Abstract
Messenger ribonucleoprotein particles (mRNPs) move randomly within nucleoplasm before they exit from the nucleus. To further understand mRNP trafficking, we have studied the intranuclear movement of a specific mRNP, the BR2 mRNP, in salivary gland cells in Chironomus tentans. Their polytene nuclei harbor giant chromosomes separated by vast regions of nucleoplasm, which allows us to study mRNP mobility without interference of chromatin. The particles were fluorescently labeled with microinjected oligonucleotides (DNA or RNA) complementary to BR2 mRNA or with the RNA-binding protein hrp36, the C. tentans homologue of hnRNP A1. Using high-speed laser microscopy, we followed the intranuclear trajectories of single mRNPs and characterized their motion within the nucleoplasm. The Balbiani ring (BR) mRNPs moved randomly, but unexpectedly, in a discontinuous manner. When mobile, they diffused with a diffusion coefficient corresponding to their size. Between mobile phases, the mRNPs were slowed down 10-to 250-fold but were never completely immobile. Earlier electron microscopy work has indicated that BR particles can attach to thin nonchromatin fibers, which are sometimes connected to discrete fibrogranular clusters. We propose that the observed discontinuous movement reflects transient interactions between freely diffusing BR particles and these submicroscopic structures.
Keywords: Balbiani ring particles, in vivo labeling, mRNP trafficking, single-molecule fluorescence microscopy, single-particle tracking
Concomitant with transcription, the growing premessenger RNA (pre-mRNA) molecules become associated with proteins to form ribonucleoprotein (RNP) particles (1). The pre-mRNA is processed to mRNA, and the reorganized messenger RNP (mRNP) particles are prepared for export (2, 3). After being released from the gene, mRNP particles move randomly within the nucleus, apparently by free diffusion (4). However, the mobility can be affected by energy depletion, which suggests that ATP-dependent processes also play a role (5). Recently, it became feasible to track individual mRNP particles in the nucleoplasm (6, 7). It could then be confirmed that the particles move by free diffusion, but the diffusion is often spatially constrained. mRNPs travel in interchromatin channels (8, 9), and the movement of mRNPs is probably hindered in tight channels and even stalled in cavities formed by chromatin. In the single-particle-tracking experiments, ATP depletion showed no effect on the individual, still-mobile mRNPs but further constrained the movement of the particles, probably because of a more condensed organization of the chromatin (6, 7). To further study the movement of mRNPs, we have now chosen an experimental system that allows us to follow the movement of individual mRNPs in large nucleoplasmic regions lacking chromatin, and thus we avoid the complex influence of chromatin. Surprisingly, we then find that the mRNP particles move in a discontinuous fashion, suggesting that the particles transiently interact with supramolecular assemblies other than chromatin.
As the experimental system, we have chosen the salivary gland cells of the dipteran Chironomus tentans (2, 10, 11). The nuclei harbor 4 well-demarcated giant chromosomes, which are separated by vast regions of nucleoplasm. The chromosomes are polytene, i.e., they consist of thousands of perfectly aligned chromatids. Transcriptionally active regions are expanded, puffed. On chromosome IV there are 2 giant puffs, Balbiani rings (BR) 1 and 2, with exceptionally high transcriptional activity. The BR genes are 30–40 kb in size and encode internally repetitive, silk-like proteins. The transcripts contain 4 short introns, which are removed predominantly cotranscriptionally. The packaging of the large BR transcripts with proteins to mRNPs can be visualized in the electron microscope, the final product being almost spherical particles (2). The BR particles, ≈50 nm in diameter, can be recognized in nucleoplasm and during their translocation through the nuclear pore complexes. Approximately a dozen BR-associated RNA-binding proteins have been identified, some of which stay associated with the transcript when transferred from the gene to the polysomes, e.g., hrp36, whereas others leave the transcript in conjunction with the passage through the nuclear pore complex (12). By immunoelectron microscopy of BrUTP-labeled BR particles, it has been possible to follow the dispersion of the BR particles from the transcription sites into the vast interchromosomal regions of the cell nucleus (13). It was concluded that the BR particles move randomly in nucleoplasm and seem to bind stochastically to the nuclear pore complexes before exit from the nucleus.
The new, powerful light-microscopy technique used to track single fluorescent molecules or particles with high time resolution provides detailed information on the molecular dynamics inside living cells (14). Messenger RNPs can be imaged, because they are large and can be brightly labeled. Two recent studies used synthetic genes to produce highly fluorescently labeled mRNA in vivo and followed the pathways of these artificial mRNA molecules by single-particle-tracking microscopy (6, 7).
In the present study, we have analyzed the intranuclear mobility of a single native mRNP species, the BR2 mRNP. Fluorescence labeling was achieved in vivo by microinjection of fluorescent oligonucleotides (DNA or RNA), which were complementary to a highly repetitive coding sequence in BR2 mRNA. In addition, we examined mRNPs labeled with fluorescent hrp36, a homologue of the hnRNP protein A1. Using high-speed laser microscopy, we followed the intranuclear trajectories of individual mRNPs and characterized their motion within the nucleoplasm. For reference, we determined the intranuclear viscosity by the mobility of inert reference particles of known sizes. Finally, a detailed analysis of the single-trajectory data provided insights into the intranuclear dynamics of mRNPs.
Results
In Vivo Fluorescence Labeling of BR2 mRNPs.
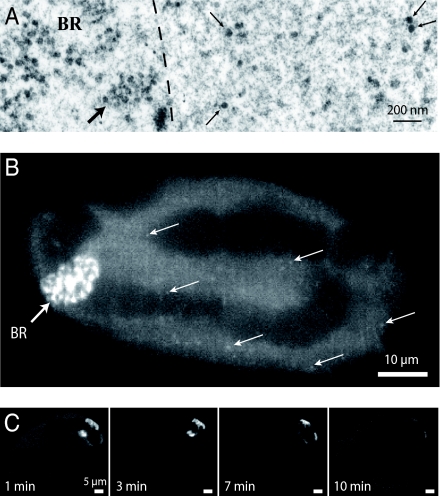
BR mRNPs are abundant in the nuclei of isolated salivary glands, and they can be readily recognized in the electron microscope as large, densely stained granules (thin arrows in Fig. 1A) (15). A quantitative analysis yields a density of approximately 10 BR particles per μm3. To label BR2 mRNPs in vivo, fluorescent DNA oligonucleotides complementary to a repetitive sequence in BR2 RNA were microinjected into the nuclei of isolated salivary glands. After injection of minimal amounts of the oligonucleotides, the BR2 transcription site on chromosome IV became clearly visible (Fig. 1B). This labeling is likely to correspond to nascent mRNA transcripts, because it disappeared when the transcription inhibitor 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB) was coinjected with the oligonucleotides (Fig. 1C). DRB specifically hinders the RNA polymerase II from leaving the promoter but does not inhibit subsequent transcription steps. Coinjection of the oligonucleotides with DRB cleared the BR from growing mRNA transcripts and, thereby, the fluorescence vanished. Hybridization occurred quickly, and the BRs appeared almost instantaneously upon microinjection of the fluorescent probe. Not only nascent mRNPs were labeled, but also mRNPs that had left the transcription site and were roaming the nucleoplasm (Fig. 1B, white arrows). The labeled particles did not access the polytene chromosomes, which appeared as dark structures within the nucleus. Experiments using Cy5-labeled control oligonucleotides of the same length but reverse sequence did not yield any labeling of the transcription site, and no large particles were detected in the nucleoplasm.
Fig. 1.
Electron- and light-microscopic visualization of BR mRNPs. (A) Electron microscopy of BR particles in the nucleoplasm of C. tentans salivary-gland cells. Four BR particles are indicated by thin arrows. A minor portion of a BR is demarcated to the left by an dashed line. A segment of an active gene with growing RNP particles has been marked by a thick arrow. (B) A C. tentans salivary-gland cell nucleus imaged in an LSM 5 Live after microinjection of LgBR-Oligo-Cy5. The white arrows indicate single BR mRNPs. (C) Time course of the fluorescence in a living C. tentans salivary-gland cell nucleus after addition of DRB, an inhibitor of RNA polymerase-II-dependent transcription. The characteristic labeling of the BR2 by LgBR-Oligo-Cy5 is lost within 10 min. This observation indicates that the oligonucleotide probe labeled growing BR2 mRNPs and not the BR2 DNA.
In summary, we can use DNA oligonucleotides to specifically label both the growing mRNPs in BR2 and the mature BR2 mRNPs released into the nucleoplasmic regions between the giant chromosomes.
Intranuclear Mobility of Single BR mRNPs.
Having shown that BR2 mRNPs can be specifically labeled in vivo, we next optimized the visualization and tracking of single particles in the nucleoplasm by using a high-speed laser scanning microscope (LSM). This line-scanning LSM yielded high signal-to-noise ratios (SNR) and frame rates. However, because of the high density of labeled mRNPs (approximately 10 per μm3), a short photobleaching phase had to be included to singularize particles for tracking. A rectangular region comprising ≈1/5 of the nucleus was bleached with high laser power. Unbleached particles moving into that region could be clearly seen. As an alternative to line-scanning LSM, a fast and ultrasensitive custom-built single-molecule microscope (SMM) was used (16). In these experiments, a circular region within the nucleus with a diameter of ≈15 μm was bleached with maximum laser intensity before movies of unbleached mRNPs tumbling into the bleached volume were acquired with reduced laser intensity (data not shown).
To properly locate the nuclei, we coinjected AlexaFluor488-labeled importin β1 (Imp β1) (green) together with red-labeled oligonucleotides. Imp β1 is an import receptor specifically interacting with nucleoporins that allowed a straightforward localization of the nuclear envelope. However, being living tissues, the glands were not completely immobile on the coverslip and could move slightly during movie acquisition.
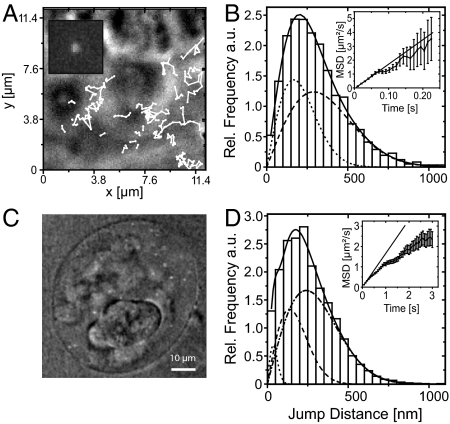
The tracking of individual BR2 mRNPs is shown in Fig. 2. Five successive frames from a single LSM movie [supporting information (SI) Movie S1, which show several single BR2 mRNPs] are displayed in Fig. 2A. Fig. 2B gives several examples of trajectories, which were deduced by tracking several BR2 mRNPs plotted onto a nuclear-envelope reference image, whereas Fig. 2C presents a plot of all observed trajectories in a sequence of 23 movies, each 5–8 s long. The particle trajectories covered the nucleoplasm quite homogeneously. Only a small area remained void of mRNPs, and it was found to be occupied by a polytene chromosome as concluded from the corresponding bright-field image. Occasionally, mRNPs attached to the nuclear envelope, but actual translocations were not recorded, probably because of the chosen time scale or bleaching of the particles.
Fig. 2.
Tracking single BR mRNPs in the nucleus of a living salivary-gland cell. (A) Five subsequent frames of a movie obtained with the LSM 5 Live. BR2 mRNPs are marked by black arrows. Their pathways could be followed by time-lapse imaging with 20 Hz. C marks the location of a polytene chromosome. (Scale bar, 2 μm.) (B) LSM image of the nuclear envelope marked by Alexa488-labeled Imp β1. Selected trajectories were plotted on the image, which provided a rough structure reference. (C) Plot of all trajectories obtained from this nucleus. (D) Frequency distribution of BR2 mRNP jumps performed within 0.05 s. Eq. 3 was used to fit the data points (bold, solid line). Thin or broken lines indicate distributions for each of the mobility fractions I–III. (E) Mean-square displacements (MSDs) plotted against the time. The initial slope of the curve was fitted by Eq. 1.
To describe the mobility of the BR2 particles, we analyzed both mean-square displacements (MSDs) and jump-distance distributions. From the single-particle trajectories, the MSD can be determined as a function of time, t, yielding the diffusion coefficient, D (see Materials and Methods). MSD plots, however, produce only averaged diffusion coefficients, which are misleading when different types of particles or particles with various mobilities are analyzed. Such heterogeneous ensembles are better investigated by analysis of jump-distance distributions, where subpopulations can be resolved by curve fitting (14).
Our jump-distance analysis was based on the trajectories of 4 independent experiments (11,256 trajectories) comprising almost 50,000 single BR2 mRNP jumps between successive frames. The analysis of these data revealed that there are at least 3 mobility fractions present (I–III) (Fig. 2D and Table 1). The fastest particles exhibited DI = 4.0 ± 1.3 μm2/s. Two-thirds of the jumps showed DII = 0.7 ± 0.1 μm2/s (fraction II), and the slowest fraction (III) yielded DIII = 0.24 ± 0.05 μm2/s. In addition to the jump-distance analysis, we performed an MSD analysis that yielded an average diffusion coefficient DMSD = 0.76 ± 0.03 μm2/s (Fig. 2E).
Table 1.
Mobility analysis of single BR2 mRNPs and reference particles
| Particle | AIII, % | DIII, μm2/s | AII, % | DII, μm2/s | AI, % | DI, μm2/s | n |
|---|---|---|---|---|---|---|---|
| DNA-labeled BR mRNP | 22 ± 9 | 0.24 ± 0.05 | 65 ± 7 | 0.7 ± 0.1 | 14 ± 4 | 4.0 ± 1.28 | 49,547 |
| Qdots 655 | 40 ± 8 | 1.43 ± 0.15 | 60 ± 7 | 4.17 ± 0.39 | 3,598 | ||
| Beads (210 nm) | 4 ± 0.9 | 0.01 ± 0.002 | 30 ± 11 | 0.13 ± 0.03 | 66 ± 11 | 0.4 ± 0.05 | 18,743 |
| FITC-dextran,* 500 kDa | 100 | 2.42 ± 0.2 |
A, relative fraction of total absorbance; n, number of single jumps from frame to frame.
*This diffusion constant was determined by a FRAP experiment; see SI Materials.
Verification of BR mRNP Identity and Integrity.
To further substantiate that the BR2 RNA-containing particles are mRNPs, we microinjected fluorescently labeled recombinant hrp36, an hnRNP A1-like protein in C. tentans. hrp36 is known to bind in many copies to BR2 RNA and remain associated with the mRNA during transport from gene to polysomes (17). The fluorescent protein clearly marked the BR transcription sites on chromosome IV, demonstrating the efficient incorporation of the labeled hrp36 into the BR2 mRNPs (data not shown). Upon injection of very low amounts of this protein into the nuclei, we detected distinct particles after a minimum incubation time of 10 min. A jump-distance analysis of the particle motion yielded a distribution that was similar to that of the oligonucleotide-labeled BR2 mRNPs (for details, see SI Text). Thus, the labeled particles analyzed are likely to be BR2 mRNPs.
The DNA-oligonucleotide-labeled particles displayed a considerably higher mobility than recorded in previous single-particle experiments (6, 7, 14). To rule out the possibility that the fast motion was caused by fragmentation of the particles by RNase H, we performed microinjection experiments using fluorescently labeled 2′-O-methyl-RNA oligonucleotides complementary to BR2 RNA. Using this approach, we could again identify and specifically track labeled BR2 mRNPs, which yielded results comparable with those from the DNA-oligonucleotide approach (see SI Text). We conclude that the DNA-oligonucleotide-labeled BR2 mRNPs are not likely to be fragmented by RNase H.
Analysis of the Nuclear Viscosity.
An important parameter defining the intranuclear mRNP mobility is the effective viscosity, η, of the nucleoplasm, which was examined with fluorescent reference particles with diameters similar to those of BR2 mRNPs. Single-particle-trajectory analysis yielded the diffusion coefficient D, which allowed the calculation of the viscosity by the Stokes–Einstein relation, D = kT/6πηRS, for a known particle radius RS and temperature T.
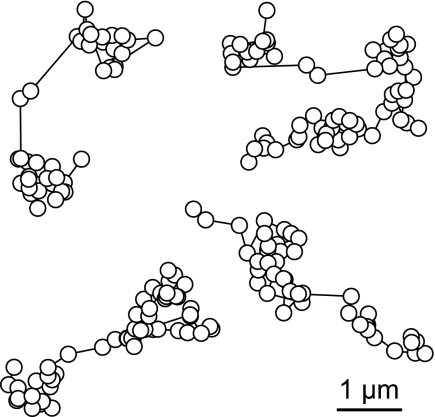
First, we analyzed the diffusion of streptavidin-conjugated quantum dots (Qdots 655-Sav) with a Stokes diameter of 26 nm (18). The Qdots could be imaged with excellent SNRs, yielding long trajectories by using the SMM at a frame rate of ≈100 Hz (Movie S2 and Fig. 3A). A jump-distance analysis revealed 2 fractions (Fig. 3B), with DI = 4.2 ± 0.4 μm2/s and DII = 1.4 ± 0.2 μm2/s. Similar experiments were performed with 210-nm microspheres (Fig. 3 C and D and Movie S3), yielding DI = 0.4 ± 0.05 μm/s2 for the majority of all jumps. Finally, we determined the intranuclear viscosity by fluorescence recovery after photobleaching (FRAP) experiments using an FITC-labeled 500-kDa dextran with a Stokes diameter of ≈57 nm. The microinjected probe yielded an intranuclear diffusion constant of D = 2.4 ± 0.2 μm2/s (see Fig. S1). All tracer mobility results are summarized in Table 1.
Fig. 3.
Measurement of the nuclear viscosity with inert reference particles. (A) Sample trajectories of microinjected Qdots in a salivary-gland cell nucleus plotted onto the corresponding bright-field image. A part of a polytene chromosome is visible in the upper image area. (Inset) A typical image of a quantum dot from the movie (length, 1 μm). (B) Jump-distance distribution of the Qdots655 fitted according to Eq. 3 (solid line) with 2 mobility fractions (dashed lines). (Inset) MSD as a function of time. (C) A movie frame showing microinjected fluorescent microbeads (diameter of 210 nm) within a salivary-gland cell nucleus obtained by the LSM 5 Live at 15 Hz. The overlay of the fluorescence and the bright field image shows the microbeads as bright dots. (D) Jump-distance distribution of the microbeads. The data were fitted (solid line) with Eq. 3 with 2 mobility fractions (dashed lines). (Inset) MSD as a function of time.
The apparent intranuclear viscosity was calculated by assuming that only the fast fractions represented particles with unrestricted mobility. Based on the quantum dots and microspheres, the intranuclear viscosity is 5 centipoise (cP) and 4 cP, respectively. The FRAP measurements yielded a viscosity of 3 cP. Thus, the effective intranuclear viscosity is in the 3- to 5-cP range.
BR mRNPs Are Transiently Retarded but Not Completely Immobilized.
Because very slow BR2 mRNPs cannot be detected at short lag times, we also evaluated the jump distances at long lag times. In addition to the distances, which were covered after 1 frame or 0.05 s (JD1) (Figs. 2D and 4A), we also analyzed those that were covered after 10, 20, and 30 frames corresponding to lag times of 0.5, 1, and 1.5 s, respectively (JD10, JD20, and JD30) (Fig. 4 B–D). Four different mobility fractions could be identified. Three of them (I–III) corresponded to the ones discussed above. In addition, a very slow fraction (IV) was revealed (Table 2). The fast fraction could not be detected after 0.5 s. The observation that particles with D = 4 μm2/s left the focal plane within 0.5 s was rationalized by a respective Monte Carlo simulation (see SI Text). The very slow fraction IV with D = ≈0.015 μm2/s became noticeable after 0.5 s and was clearly seen after 1 and 1.5 s (Fig. 4 B–D). Notably, this fraction was not completely immobile but showed a distinct increase in MSDs with time.
Fig. 4.
Jump-distance analysis at different lag times. (A) BR2 mRNP jump-distance distribution from frame to frame. a.u., Arbitrary units. (B) Jump-distance distribution from frame i to i + 10. (C) Jump-distance distribution from frame i to i + 20. (D) Jump-distance distribution from frame i to i + 30. A satisfactory fit to the data required 3 mobility components, from which the fraction sizes and the diffusion coefficients were extracted. The broken lines quantify the contributions of the three mobility fractions, whereas the solid line represents their sum.
Table 2.
Jump-distance (JD) analysis of single mRNPs for various lag times
| AIV, % | DIV, μm2/s | AIII, % | DIII, μm2/s | AII, % | DII, μm2/s | AI, % | DI, μm2/s | n | |
|---|---|---|---|---|---|---|---|---|---|
| JD1* | 0.7 ± 0.3 | 0.015 | 19 ± 3 | 0.23 | 66 ± 2 | 0.64 ± 0.04 | 15 ± 3 | 3.74 ± 1 | 49,547 |
| JD10 | 3.5 ± 0.5 | 0.015 ± 0.003 | 32 ± 7 | 0.22 ± 0.03 | 65 ± 7 | 0.63 ± 0.04 | 13,724 | ||
| JD20 | 4.5 ± 0.7 | 0.014 ± 0.003 | 41 ± 15 | 0.23 ± 0.04 | 54 ± 15 | 0.58 ± 0.09 | 6,487 | ||
| JD30 | 6.6 ± 1 | 0.015 ± 0.002 | 19 ± 6 | 0.13 ± 0.03 | 74 ± 6 | 0.43 ± 0.03 | 3,806 |
A, relative fraction; n, number of single jumps from frame to frame.
*For this fit, a fourth component with DIV and DIII were held fixed.
BR mRNPs Move in a Discontinuous Fashion.
The 4 BR2 mRNP fractions with different mobilities could represent either (i) 4 classes of BR2 mRNPs, each with a characteristic constant mobility, or (ii) a single class of BR2 mRNP particles with varying mobility along their trajectories. To decide between the two possibilities, we performed a detailed data analysis aided by extensive Monte Carlo simulations (Fig. S2). We selected all mRNP trajectories containing jump distances >1,000 nm within 50 ms. Only the mRNPs in the fast fraction (4 μm2/s) performed such long jumps. In the case of scenario i, a jump-distance histogram compiled from the selected trajectories would recover only a single—the fast—mobility fraction. However, upon application of this filter to the total experimental trajectory data, we regained at least 2 mobility fractions, thus proving that the trajectories of fast particles also contain retarded sections. This result was supported by visual inspection of trajectories. In Fig. 5, 4 long trajectories are shown, where segments of high mobility appear to be interrupted by phases of very low mobility. Hence, BR mRNPs varied their mobility along the track.
Fig. 5.
Discontinuous movement of BR2 mRNPs. Four examples of long trajectories, each containing sections corresponding to strongly retarded mobility and others to free mobility. Interval between 2 points, 50 ms.
Discussion
In this study, we have investigated, at the single-particle level, the intranuclear trafficking of native mRNPs in living tissue by using a well-established model system for RNA biogenesis (19). A specific endogenous mRNP particle, BR2 mRNP, was labeled in vivo by fluorescent oligonucleotides complementary to a highly repetitive sequence in BR2 RNA. Control experiments with DRB and oligonucleotides with the reversed sequence proved the specificity of our approach.
Two additional fluorescence-labeling strategies, employing either 2′-O-methyl-RNA oligonucleotides or an hnRNP protein (hrp36), labeled predominantly the same particles, mostly BR mRNPs. Labeling by these quite distinct approaches yielded particles with very comparable mobilities, suggesting that the labeling did not change the dynamic behavior. Therefore, a mobility analysis using either approach yields valid results.
High-speed imaging enabled us to trace the intranuclear trajectories of individual BR2 mRNPs. The Stokes diameter of the BR2 mRNPs was determined to be 36 ± 12 nm, which is in good agreement with previous electron-microscopy estimations (13), verifying that we indeed visualized single mRNPs and not aggregates or fragments of mRNPs. The huge amount of data and the considerable length of the trajectories permitted a detailed analysis of the mobility of the BR2 mRNPs. A statistical analysis of all tracks with more than 3 positions revealed that the mRNP trajectories did not contain more linear, directional sections than trajectories produced by randomly moving simulated particles (see SI Text). Hence, we could rule out the existence of directed motion for BR2 mRNPs, which confirms earlier studies that have shown that mRNPs move randomly in the nucleus, most likely by Brownian motion.
We found that the mobility of BR2 particles varied widely. Four different mobility fractions were identified (I–IV). The fastest BR2 mRNPs (fraction I) had a diffusion coefficient in the range between 2.7 and 4.7 μm2/s. The high mobility was not due to a low viscosity in the salivary-gland cell nucleoplasm; the effective viscosity was 3–5 cP, i.e., a viscosity only slightly lower than that in mammalian-cell nuclei (4–8 cP) (20). BR2 mRNPs in fractions II–IV moved considerably slower, with diffusion coefficients of 0.6, 0.2, and 0.015 μm2/s, respectively. Notably, we found no particles that were completely immobilized. We conclude that BR2 mRNPs in fraction I are likely to diffuse freely in the nucleoplasm, whereas those in fractions II, III, and IV are retarded to an increasing extent.
The high diffusion coefficient of the fast BR2 mRNPs confirms earlier results on the most-mobile fraction of RNP particles within cell nuclei (4). Poly(A)+ mRNA was loaded with fluorescent oligo(dT), and the local intranuclear mobility of the resulting complexes was analyzed by fluorescence correlation spectroscopy (FCS). Politz et al. (9) speculated that the fast mRNPs rapidly moved within interchromatin channels. In further studies employing photoactivation or photobleaching techniques (FRAP), poly(A)+ mRNA was loaded with fluorescent probes, oligo(dT) (21), 2′-O-methyl oligoribonucleotides (22), or poly(A)+ RNA-binding proteins (5). These studies reported diffusion coefficients in the range from 0.03 to 0.7 μm2/s only. All numbers represented averages over the complete poly(A)+ mRNA population and referred to mRNPs significantly smaller than the especially large BR2 mRNPs. Despite this fact, the diffusion constants were at least an order of magnitude smaller than those in our study. It is known, however, that when using FRAP it is very difficult to correctly account for restriction of the movement because of interactions (23). In addition, it is not straightforward to analyze mRNP mobility by FRAP if small fluorescent ligands are used to tag the particles, because the ongoing exchange of bound and free labels leads to complex recovery kinetics (24). These problems are avoided by using single-particle tracking, where fast, slow, or bound molecules are directly discernible. Only 2 recent studies have followed individual mRNPs, which were designed to bind multiple fluorophores, either GFP-tagged MS2, a viral RNA binding protein (6), or molecular beacons (7). In this way, highly fluorescent artificial mRNPs that could be visualized and traced one at a time were constructed. In both approaches, diffusion constants that were ≈100-fold smaller than the values from the native mRNPs used in this study were obtained. The reported diffusion coefficients were conspicuously low, especially considering the shorter length of the mRNAs studied (see discussion of this issue in SI Text). However, these low values were recorded in mammalian nuclei with their complex chromatin network interfering with the mobility of the mRNPs. We propose that the constraining effect of chromatin is substantial or that the mRNPs in mammalian nuclei also interact with major nonchromosomal structures in the nucleoplasm.
Using single-particle tracking, we detected 4 different mobility fractions. These fractions could be due either to different classes of mobile mRNPs or alternatively to mRNPs altering their mobility along the track. The question of which possibility was the case was answered by a detailed statistical analysis of the trajectory data and illustrated by the corresponding Monte Carlo simulations. This approach revealed unambiguously that the mRNPs with high mobility also exhibited extended retarded phases along their trajectories (see Fig. 5 and SI Text). Hence, the particles moved in a discontinuous manner but never completely stopped. Phases of very slow diffusive motion occurred all over the nucleoplasm and were not confined to regions close to the polytene chromosomes (Fig. S3). In principle, the transient retardation of the BR2 mRNPs could imply that the BR2 particles are temporarily corralled in a small volume or transiently bound to a large submicroscopic structure. Although we cannot exclude the former alternative, we favor the latter because it has been observed by 3D electron tomography that a considerable fraction of the BR mRNPs in the nucleoplasm are associated with thin nonchromatin fibers. These fibers are sometimes connected to discrete, complex meshworks of interconnected fibers and granules, called fibrogranular clusters (25). The fact that the BR particles never completely stop moving would then suggest that the thin fibers and the associated structures are mobile and not likely to be part of a rigid nuclear matrix. Taken together, the morphology and mobility data suggest that BR particles bind transiently to thin nonchromatin fibers, which results in a drastic temporary slow-down of the particles. Miralles et al. (25) speculated that the submicroscopic structures might modulate the intranuclear mobility of the BR RNPs. If so, one would expect to find that the interactions between the BR particles and the thin fibers are nonrandom.
In summary, we have demonstrated that native BR2 mRNPs move randomly in the chromatin-free nucleoplasmic regions in the C. tentans salivary-gland cell nuclei. However, they travel in a discontinuous fashion. When mobile, the particles move as fast as expected from the intranuclear viscosity. We propose that the transient restrictions in mobility are due to interactions between the BR particles and macromolecular, nonchromatin structures. Such a conclusion is supported by the electron-microscopic observation that, at a given moment, a considerable number of the BR particles are associated with thin fibers that at least sometimes connect to complex fibrogranular clusters in the nucleoplasm.
Materials and Methods
Buffer and Reagents.
Transport buffer was used for the dilution of microinjection probes [20 mM Hepes/KOH (pH 7.3), 110 mM potassium acetate, 5 mM sodium acetate, 2 mM magnesium acetate, 1 mM EGTA, and 2 mM DTT]. ZO medium was prepared according to ref. 26. 5,6-Dichloro-1-β-d-ribofuranosylbenzimidazole (DRB, Sigma) was dissolved in DMSO and coinjected at a final concentration of 1 μM. PBS was prepared from a commercially available stock solution (Biochrom). Fluorescent DNA and RNA probes used for in vivo hybridization of the BR mRNA were from IBA BioTAGnologies. The 30-oligomer BR2.1 DNA probe (ACT TGG CTT GCT GTG TTT GCT TGG TTT GCT) was either Cy5- or AlexaFluor647-labeled on its 5′ end. An estimate of the number of oligonucleotides that can maximally bind to a single BR2 mRNP is given in SI Text.
As control, Cy5-labeled DNA oligonucleotides of the reversed sequence were used. A corresponding 2′-O-methyl RNA was labeled by Atto 647N (AttoTec). All oligonucleotides were dissolved in water (0.1 nmol/μl) and diluted 1:10 in transport buffer before microinjection. The human Imp β1 was bacterially expressed and labeled with AlexaFluor488. The hrp36 cDNA was kindly provided by N. Visa (Stockholm University, Stockholm) and subcloned into the pGEX-2T vector (Amersham Pharmacia), thereby introducing an additional cysteine at the hrp36 C terminus. The protein was bacterially expressed as a GST fusion protein, and the GST tag was proteolytically removed. The hrp36 protein was labeled on the introduced cysteine by using maleimide-derivatized dyes.
Preparation of Salivary Glands.
C. tentans larvae were raised in water-filled, aerated plastic dishes and fed with fermented nettle powder (10). Salivary glands were isolated from rapidly growing fourth-instar larvae and kept in PBS during preparation. For microinjection and microscopy, the glands were transferred onto a polylysine-coated coverslip embedded in a cell culture dish (MatTek). For incubation under the microscope, either ZO medium or PBS was used. Usually it took 5–10 min to prepare the gland and an additional 5–10 min to microinject 3–6 nuclei. Microinjection was carried out with an Eppendorf injection and micromanipulation setup, using a holding pressure of 20 hPa and manual injection procedure. All injection solutions were diluted in transport buffer and centrifuged at 22,000 × g for 30 min at 4 °C.
Fluorescence Microscopy.
High-speed confocal imaging was performed with an LSM 5 LIVE microscope (Zeiss). Otherwise, an LSM 510-META (Zeiss) was used. For single-molecule fluorescence microscopy, a custom-built instrument based on a commercial inverted microscope was used (16).
Determination of the Intranuclear Viscosity with Fluorescent Particles.
The fluorescent particles were highly diluted in transport buffer (1:1,000 to 1:10,000) and visualized by the LSM5 LIVE or the SMM. We used dark-red fluorescent polystyrol microspheres with a diameter of 210 nm and streptavidin-coated Quantum dots emitting at 655 nm (Invitrogen).
Data Analysis.
Identification and tracking of the single-particle signals were accomplished by using Diatrack 3.01 (Semasopht), a commercial image-processing program for the identification and localization of single-particle signals and trajectories. All further data processing was performed by using Origin 7.5 (OriginLab) and ImageJ (27) as described in ref. 14. In the case of Brownian motion, the MSDs, 〈r2(tc)〉, are linearly related to time and D:
Heterogeneous mobility populations are more appropriately analyzed by a jump-distance analysis. The probability for a particle starting at a specific position to be encountered within a shell of radius r and width dr at time t from that position is given as
when starting at the origin. The probability distribution function can be approximated by a frequency distribution by counting the jump distances within respective intervals [r, r + dr] traveled by single particles in a given time. When particles move discontinuously, the jump-distance distributions cannot satisfactorily be fitted by Eq. 2. Different mobility fractions can be quantified by summing up several diffusion terms according to Eq. 2. We mostly used 3 fractions with different diffusion constants,
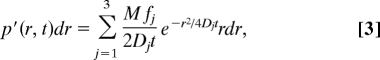 |
where M is related to the number of jumps considered in the analysis, and f1, f2, and f3 denote the fractions with diffusion constants D1, D2, and D3, respectively.
Computer Simulation.
A computer simulation was designed to simulate diffusing and binding particles in a confined volume representing a nucleus. The particles moved according to a Gaussian random walk in 3D space. The nucleus was modeled as a sphere of the size of a salivary-gland cell nucleus (radius, 35 μm). Particles hitting the boundaries were reflected, and export was not taken into account. For details, see SI Text.
Electron Microscopy.
Salivary glands were isolated from fourth-instar larvae. They were fixed for 2 h at 4 °C in 2% glutaraldehyde in 0.05 M sucrose and 0.1 M sodium cacodylate buffer (pH 7.2) and postfixed for 1 h at 4 °C in 1% OsO4. The glands were embedded in an agar 100 resin and sectioned in a Reichert Ultracut S ultramicrotome. The ultrathin sections were stained with uranyl acetate and lead citrate and examined in a Philips CM 120 electron microscope.
SI.
Further information is available in Supporting Information including Figs. S4 and S5 and Table S1.
Supplementary Material
Acknowledgments.
We thank Reiner Peters and Nathalie Fomproix for contributions to the first project phase and Lars Wieslander and Neus Visa for stimulating discussions. This work was supported by BioImaging Network Munich (A.D.), Swedish Research Council and the Knut and Alice Wallenberg Foundation (B.D.), and Volkswagen Foundation and German Research Foundation (U.K. and H.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810692105/DCSupplemental.
References
- 1.Dreyfuss G, Matunis MJ, Pinol-Roma S, Burd CG. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. 1993;62:289–321. doi: 10.1146/annurev.bi.62.070193.001445. [DOI] [PubMed] [Google Scholar]
- 2.Daneholt B. Assembly and transport of a premessenger RNP particle. Proc Natl Acad Sci USA. 2001;98:7012–7017. doi: 10.1073/pnas.111145498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohler A, Hurt E. Exporting RNA from the nucleus to the cytoplasm. Nature Rev. 2007;8:761–773. doi: 10.1038/nrm2255. [DOI] [PubMed] [Google Scholar]
- 4.Politz JC, Browne ES, Wolf DE, Pederson T. Intranuclear diffusion and hybridization state of oligonucleotides measured by fluorescence correlation spectroscopy in livings cells. Proc Natl Acad Sci USA. 1998;95:6043–6048. doi: 10.1073/pnas.95.11.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calapez A, et al. The intranuclear mobility of messenger RNA binding proteins is ATP dependent and temperature sensitive. J Cell Biol. 2002;159:795–805. doi: 10.1083/jcb.200203046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shav-Tal Y, et al. Dynamics of single mRNPs in nuclei of living cells. Science. 2004;304:1797–1800. doi: 10.1126/science.1099754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vargas DY, Raj A, Marras SA, Kramer FR, Tyagi S. Mechanism of mRNA transport in the nucleus. Proc Natl Acad Sci USA. 2005;102:17008–17013. doi: 10.1073/pnas.0505580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cremer T, et al. Role of chromosome territories in the functional compartmentalization of the cell nucleus. Cold Spring Harbor Symp Quant Biol. 1993;58:777–792. doi: 10.1101/sqb.1993.058.01.085. [DOI] [PubMed] [Google Scholar]
- 9.Politz JC, Pederson T. Review: Movement of mRNA from transcription site to nuclear pores. J Struct Biol. 2000;129:252–257. doi: 10.1006/jsbi.2000.4227. [DOI] [PubMed] [Google Scholar]
- 10.Case ST, Daneholt B. The size of the transcription unit in Balbiani ring 2 of Chironomus tentans as derived from analysis of the primary transcript and 75 S RNA. J Mol Biol. 1978;124:223–241. doi: 10.1016/0022-2836(78)90157-2. [DOI] [PubMed] [Google Scholar]
- 11.Wieslander L. The Balbiani ring multigene family: Coding repetitive sequences and evolution of a tissue-specific cell function. Progr Nucl Acid Res Mol Biol. 1994;48:275–313. doi: 10.1016/s0079-6603(08)60858-2. [DOI] [PubMed] [Google Scholar]
- 12.Kiesler E, Visa N. Intranuclear pre-mRNA trafficking in an insect model system. In: Jeanteur P, editor. RNA Trafficking and Nuclear Structure Dynamics. Berlin: Springer; 2003. pp. 99–118. [Google Scholar]
- 13.Singh OP, Bjorkroth B, Masich S, Wieslander L, Daneholt B. The intranuclear movement of Balbiani ring premessenger ribonucleoprotein particles. Exp Cell Res. 1999;251:135–146. doi: 10.1006/excr.1999.4490. [DOI] [PubMed] [Google Scholar]
- 14.Grunwald D, et al. Probing intranuclear environments at the single-molecule level. Biophys J. 2008;94:2847–2858. doi: 10.1529/biophysj.107.115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skoglund U, Andersson K, Bjorkroth B, Lamb MM, Daneholt B. Visualization of the formation and transport of a specific hnRNP particle. Cell. 1983;34:847–855. doi: 10.1016/0092-8674(83)90542-1. [DOI] [PubMed] [Google Scholar]
- 16.Siebrasse JP, Grunwald D, Kubitscheck U. Single-molecule tracking in eukaryotic cell nuclei. Anal Bioanal Chem. 2007;387:41–44. doi: 10.1007/s00216-006-0763-0. [DOI] [PubMed] [Google Scholar]
- 17.Visa N, et al. A pre-mRNA-binding protein accompanies the RNA from the gene through the nuclear pores and into polysomes. Cell. 1996;84:253–264. doi: 10.1016/s0092-8674(00)80980-0. [DOI] [PubMed] [Google Scholar]
- 18.Grunwald D, Hoekstra A, Dange T, Buschmann V, Kubitscheck U. Direct observation of single protein molecules in aqueous solution. ChemPhysChem. 2006;7:812–815. doi: 10.1002/cphc.200500632. [DOI] [PubMed] [Google Scholar]
- 19.Kiesler E, Visa N. Intranuclear pre-mRNA trafficking in an insect model system. Prog Mol Subcell Biol. 2004;35:99–118. doi: 10.1007/978-3-540-74266-1_5. [DOI] [PubMed] [Google Scholar]
- 20.Seksek O, Biwersi J, Verkman AS. Translational diffusion of macromolecule-sized solutes in cytoplasm and nucleus. J Cell Biol. 1997;138:131–142. doi: 10.1083/jcb.138.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Politz JC, Tuft RA, Pederson T, Singer RH. Movement of nuclear poly(A) RNA throughout the interchromatin space in living cells. Curr Biol. 1999;9:285–291. doi: 10.1016/s0960-9822(99)80136-5. [DOI] [PubMed] [Google Scholar]
- 22.Molenaar C, Abdulle A, Gena A, Tanke H, Dirks R. Poly(A)+ RNAs roam the cell nucleus and pass through speckle domains in transcriptionally active and inactive cells. J Cell Biol. 2004;165:191–202. doi: 10.1083/jcb.200310139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mueller F, Wach P, McNally JG. Evidence for a common mode of transcription factor interaction with chromatin as revealed by improved quantitative fluorescence recovery after photobleaching. Biophys J. 2008;94:3323–3339. doi: 10.1529/biophysj.107.123182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braga J, McNally JG, Carmo-Fonseca M. A reaction-diffusion model to study RNA motion by quantitative fluorescence recovery after photobleaching. Biophys J. 2007;92:2694–2703. doi: 10.1529/biophysj.106.096693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miralles F, et al. Electron tomography reveals posttranscriptional binding of pre-mRNPs to specific fibers in the nucleoplasm. J Cell Biol. 2000;148:271–282. doi: 10.1083/jcb.148.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soop T, et al. Nup153 affects entry of messenger and ribosomal ribonucleoproteins into the nuclear basket during export. Mol Biol Cell. 2005;16:5610–5620. doi: 10.1091/mbc.E05-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophot Int. 2004;11:36–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.