE, Gordon DM. Community disassembly an invasive ant species. Proc Natl Acad Sci USA. 2003;100:2474–2477. doi: 10.1073/pnas.0437913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibb H, Hochuli DF. Removal experiment reveals limited effects of a behaviorally dominant species on ant assemblages. Ecology. 2004;85:648–657. [Google Scholar]
- 25.Menke SB, Holway DA. Abiotic factors control invasion by Argentine ants at the community scale. J Anim Ecol. 2006;75:368–376. doi: 10.1111/j.1365-2656.2006.01056.x. [DOI] [PubMed] [Google Scholar]
- 26.Lebrun EG, et al. An experimental study of competition between red imported fire ants and Argentine ants in their native range. Ecology. 2007;88:63–75. doi: 10.1890/0012-9658(2007)88[63:aesocb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.King JR, Tschinkel WR. Experimental evidence that the introduced fire ant, Solenopsis invicta, does not competitively suppress co-occurring ants in a disturbed habitat. J Anim Ecol. 2006;75:1370–1378. doi: 10.1111/j.1365-2656.2006.01161.x. [DOI] [PubMed] [Google Scholar]
- 28.King JR, Porter SD. Body size, colony size, abundance, and ecological impact of exotic ants in Florida's upland ecosystems. Evol Ecol Res. 2007;9:757–774. [Google Scholar]
- 29.Bolger DT. Spatial and temporal variation in the Argentine edge effect: Implications for the mechanism of edge limitation. Biol Conserv. 2007;136:295–305. [Google Scholar]
- 30.Myers RL, Ewel JJ, editors. Ecosystems of Florida. Orlando, FL: Univ of Central Florida Press; 1990. [Google Scholar]
- 31.Tschinkel WR, King JR. Targeted removal of ant colonies in ecological experiments, using hot water. J Ins Sci. 2007;7:41. doi: 10.1673/031.007.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King JR, Porter SD. Evaluation of sampling methods and species richness estimators for ants in upland ecosystems in Florida. Environ Entomol. 2005;34:1566–1578. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.