Abstract
Transcriptional regulators such as the glucocorticoid receptor (GR) recruit multiple cofactors to activate or repress transcription. Although most cofactors are intrinsically bifunctional, little is known about the molecular mechanisms dictating the specific polarity of regulation. Furthermore, chromatin modifications thought to be confined to silent loci appear in actively transcribed genes suggesting that similar enzymatic activities may mediate constitutive and transient chromatin states. GRIP1, a GR ligand-dependent coregulator of the p160 family can potentiate or inhibit transcription but the molecular contexts and mechanisms that enable GRIP1 corepressor activity are poorly understood. In a yeast 2-hybrid screen with GRIP1 repression domain (RD)-containing fragment, we repeatedly isolated the C-terminal region of a SET domain-containing protein subsequently identified as histone H4 lysine 20 trimethyltransferase, Suv4-20h1. We cloned a full-length Suv4-20h1 and dissected its interaction with GRIP1 in yeast, in vitro, and in mammalian cells. Strict nuclear localization and high salt concentration required for Suv4-20h1 extraction were consistent with its tight association with chromatin. Overexpression of Suv4-20h1 in human U2OS and A549 cells expressing integrated and endogenous GR, respectively, antagonized ligand-dependent induction of a subset of GR target genes, whereas Suv4-20h1 siRNA-mediated depletion had a reciprocal effect. Inhibition of GR transactivation required both the GRIP1 interacting region of Suv4-20h1 and its catalytic activity. Thus, Suv4-20h1 known exclusively as a factor involved in constitutive heterochromatin maintenance, actively participates in hormone-dependent transcriptional regulation affecting GR target gene expression in a promoter- and cell type-specific manner.
Keywords: GRIP1 cofactor, histone methyltransferase, Suv4-20h1, transcriptional regulation
Histones, chromatin-associated proteins and transcription factors in Eukaryotes and Archaea are subject to multiple posttranslational modifications including acetylation, phosphorylation, methylation, ADP-ribosylation and ubiquitination (reviewed in ref. 1), which define chromatin packaging and transcriptional status of a given gene. Thus, tri-methylation of histone H3 lysine 27 or 9 (H3K27 or H3K9) and H4K20 has been associated with transcriptionally silent heterochromatic regions (2–4), whereas methylation of H3K4 is generally observed in transcriptionally active domains (5). Acetylation of several lysine residues in core histones correlates with activation of transcription whereas deacetylation effects repression [(6) and references therein]. H3S10 phosphorylation correlates with activation often in combination with acetylation of H3K14 (7). In contrast to easily reversible acetylation and phosphorylation, methylation has been considered a stable, long-term epigenetic mark. This notion, however, has been recently challenged by the discovery of several demethylases capable of rapidly and specifically reversing histone methylation in response to various signaling cues (8, 9). Combined, specific modification patterns referred to as the “histone code” form unique surfaces recognized by chromatin-associated factors, which, in turn, change the accessibility of regulatory regions.
Nuclear receptors (NRs), the largest family of transcription factors, sense hormone availability and alter target gene expression by recruiting multiprotein complexes with coactivator or corepressor activities (reviewed in ref. 10). One of the best understood families of NR coactivators, the p160 proteins (SRC1, SRC2/GRIP1/TIF2 and SRC3/AIB1/RAC3/ACTR) (11), interact with receptors in a hormone-dependent fashion and serve as platforms for the assembly of numerous chromatin-modifying enzymes including histone acetyltransferases (p300, CBP, pCAF) and arginine and lysine methyltransferases (CARM1, PMRT1, G9a). Other factors reported to associate with NRs include histone deacetylases (HDAC) 1/3 recruited by corepressors NCoR/SMRT, histone demethylases LSD1 and JMJD2C, and ATP-dependent chromatin remodellers Brg1/Brm (9, 10). Such an extensive array of cofactors with chromatin modifying activities suggests a tight link between NR-mediated transcription and chromatin state built on an intricate network of protein:protein interactions that can be regulated at different levels, thereby adjusting specific complex composition and transcriptional responses to a particular genetic and cellular environment.
Glucocorticoid receptor (GR) when bound to palindromic glucocorticoid response elements (GREs) on DNA interacts with all 3 p160 proteins to activate transcription (11). GR can also repress, in a hormone-dependent manner, a subset of genes via “tethering” GREs whereby GR does not bind DNA directly but is recruited through protein:protein interactions with unrelated transcription factors, e.g., AP1 or NFκB (reviewed in ref. 12). Unexpectedly, the p160 GRIP1 was found to participate in GR-mediated repression at tethering GREs (13). The repression domain (RD) responsible for GRIP1 corepressor activity is located between its NR interaction domain (NID) and activation domain (AD) 1 and does not resemble any known protein including other p160 family members (14). Interestingly, GRIP1 RD mediates the interactions between GRIP1 and transcriptional activator IRF3, suggesting that the RD activity is context-specific and not limited to corepression at tethering GREs (15).
To obtain further insight into the possible function of RD, we performed a yeast 2-hybrid screen with the GRIP1 RD-containing “bait.” In this screen, we repeatedly isolated a cDNA corresponding to the C terminus of the putative protein CGI-85 (16). Primary amino acid sequence analysis revealed that the N-terminal part of CGI-85 contained a SET domain typical of a histone methyltransferase (HMTase). Indeed, while this study was ongoing, CGI-85 was shown to specifically tri-methylate histone H4K20 and was therefore renamed Suv4-20h1 (4). Little is known about biological activities of Suv4-20h1. Tri-methylation of H4K20 is enriched within the silent loci and was implicated in the definition and maintenance of transcriptionally inactive heterochromatin at pericentromeric and telomeric regions (4, 17). The loss of H4K20–3Me, a common feature of leukemic cell lines, primary lymphomas and colorectal adenocarcinomas, is associated with increased genomic instability (18). The role of Suv4-20h1 in transcriptional regulation has not been reported.
Here, we dissect the GRIP1:Suv4-20h1 interaction in vitro and in vivo and examine its potential involvement in hormone-dependent transcriptional regulation by GR. Our studies identify Suv4-20h1 as a GRIP1-associated “secondary” cofactor for GR, thereby establishing the function of this HMTase outside of constitutive heterochromatin maintenance; instead, Suv4-20h1 appears to be an active modulator of hormone-dependent gene expression.
Results
Isolation of Suv4-20h1 in a Yeast 2-Hybrid System.
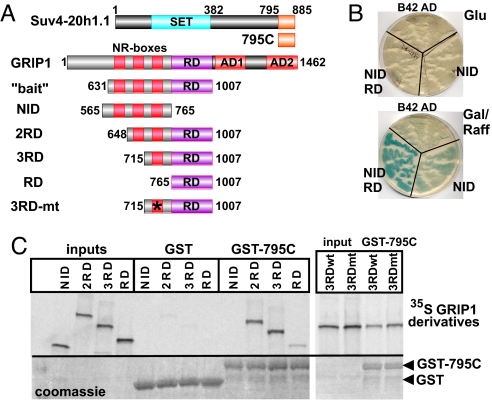
To identify GRIP1 RD-interacting factors, we performed a yeast 2-hybrid screen (15). Briefly, an LNCaP cell cDNA library fused to the LexA DNA Binding Domain (DBD) was screened with the NID-RD region of GRIP1 (amino acids 631-1007, Fig. 1A) fused to B42 AD. One cDNA isolated 13 times throughout the screen conferred a dark blue color to yeast colonies in the presence of galactose (when the GRIP1 bait was expressed) but not glucose, and contained a single ORF corresponding to LexA DBD fused to the C-terminal 91aa (Fig. 1A; hereafter, 795C) and the 3′ UTR of a factor termed CGI-85 (16), subsequently renamed Suv4-20h1 (4). This region is specific for Suv4-20h1 and bears no obvious structural or functional motifs. The fragment was reintroduced into yeast where it bound under galactose conditions, the GRIP1 NID-RD bait, but not a shorter NID (amino acids 631–765; Fig. 1 A and B), suggesting that the RD region (amino acids 765-1007) was required for binding.
Fig. 1.
GRIP1 and Suv4-20h1 interact in yeast and in vitro. (A) GRIP1 and Suv4-20h1 derivatives used in binding assays. Suv4-20h1 SET domain and the original yeast 2-hybrid isolate 795C are diagrammed. GRIP1 functional domains including NR boxes 1, 2, and 3, the repression domain (RD) and activation domains (AD) 1 and 2 are indicated. GRIP1 deletion mutants included the NID-RD bait, NID, 2RD, 3RD, RD and 3RD-mt (where asterisk denotes the NR box-3 LL → AA substitution). (B) The C-terminal fragment of Suv4-20h1.1 interacts with GRIP1 NID-RD in yeast. LexOP-driven LacZ reporter and Suv4–20 795C fused to LexA DBD were cotransformed into yeast with B42AD alone, fused to GRIP1 NID or NID-RD (as indicated) and replica-plated onto glucose (Upper) or galactose/raffinose (Lower) in the presence of X-Gal. (C) Suv4-20h1 795C interacts with GRIP1 in vitro. 35S-labeled GRIP1 derivatives (shown above the autoradiogram) were produced in vitro (“inputs”) and tested for their ability to interact with recombinant GST or GST-795C, as indicated. Equal amounts of GST or GST-795C in binding reactions were assessed by Coomassie blue staining (Lower).
Recombinant Suv4-20h1 795C Binds GRIP1 in Vitro.
To recapitulate GRIP1:Suv4-20h1 interactions in a purified in vitro system, we recombinantly expressed Suv4–20 795C in E. coli as a GST-fusion protein and produced in vitro several 35S-labeled derivatives of GRIP1 (Fig. 1C). The NID LxxLL motifs (NR boxes 1, 2 and 3) were progressively removed to create NID (amino acids 565–765), 2RD (amino acids 648-1007), 3RD (amino acids 715-1007) and RD (amino acids 765-1007) containing 3, 2, 1 or 0 LxxLL motifs, respectively (Fig. 1A). Immobilized GST-795C (but not GST alone) retained equally well 2RD and 3RD, and to a lesser degree RD (Fig. 1C). As in yeast 2-hybrid, no interactions were observed between GST-795C and NID. Furthermore, mutation of the 3rd LxxLL motif to LxxAA in the context of 3RD did not impair binding (Fig. 1C), suggesting that GRIP1 RD is a predominant site of interaction with Suv4-20h1 and is separable from NR box-3 responsible for binding GR.
Suv4-20h1.1 Is a Nuclear Chromatin-Associated Protein.
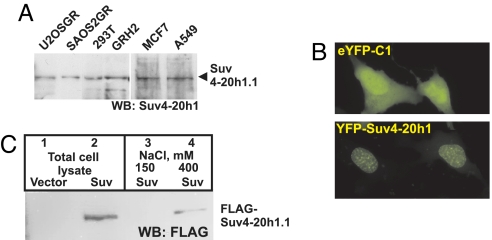
Suv4-20h1 is encoded by a single gene located on human chromosome 11. Two alternatively spliced mRNA variants transcribed from this gene give rise to the long (Suv4-20h1.1, 885aa, Fig. 2A) and short (Suv4-20h1.2, 393aa) isoforms (4) widely expressed in various human cell types. The most striking feature of the Suv4-20h1 proteins is a conserved SET domain (Fig. 1A) located in the N-terminal half common to both isoforms. SET domains, the hallmark of many N-methyltransferases, catalyze methyl group transfer from AdoMet to the ε-aminogroup of lysine residues in histone or nonhistone substrates (reviewed in ref. 19). The Suv4-20h1 yeast 2-hybrid isolate comprised the unique C terminus of the long Suv4-20h1.1 isoform (Fig. 1A).
Fig. 2.
Suv4-20h1 expression in mammalian cells. (A) Suv4-20h1 is a ubiquitous protein. Extracts from indicated cell lines were separated by SDS/PAGE and probed with antibodies to Suv4-20h1. (B) Suv4-20h1 resides in the nucleus. YFP or YFP-Suv4-20h1 were transfected into U2OS-GR cells and images were acquired 18 h later under fluorescent microscope with the FITC filter set. (C) Suv4-20h1 tightly associates with chromatin. U2OS-GR cells were transfected with FLAG-Suv4-20h1 (or “vector” control) and either boiled in 2× SDS sample buffer (total cell lysate) or extracted in a lysis buffer containing 150 or 400 mM NaCl, as indicated, before boiling in 2× SDS. Proteins were separated by SDS/PAGE and probed with anti-FLAG antibodies.
We reverse-transcribed and amplified the Suv4-20h1.1 cDNA from the total LNCaP cell RNA with the 5′ and 3′ UTR-specific primers and confirmed the sequence identity of the 2.9 kb PCR product. Suv4-20h1.1 cDNA was then fused to YFP and transfected into human U2OS-GR osteosarcoma cells (20). The resultant protein displayed nuclear localization (Fig. 2B) as predicted for the chromatin-binding factor.
FLAG-tagged Suv4-20h1.1 overexpressed in U2OS-GR cells was undetectable by immunoblotting after the extraction with normal salt (150 mM NaCl)-containing lysis buffer; yet, the protein was abundant in total cell lysate (Fig. 2C, lanes 3 vs. 2). Interestingly, high-salt (0.4 M NaCl) extraction began to recover FLAG-tagged protein (Fig. 2C, lane 4), consistent with reported chromatin internment of Suv4-20h1 (4) and resembling biochemical properties of other chromatin-associated proteins (21).
Suv4-20h1.1:GRIP1 Interactions in Mammalian Cells.
Given that Suv4.20h1 extraction required stringent conditions disrupting most protein:protein interactions, we used a transcriptional readout of the mammalian 2-hybrid system to test for Suv4-20h1.1:GRIP1 interactions in mammalian cells.
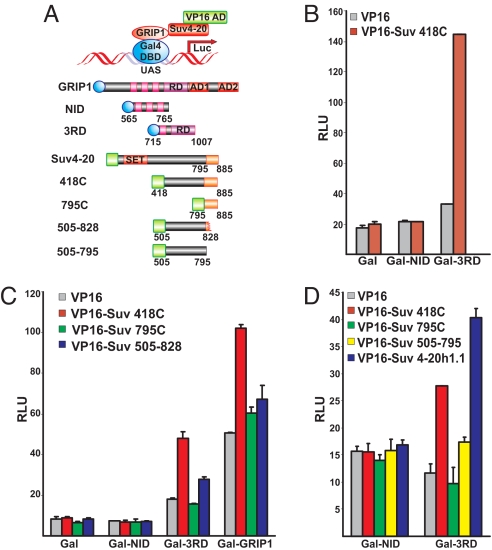
We fused a series of GRIP1 and Suv4-20h1.1 derivatives to the yeast Gal4 DBD and VP16 AD, respectively (Fig. 3A), which were cotransfected into U2OS-GR cells along with a UAS-controlled luciferase reporter. Gal-GRIP1 chimeric constructs included NID (which failed to bind Suv4-20h1 in yeast and in vitro), 3RD (which interacted with it well) and full-length GRIP1. In addition to the originally isolated Suv4–20 795C, we tested several larger derivatives: Suv4–20 418–885 (418C) representing the C-terminal half of the protein; 505–828 and 505–795 comprising the C-terminal half of Suv4-20h1.1 but lacking a part (57aa) or all (91aa) of our original yeast 2-hybrid isolate, and full-length Suv4-20h1.1 (Fig. 3A). Cotransfection of VP16–418C with Gal-3RD but not Gal-NID cells yielded a 5-fold increase in luciferase activity over the baseline of Gal alone (Fig. 3B) indicating that, consistent with the in vitro results (Fig. 1C), 3RD is sufficient for the interaction with Suv4-20h1 and that the C-terminal half of Suv4-20h1.1 contains the GRIP1-interacting surface. Similar results were obtained with VP16–418C and Gal-fused full-length GRIP1 (Fig. 3C, red bars), except that Gal-GRIP1 significantly activated transcription even in the absence of Suv4–20 (Fig. 3C, gray bars), as expected from the DNA-tethered coactivator. Surprisingly, VP16–795C failed to bind Gal-3RD and Gal-GRIP1 (Fig. 3C, green bars) suggesting that GRIP1:Suv4-20h1.1 interface in vivo includes or is stabilized by the Suv4-20h1 sequences in addition to its C-terminal 91aa. As expected, the VP16-Suv4–20 505–828 derivative lacking the C-terminal 57aa was ineffective at binding Gal-3RD and Gal-GRIP1 relative to the VP16–418C (Fig. 3C, blue vs. red bars). Hence, we conclude that the C-terminal 91aa region of the Suv4-20h1.1 is necessary but not sufficient for the GRIP1:Suv4-20h1.1 interaction in mammalian cells. Finally, Gal-3RD (and not Gal-NID) bound full-length Suv4-20h1.1 (Fig. 3D, blue bars), further validating interactions established in vitro and in yeast, and those observed with smaller fragments of each partner in mammalian cells.
Fig. 3.
Suv4-20h1.1 interacts with GRIP1 in mammalian cells. (A) A schematic of the mammalian 2-hybrid system with the diagrammed GRIP1 and Suv4-20h1 derivatives. Blue circles and green rectangles indicate Gal4 DBD and VP16 AD, respectively. (B) GRIP1 RD mediates the interaction with Suv4-20h1.1. U2OS-GR cells were cotransfected with Gal4 DBD alone or fused to NID or 3RD (Gal, Gal-NID and Gal-3RD), VP16 or VP16 418C, as indicated, a UAS-driven luciferase reporter and β-actin LacZ. Luciferase activity was assayed 16 h later, normalized to β-Gal activity and expressed as relative luminescence units (RLU). (C) The C-terminal 91aa of Suv4-20h1.1 are necessary but not sufficient for binding 3RD or full-length GRIP1 in U2OS cells. Indicated GRIP1 and Suv4-20h1 fragments were cotransfected into U2OS cells and luciferase activity was determined as in B. (D) Full-length Suv4-20h1.1 interacts with GRIP1 in U2OS cells. The assay was performed as in B.
Suv4-20h1.1 Attenuates Transcription from the GR-Inducible Integrated Reporter Construct.
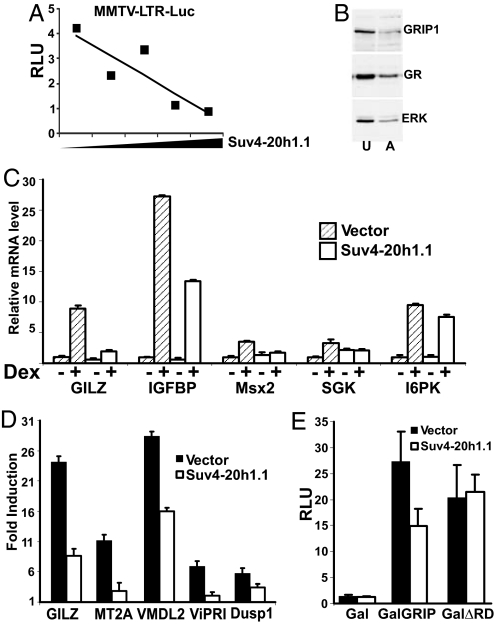
Suv4-20h1 has been studied exclusively as a factor involved in the maintenance of constitutive heterochromatin (4, 17). Because we isolated Suv4-20h1.1 as a GRIP1-interacting protein, and GRIP1 is an established NR coregulator rather than a chromatin remodeller, we focused on the possible role of Suv4-20h1.1 in NR signaling. We first examined whether overexpressing Suv4-20h1.1 in mammalian cells affects the activity of the transiently transfected glucocorticoid-inducible MMTV-Luc and XG46TL reporters containing idealized palindromic GREs. No consistent effect on GR-dependent activation was observed in U2OS, 293T or CV1 cells (data not shown). Because Suv4-20h1 normally functions in the context of chromatin and specifically alters its structure, we reasoned that this apparent lack of effect could be due to an improper chromatin environment surrounding the transiently transfected plasmids. To address this possibility, we used U2OS-GR-derived UL3 cells containing a stably integrated chromatinized glucocorticoid-responsive MMTV-LTR-Luc cassette (22). As expected, dexamethasone (Dex) treatment robustly activated the MMTV-LTR-Luc reporter; this induction was attenuated by the overexpressed Suv4-20h1.1 in a dose-dependent manner (Fig. 4A) suggesting that Suv4-20h1.1 may negatively regulate GR-dependent transcriptional activation in mammalian cells.
Fig. 4.
Overexpression of Suv4-20h1.1 antagonizes GR-dependent activation. (A) Suv4-20h1.1 abolishes glucocorticoid activation of the chromatinized GR-responsive reporter. UL3 cells containing an integrated MMTV-LTR-Luc reporter were transfected with increasing amounts of pCDNA3-Suv4-20h1.1 (balanced by “empty” pCDNA3) along with the β-actin LacZ normalization vector and induced with Dex for 18 h. Luciferase activity was assessed as in Fig. 2B. (B) GRIP1 and GR are abundantly expressed in U2OS-GR (U) and A549 (A) cells. Protein extracts were separated by SDS/PAGE and probed with antibodies to GRIP1, GR, or ERK as a loading control. (C) Suv4-20h1.1 overexpression antagonizes hormonal induction of endogenous GR targets in U2OS-GR cells. U2OS-GR cells were transfected with pCDNA3 vector or pCDNA3-Suv4-20h1.1 (400 ng per 3 × 105 cells) and 18 h later, treated with vehicle or 100 nM Dex for 2 h. Total RNA was isolated, reverse-transcribed and mRNA levels of indicated genes were measured by qPCR, using β-actin as normalization control. mRNA level of each gene in untreated cells transfected with pCDNA3 was set as 1. Error bars represent standard deviations of duplicated experiments calculated as in Applied Biosystems User Bulletin no. 2 (http://www3.appliedbiosystems.com/cms/groups/mcb_support/documents/generaldocuments/cms_040980.pdf). (D) Glucocorticoid induction of endogenous GR targets in A549 cells is diminished by Suv4-20h1.1 overexpression. Transfections were performed as in C, using 2 μg of DNA per 3 × 105 cells. qPCR data (collected as in C) were expressed as a fold induction of each mRNA in Dex-treated over untreated cells. (E) Suv4-20h1.1 inhibits GRIP1-mediated activation in heterologous context in GRIP1 RD-dependent manner. Gal4DBD alone (Gal) or fused to GRIP1 or ΔRD were transfected into U2OS-GR cells along with UAS-Luc reporter, β-actin LacZ (40 ng per 2 × 104 cells each) and pCDNA3 vector or pCDNA3-Suv4-20h1.1 (20 ng per 2 × 104 cells). Luciferase activity was assayed as in Fig. 3A.
Suv4-20h1.1 Antagonizes Glucocorticoid-Dependent Activation of GR Target Genes.
To determine whether Suv4-20h1.1 affects transcription of endogenous glucocorticoid-responsive genes, we chose the human cell lines U2OS-GR and A549. Both express GR and GRIP1 (Fig. 4B), but U2OS cells express stably integrated rat GR, whereas in A549 cells, GR is endogenous. In addition, the two cell lines are derived from different cell types (osteoblasts vs. lung epithelium) and have distinct repertoires of glucocorticoid-inducible genes (23), which enabled us to monitor the effect of Suv4-20h1.1 in different cellular environments. We introduced Suv4-20h1.1 into U2OS-GR or A549 cells and determined the mRNA levels of endogenous GR target genes in the absence or presence of Dex. As shown in Fig. 4C, Suv4-20h1.1 overexpression in U2OS-GR cells markedly decreased glucocorticoid induction of GILZ, IGFBP1, Msx2 and SGK, but not I6PK, whereas the “basal” levels of these mRNAs in untreated cells remained unaffected. Similarly, overexpression of Suv4-20h1.1 in A549 cells dampened hormonal response of GILZ, MT2A, VMDL2 and ViPRI, with DUSP1 displaying a more modest decrease (Fig. 4D). Hence, overexpressed Suv4-20h1.1 appears to antagonize glucocorticoid-dependent induction of a subset of GR targets in the two cell lines tested.
In contrast, no consistent pattern of regulation by Suv4-20h1.1 was observed on a diverse group of glucocorticoid-sensitive genes (cMyc, Fra, JunB, IL8, EphA1, PacI); it was difficult to uncouple the variable effects of this HMTase on their basal expression in resting cells vs. their glucocorticoid inhibition. Interestingly, when cells were activated with phorbol ester to mimic broad-spectrum proinflammatory signaling, the levels of induction or repression of these genes were completely unaffected by Suv4-20h1.1 (Fig. S1).
We used a heterologous system to evaluate whether GRIP1 coactivator properties per se were a target of Suv4-20h1.1. Transfection of Gal-GRIP1 into U2OS-GR cells led to a dramatic induction of UAS-luciferase reporter (Fig. 4E); strikingly, overexpression of wtSuv4-20h1.1 attenuated this activation by 40–50%. Furthermore, deletion of GRIP1 RD, the major site of Suv4-20h1.1 binding, did not alter GRIP1-mediated activation but abolished the inhibitory effect of this HMTase (Fig. 4E). These data suggest that Suv4-20h1.1 specifically binds GRIP1 via RD and interferes with its activation functions.
Suv4-20h1.1 Depletion Potentiates Hormonal Induction of a Subset of GR Target Genes.
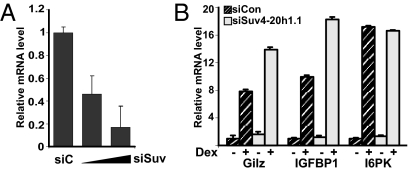
To rule out that the observed effects of Suv4-20h1 on GR transcriptional regulation were due to overexpression and do not occur under physiological concentration of this protein, we siRNA-depleted the endogenous Suv4-20h1.1 in U2OS-GR cells. The siRNA targeted a sequence specific to the long Suv4-20h1.1 splice variant to preserve the function of the Suv4-20h1.2 short isoform, which does not bind GRIP1 and is therefore not predicted to affect GR action. We obtained most consistent results with 60–80% knockdown of Suv4-20h1.1 mRNA and no off-target effects on GRIP1 or GR expression (Fig. 5A and Fig. S2); greater down-regulation adversely affected cell viability and basal levels of multiple mRNAs. As shown in Fig. 5B, depletion of endogenous Suv4-20h1.1 resulted in superinduction of GILZ and IGFBP1, but not I6PK (the latter was similarly unaffected by Suv4-20h1.1 overexpression, Fig. 4C). Thus, overexpression and knockdown of Suv4-20h1.1 exert reciprocal effects on glucocorticoid-activated gene expression, and the same subset of GR target genes is sensitive to the alterations of the Suv4-20h1.1 levels in a cell.
Fig. 5.
Depletion of endogenous Suv4-20h1.1 potentiates hormonal induction of a subset of GR target genes. U2OS-GR cells were nucleofected with scrambled (siC, 1 μg per 2 × 106 cells) or Suv4-20h1.1-specific (siSuv; 1 or 2 μg per 2 × 106 cells) siRNA. Twenty-four hours later, cells were treated with 10 nM Dex for 2 h, as indicated, and the expression of Suv4-20h1.1 (A) and IGFBP1, GILZ, and I6PK (B) was analyzed as in Fig. 4C. In A, relative amount of Suv4-20h1.1 mRNA in siC-transfected cells is set as 1. In B, mRNA level of each gene in untreated cells transfected with siCon is set as 1.
Suv4-20h1.1 Effects on GR Transcriptional Regulation Require Interaction with GRIP1 and Catalytic Activity.
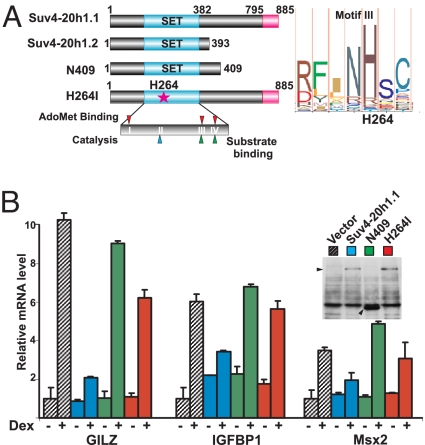
Suv4-20h1 is a multifunctional protein that contains a histone methyltransferase SET domain near the N terminus and a GRIP1 interacting domain at the C terminus. We demonstrated that GRIP1 and Suv4-20h1.1 interact in vitro and in cells (Figs. 1–3), and that Suv4-20h1.1 antagonizes the hormone inducibility of endogenous GR targets and specifically interferes with GRIP1-dependent transcriptional activation (Figs. 4 and 5). To establish a mechanistic link between these observations, we deleted the Suv4-20h1.1 C-terminal half including the GRIP1 interaction domain, thereby creating a derivative reminiscent of the 393aa Suv4-20h1.2 isoform (Suv4-20h1 N409; Fig. 6A), and assessed the effect of its overexpression on GR-mediated transcription. As shown in Fig. 6B, whereas full-length Suv4-20h1.1 nearly abolished Dex induction of GILZ, IGFBP1 and MSX2, N409 failed to do so (blue vs. green bars) suggesting that the GRIP1-interacting domain is required for inhibition.
Fig. 6.
GRIP1 binding and catalytic activities are required for Suv4-20h1-mediated regulation of GR target genes. (A) Suv4-20h1 long and short isoforms, the mutant proteins deficient in GRIP1 binding (N409) or catalysis (H264I), and the SET domain core with 4 conserved motifs (I-IV) are shown on the Left. We replaced a conserved histidine in motif III (H264, Right), involved in AdoMet binding with isoleucine. (B) U2OS-GR cells were transfected with an empty vector, wt Suv4-20h1, or its N409 or H264I mutants, as indicated. Twenty hours later, cells were treated with Dex for 2 h as shown, and the expression of indicated GR target genes were assessed as in Fig. 4C. Ectopic expression of Suv4-20h1.1 derivatives was assessed by anti-Flag immunoblotting (Inset).
HMTases activate or repress transcription by covalently modifying histones or associated factors. To address the importance of Suv4-20h1.1 enzymatic activity for the observed effects on GR-dependent transcriptional regulation, we mutated 2 conserved amino acids in its SET domain: H264 (Fig. 6A) and E293, which are essential for the AdoMet cofactor and substrate binding, respectively. Mutation of either residue abolishes methyltransferase activity in other SET domain HMTases (3). We found that overexpression of either H264I (Fig. 6B, red bars) or E293A (data not shown) had little effect on Dex-inducible genes in U2OS-GR cells. Similar results were obtained with the Suv4-20h1.1 deletion mutant lacking SET domain core (amino acids 183–271, Fig. S3), suggesting that the catalytic activity of Suv4-20h1.1 is required for the antagonism with GR.
Discussion
Chromatin modifying enzymes alter DNA accessibility and affect numerous cellular activities including transcription, DNA replication and repair. HMTases were discovered as large-scale chromatin modifiers promoting the assembly and spread of transcriptionally silent heterochromatin (3). Later, however, it became apparent that the functions of these enzymes are not confined to the maintenance of perpetually inactive chromatin domains. Several HMTases have been implicated in transcriptional regulation. For example Suv39h1, a prototypic enzyme responsible for heterochromatin organization and spread by methylating H3K9 (3), is involved in Rb protein-dependent silencing of S-phase specific genes and in gene repression by unliganded thyroid hormone receptors (24, 25). The E(Z) complex containing HMTases of the Polycomb family that methylate H3K27 and mediate X-chromosome inactivation, were found to repress Hox genes during development (2). Our study demonstrates that the H4K20 HMTase Suv4-20h1 has a role unrelated to heterochromatin maintenance, as a GRIP1-associated NR coregulator.
GRIP1 binds NRs in a ligand-dependent manner and can potentiate or inhibit transcription depending on the gene, promoter organization and signaling environment. GRIP1 appears to have no enzymatic activities of its own, and secondary cofactors recruited by GRIP1 (CBP/p300, CoCoA, CARM1) mediate primarily transcriptional activation. Hence, its association with an inhibitory HMTase was surprising; even less expected was the fact that Suv4-20h1.1 in conjunction with GRIP1 dramatically inhibited transcriptional activation of several conventional GR-inducible genes. Notably, basal levels of these transcripts and the expression of housekeeping controls (actin and Rpl19) were unaffected by overexpressed Suv4-20h1, suggesting that it did not cause a general transcriptional shutdown and was specifically recruited as a consequence of ligand-dependent recruitment of GRIP1. Further corroborating specificity, inhibition of GR-mediated activation by Suv4-20h1 required its GRIP1 interacting domain, depletion of endogenous Suv4-20h1 had a reciprocal effect on GR-inducible genes and, finally, only a subset of GR targets was affected by either overexpression or knockdown.
These observations raise an intriguing question: What is the function of the inhibitory HMTase Suv4-20h1.1 in GR activation complexes? It is tempting to speculate that a repressor incorporated into such a complex is part of a negative feedback loop that prevents exaggerated response to ligand or enables rapid cessation of transcription upon ligand withdrawal or an unrelated signaling event. A similar mechanism has been proposed for the regulation of ARG1 transcription in yeast. The expression of ARG1 is induced by the transcriptional activator Gcn4p in response to amino acid starvation and inhibited by the ArgM/Mcm1p repressor in the presence of arginine (26). Curiously, ArgM/Mcm1 is constitutively recruited to the ARG1 promoter by Gcn4p (26), thus ensuring a balanced, near-quantitative response to the concentration of arginine in the environment.
The exact mechanism engaged by Suv4-20h1.1 to affect GR transcriptional activity is unclear. Regulation of transcription involves multiprotein complexes whose ultimate function may not rely on the primary activities of participating factors. For example, the G9a HMTase typically confers repression yet, when recruited by GRIP1:CARM1, it serves as a coactivator and its enzymatic function is dispensable (27). In our study, deletion of the SET domain core or mutation of the conserved H264 involved in catalysis impairs the ability of Suv4-20h1.1 to attenuate transcription of GR-inducible genes. Hence, the Suv4-20h1.1 catalytic activity is required for its antagonism with GR; however, the target of this activity remains to be determined. H4K20, the only established Suv4-20h1 substrate, is typically not tri-methylated in transcriptionally active domains, and our preliminary studies did not reveal appreciable levels of H4K20-Me3 at glucocorticoid-inducible loci (data not shown). Recent findings, however, indicate that short heterochromatic regions enriched for H4K20-Me3 can be found within actively transcribed genes (24, 28). Alternatively, Suv4-20h1 could modify nonhistone factors. Indeed, CARM1 that methylates H3R17 also targets specific arginine residues in CBP/p300 (29, 30). The related enzyme PRMT1 methylates H4R3 (31) and also modifies the PolyA-binding protein NAB2p (32). The H3K9 HMTase G9a is capable of automethylation (33). Finally, Set9 and Smyd2, the H3K4 and H3K36 HMTases, methylate distinct residues in p53, which results in opposite effects on its transcriptional activity (34, 35). Further analysis is needed to establish whether H4K20 and/or other factors are modified by Suv4-20h1.1 in the context of GR target genes.
Notably, although GRIP1 likely serves as a primary determinant of selective Suv4-20h1.1 recruitment into only a subset GR regulatory complexes, the availability of potential Suv4-20h1.1 substrates may itself predispose a particular gene for being regulated by this HMTase. An additional layer of specificity could be provided by the interactions between Suv4-20h1 and other cofactors bound at the site potentially stabilizing the GRIP1:Suv4-20h1.1 complex and bringing Suv4-20h1 in proximity to its substrates. The promoter architecture, type and sequence of a given GRE will collectively promote the recruitment of such cofactors and substrates, ultimately enabling the multivalent interactions within a repressosome in a gene-specific manner (36).
It is becoming increasingly apparent that many activators and repressors can carry out either function depending on a gene or even reverse their activities at the same promoter in response to a particular signaling cue. Such a duality of functions presents a formidable challenge when one seeks to affect one activity without altering the other. The ability of GR to attenuate transcription of cytokines, chemokines and other proinflammatory mediators underlies the widespread use of glucocorticoids as a therapy for inflammation and autoimmunity (12, 37). Conversely, a number of debilitating side effects associated with excessive glucocorticoid exposure have been traditionally attributed to the transcriptional activation by GR (37). Even though such a polar view is a vast oversimplification, a lack of solid mechanistic criteria distinguishing the two activities obstructs drug design efforts. Not surprisingly, cofactors are increasingly recognized not only as predictors of GR actions but also as potentially “druggable” targets, whose interaction with defined molecular surfaces within GR regulatory complexes can be modified therapeutically. Thus, the identification of Suv4-20h1.1 as part of GR regulatory circuitry uncovers a new link between chromatin and hormone-regulated transcription and an unexpected molecular interface to be dissected with respect to modulating GR-dependent gene expression.
Methods
Plasmid Construction.
See Sl Methods.
Yeast 2-Hybrid Set-Up.
The modified yeast 2-hybrid screen was as described in ref. 15. In brief, the EGY188 (trp1 his3 ura3 leu2) strain of S. cerevisiae with a chromosomally integrated leucine reporter gene driven by a single LexA operator was transformed with pJG4-5-GRIP1 NID-RD bait, pEG202-LNCaP prostate cell cDNA library and pJK103 β-galactosidase reporter gene (controlled by galactose-inducible GAL1–10 promoter, constitutive ADH promoter and a single LexA operator, respectively). Positive clones grew on leucine-deficient plates and turned blue on chromogenic X-Gal substrate in the presence of galactose but not glucose. pEG202-Suv4-20h1.1 795C isolate was retransformed into yeast and tested against pJG4-5, pJG4-5 GRIP1 NID-RD or pJG4-5 GRIP1 NID in the presence of glucose or galactose.
In Vitro Binding Assays.
GST pull-down assays were performed as in ref. 15.
Cell Culture, Transfections, and siRNA.
Human U2OS-GR, Saos2-GR (20) and UL3 (22) osteosarcoma, A549 lung carcinoma, 293T embryonic kidney, MCF7 breast cancer cells, Grh2 rat hepatoma, and CV1 monkey kidney fibroblasts were maintained in DMEM (Invitrogen) with 10% FBS (HyClone), supplemented with 350 μg/ml G418 (Invitrogen; U2OS-GR, Saos2-GR, and UL3) and 1 μg/ml puromycin (Invivogene; UL3). U2OS-GR cell transfections were performed using lipofectamine-PLUS reagent (Invitrogen) as described (14, 23).
For Suv4-20h1.1 siRNA, 106 U2OS-GR cells were nucleofected with 1 or 2 μg of annealed RNA duplex r(CGAUAACAAUCUCUAUGUA)dTdT with kit V AMAXA transfection system, treated with Dex 24 h later and harvested for RNA preparation.
Cell Imaging, Immunoblotting, RNA Isolation and Real-Time PCR.
See Sl Methods.
Supplementary Material
Acknowledgments.
We thank C. Pantoja and M. Garabedian for help with the yeast 2-hybrid screen, M. Reily and M. Kennedy for technical assistance, and J. Flammer and A. Tulin for critical comments on the manuscript. This work was supported by National Institutes of Health Grants T32 AR07517 (to Y.C.), AI068820 (to I.R.), and CA20535 (K.R.Y.); a Leukemia and Lymphoma Society Special Fellowship (to I.R.), and Lupus Research Institute grants (to I.R.). M.A.S. and A.R.C. were Gateways to The Laboratory Undergraduate Fellows at Weill Cornell.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810863105/DCSupplemental.
References
- 1.Ruthenburg AJ, Li H, Patel DJ, Allis CD. Multivalent engagement of chromatin modifications by linked binding modules. Nat Rev Mol Cell Biol. 2007;8:983–994. doi: 10.1038/nrm2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao R, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 3.Rea S, et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- 4.Schotta G, et al. A silencing pathway to induce H3–K9 and H4–K20 trimethylation at constitutive heterochromatin. Genes Dev. 2004;18:1251–1262. doi: 10.1101/gad.300704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishioka K, et al. Set9, a novel histone H3 methyltransferase that facilitates transcription by precluding histone tail modifications required for heterochromatin formation. Genes Dev. 2002;16:479–489. doi: 10.1101/gad.967202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang XJ, Seto E. HATs and HDACs: From structure, function and regulation to novel strategies for therapy and prevention. Oncogene. 2007;26:5310–5318. doi: 10.1038/sj.onc.1210599. [DOI] [PubMed] [Google Scholar]
- 7.Sassone-Corsi P, et al. Requirement of Rsk-2 for epidermal growth factor-activated phosphorylation of histone H3. Science. 1999;285:886–891. doi: 10.1126/science.285.5429.886. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Wissmann M, et al. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat Cell Biol. 2007;9:347–353. doi: 10.1038/ncb1546. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: A coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- 11.Xu J, Li Q. Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol. 2003;17:1681–1692. doi: 10.1210/me.2003-0116. [DOI] [PubMed] [Google Scholar]
- 12.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: Molecular mechanisms for gene repression. Endocr Rev. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 13.Rogatsky I, Zarember KA, Yamamoto KR. Factor recruitment and TIF2/GRIP1 corepressor activity at a collagenase-3 response element that mediates regulation by phorbol esters and hormones. EMBO J. 2001;20:6071–6083. doi: 10.1093/emboj/20.21.6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogatsky I, Luecke HF, Leitman DC, Yamamoto KR. Alternate surfaces of transcriptional coregulator GRIP1 function in different glucocorticoid receptor activation and repression contexts. Proc Natl Acad Sci USA. 2002;99:16701–16706. doi: 10.1073/pnas.262671599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reily MM, Pantoja C, Hu X, Chinenov Y, Rogatsky I. The GRIP1:IRF3 interaction as a target for glucocorticoid receptor-mediated immunosuppression. EMBO J. 2006;25:108–117. doi: 10.1038/sj.emboj.7600919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Twells RC, et al. The sequence and gene characterization of a 400-kb candidate region for IDDM4 on chromosome 11q13. Genomics. 2001;72:231–242. doi: 10.1006/geno.2000.6492. [DOI] [PubMed] [Google Scholar]
- 17.Benetti R, et al. Suv4-20h deficiency results in telomere elongation and derepression of telomere recombination. J Cell Biol. 2007;178:925–936. doi: 10.1083/jcb.200703081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraga MF, et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet. 2005;37:391–400. doi: 10.1038/ng1531. [DOI] [PubMed] [Google Scholar]
- 19.Cheng X, Collins RE, Zhang X. Structural and sequence motifs of protein (histone) methylation enzymes. Annu Rev Biophys Biomol Struct. 2005;34:267–294. doi: 10.1146/annurev.biophys.34.040204.144452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogatsky I, Trowbridge JM, Garabedian MJ. Glucocorticoid receptor-mediated cell cycle arrest is achieved through distinct cell-specific transcriptional regulatory mechanisms. Mol Cell Biol. 1997;17:3181–3193. doi: 10.1128/mcb.17.6.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kellum R, Raff JW, Alberts BM. Heterochromatin protein 1 distribution during development and during the cell cycle in Drosophila embryos. J Cell Sci. 1995;108:1407–1418. doi: 10.1242/jcs.108.4.1407. [DOI] [PubMed] [Google Scholar]
- 22.Fryer CJ, Kinyamu HK, Rogatsky I, Garabedian MJ, Archer TK. Selective activation of the glucocorticoid receptor by steroid antagonists in human breast cancer and osteosarcoma cells. J Biol Chem. 2000;275:17771–17777. doi: 10.1074/jbc.M908729199. [DOI] [PubMed] [Google Scholar]
- 23.Rogatsky I, et al. Target-specific utilization of transcriptional regulatory surfaces by the glucocorticoid receptor. Proc Natl Acad Sci USA. 2003;100:13845–13850. doi: 10.1073/pnas.2336092100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen SJ, et al. Rb targets histone H3 methylation and HP1 to promoters. Nature. 2001;412:561–565. doi: 10.1038/35087620. [DOI] [PubMed] [Google Scholar]
- 25.Li J, et al. Involvement of histone methylation and phosphorylation in regulation of transcription by thyroid hormone receptor. Mol Cell Biol. 2002;22:5688–5697. doi: 10.1128/MCB.22.16.5688-5697.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon S, et al. Recruitment of the ArgR/Mcm1p repressor is stimulated by the activator Gcn4p: A self-checking activation mechanism. Proc Natl Acad Sci USA. 2004;101:11713–11718. doi: 10.1073/pnas.0404652101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee DY, Northrop JP, Kuo MH, Stallcup MR. Histone H3 lysine 9 methyltransferase G9a is a transcriptional coactivator for nuclear receptors. J Biol Chem. 2006;281:8476–8485. doi: 10.1074/jbc.M511093200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regha K, et al. Active and repressive chromatin are interspersed without spreading in an imprinted gene cluster in the mammalian genome. Mol Cell. 2007;27:353–366. doi: 10.1016/j.molcel.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu W, et al. A transcriptional switch mediated by cofactor methylation. Science. 2001;294:2507–2511. doi: 10.1126/science.1065961. [DOI] [PubMed] [Google Scholar]
- 30.Lee YH, Coonrod SA, Kraus WL, Jelinek MA, Stallcup MR. Regulation of coactivator complex assembly and function by protein arginine methylation and demethylimination. Proc Natl Acad Sci USA. 2005;102:3611–3616. doi: 10.1073/pnas.0407159102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strahl BD, et al. Methylation of histone H4 at arginine 3 occurs in vivo and is mediated by the nuclear receptor coactivator PRMT1. Curr Biol. 2001;11:996–1000. doi: 10.1016/s0960-9822(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 32.Lee J, Bedford MT. PABP1 identified as an arginine methyltransferase substrate using high-density protein arrays. EMBO Rep. 2002;3:268–273. doi: 10.1093/embo-reports/kvf052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chin HG, et al. Automethylation of G9a and its implication in wider substrate specificity and HP1 binding. Nucleic Acids Res. 2007;35:7313–7323. doi: 10.1093/nar/gkm726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chuikov S, et al. Regulation of p53 activity through lysine methylation. Nature. 2004;432:353–360. doi: 10.1038/nature03117. [DOI] [PubMed] [Google Scholar]
- 35.Huang J, et al. Repression of p53 activity by Smyd2-mediated methylation. Nature. 2006;444:629–632. doi: 10.1038/nature05287. [DOI] [PubMed] [Google Scholar]
- 36.Ratnaparkhi GS, Jia S, Courey AJ. Uncoupling dorsal-mediated activation from dorsal-mediated repression in the Drosophila embryo. Development. 2006;133:4409–4414. doi: 10.1242/dev.02643. [DOI] [PubMed] [Google Scholar]
- 37.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 38.So AY, Chaivorapol C, Bolton EC, Li H, Yamamoto KR. Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. PLoS Genet. 2007;3:e94. doi: 10.1371/journal.pgen.0030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.