I. Introduction
Intensive care unit (ICU) teams are a critical part of the solid organ transplant process. Although kidney transplant recipients usually do not require recovery time in the ICU, virtually all other solid organ recipients receive care from these teams at some point either pre- or post-transplantation. The ICU team is essential in the preparation, stabilization, and recovery of patients undergoing these extraordinary surgical procedures. In addition transplant recipients may experience medical decompensation requiring ICU treatment years following the initial transplant hospitalization. The psychosocial issues involved during these critical periods of transplantation are important for intensive care physicians and clinicians to understand in order to provide comprehensive care to transplant patients.
In this paper we will provide a brief overview of transplant epidemiology, followed by a review of the psychosocial issues relevant to the phases of the transplant process. We will consider the pre-transplant evaluation phase, psychiatric disorders in transplant patients, and cognitive impairments and delirium with additional issues specific to particular organs. In addition we will cover the side effects of immunosuppressive medications and special issues arising with living donors. The relevance of these issues to ICU care will be emphasized.
II. Epidemiology of Organ Transplantation in the United States
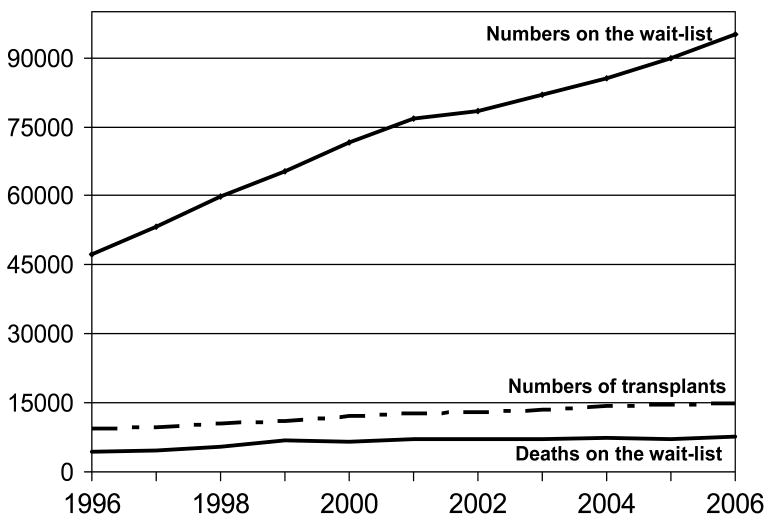
For most organ types the numbers of candidates added to the wait list each year exceeds the numbers receiving transplants (see figure 1) [1]. In some areas (e.g. kidney, liver, lung transplantation) living organ donation has become one option to address the organ shortage (see section on special issues in living donors below). Without an identified living donor, transplant candidates routinely wait for years for an organ, and living donation is not a possibility for all types of transplantation (e.g., heart). For all major organ types over 40% of US wait-listed candidates waited 2 years or more for an organ [2]. While only 0.5% become medically unsuitable and are removed from the wait-list, 2% refuse transplant after being wait-listed, and 10–18% die on the wait-list [2].
Figure 1.
Waiting List Statistics in the United States: 1996 – 2006
While the majority of transplant candidates are not in the ICU prior to transplantation, the ICU staff will occasionally care for critically ill transplant candidates on the wait list (see pre-transplant section below). For example, the highest transplant status listing for liver and heart transplant candidates is defined as requiring critical care and these patients have the highest priority to receive donated organs. For liver candidates <0.01% are in the highest status (status 1A or B). Less than 10% of heart candidates have the status (status 1A). Of the Status 1 liver candidates (fulminant failure not expected to survive 7 days) over 50% will receive an organ within a week and 10% will die. Of the status 1A heart candidates 37% will be transplanted within 30 days while 11% will die within that time [2]. For lung transplant candidates respiratory failure requiring continuous mechanical ventilation is only a relative contraindication to lung transplantation and the allocation of lungs depends on a complex algorithm of which mechanical ventilation is only one factor.
Following transplantation, recipients of living liver and kidney grafts show the highest long term survival rates (76% alive 10 years post-transplantation) with deceased liver and heart recipients having somewhat lower 10-year survival (59% and 53% respectively) and lung and intestine recipients have the poorest 10-year survival (41% and 26% respectively)[2]. However, these survival statistics are from transplants performed over 10 years ago and advances in technology, immunosuppression, and medical care have improved the survival rates over time. Graft survival rates can be significantly lower than patient survival rates (e.g. 43% for kidney graft survival and 52% for liver graft survival after 10 years), demonstrating that many transplant recipients could face re-transplantation 5–10 years after their first organ [2] or eventually may require kidney transplantation due to the chronic use of nephrotoxic immunosuppressive medications.
III. Pre-Transplant Period
A. Transplant Evaluation
The primary goal of a pre-transplant psychosocial evaluation is to determine whether a patient has physiological or psychosocial characteristics that may negatively affect post-transplant outcomes (Table 1). Psychosocial factors include cognitive, behavioral, psychological, and social issues which may interfere with adjustment to transplantation or ability to adhere to post-transplant medical directives. Many candidates present with at least a few psychosocial issues that will require additional attention. Early identification of these issues during the pre-transplant evaluation allows transplant teams the opportunity to develop treatment plans which minimize any negative impact of these factors, while also optimizing patient and caregiver preparedness for transplantation.
Table 1.
Purpose and Goals of A Transplant Psychosocial Evaluation
|
In an optimal situation, the psychosocial evaluation will consist of a thorough patient interview exploring a variety of issues relevant to transplantation (Table 2). Family members or other caregiving individuals, who will help to provide care to the patient following transplantation, may also be interviewed. ICU staff can assist in the pre-transplant information gathering process. During their own interviews with patients and families about ICU care they may learn important aspects of how the patient and family are dealing with the immediate stresses and preparing for the future. They may also identify the patient’s and family’s level of sophistication with medical information. In addition, the ICU staff can provide valuable information about a patient’s symptoms and behaviors while in the ICU along with observations about the availability and appropriateness of family/caregiver support. In some cases, the patient will not be able to be interviewed and the psychosocial evaluator will have to rely solely on other sources (family, caregivers, medical care providers, and records) to gather relevant information (see cognitive functioning and acute/fulminate organ failure sections below).
Table 2.
Content of A Comprehensive Psychosocial Evaluation
|
B. Acute/Fulminant Organ Failure
Under conditions such as fulminant liver failure or acute cardiomyopathy, patients may require emergent evaluation for transplantation. In these situations, patients often play a minimal role in their evaluation due to the presence of stupor, coma, or mechanical ventilation. Both patients and family members may be overwhelmed with the seriousness of the situation, as well as the task of having to learn and decide about transplantation.
For patients with fulminant hepatic failure from acetaminophen (representing 96% transplants due to acute drug induced hepatotoxicity) [3] or other toxic ingestion/overdose, a thorough psychiatric evaluation is necessary to determine whether the overdose was accidental or intentional. Details regarding the ingestion, prior history of suicide attempts or other self-destructive behaviors, substance abuse, psychiatric disorders, current stressors, and other risk factors for future suicide attempts must be obtained. Many of these patients will recover to the point of avoiding transplant, but a small group proceeds on to transplant and require careful consideration about their candidacy.
C. Decisions to List Patients and Potential Dilemmas
Following completion of the pre-transplant evaluation, all information and test results are reviewed, often in a transplant team meeting, in order to decide whether a patient can be listed for transplantation. For patients with significant psychosocial risk factors, transplant teams may request that additional requirements (e.g. addiction counseling, psychiatric treatment, behavioral changes, establishing an adequate support system) be met as a condition to being listed for transplantation. In some cases, patients will not be able to complete these requirements due to becoming too ill or will die while attempting to meet candidacy requirements. This is especially likely for patients being evaluated for transplantation while in the ICU. At times, differences in opinion among transplant team members as well as other healthcare providers arise regarding a particular patient’s candidacy for transplantation. Resolution of these differences requires open discussion among team members and others involved in the patient’s care. These discussions not only offer an opportunity to resolve differences, but help to ease the anxiety and discomfort that accompanies declining a patient for transplantation. Additional consultations with medical ethics teams, risk management, and the hospital’s legal department may be necessary and instructive with difficult cases (e.g. when a candidate or his/her family is challenging candidacy requirements or the candidacy decision of the transplant team). Thorough documentation is essential to delineate the specific issues involved, expectations of the team for transplantation candidacy, and efforts to work with the patient and/or family.
D. Specific Coping Challenges During the Pre-Transplant Period
1. Waiting Period
After patients are listed for transplantation, they may experience a period of elation and relief. Following this, new concerns arise as the realities of the waiting period become evident. Many patients and their families perceive the wait period to be the most psychologically stressful part of the transplant experience. This stress is especially heightened if the candidates are waiting in the ICU. Patients and their families must endure the uncertainty of whether a donor organ will arrive in time and the degree of medical deterioration or loss of functioning the patient will experience before transplantation. For some, health continues a slow decline, while others suffer through repeated exacerbations or rapid progression of their disease. Some will experience recurrent hospitalizations or prolonged stays in an ICU until a donor organ becomes available. Helping patients to weather the uncertainty of the waiting period requires healthcare providers to be aware of the stresses unique to this stage of the transplant process.
2. Preparing for Death/Maintaining Hope
The realization that patients listed for transplantation are also facing terminal illness is often overshadowed by the focus on continued medical care and the pursuit of a donor organ. Patients, families, transplant teams, and other healthcare providers may overlook or delay discussions on issues relating to end-of-life care such as living wills, power-of-attorneys, palliative care, and do-not-resuscitate orders [4,5]. Instead, staff energy is often directed at the stabilization and preparation of patients for transplant surgery and post-operative care. Patients and families may resist attempts to address end-of-life issues, partly due to denial. They may also feel that acknowledgement of these issues reflects a sense of hopelessness about transplantation or that the transplant team has become less committed to the pursuit of an organ for them. These concerns can be addressed in collaboration with the transplant team with respect to balancing a hopeful outlook with appropriate acknowledgment of the potential for an undesired outcome [6, 7]. Additionally, wait-listed candidates may develop medical contraindications to transplantation (e.g. infection, serious stroke or brain damage, hemodynamic instability) and both patient and family should be made aware that their eligibility might change over time for many reasons. By encouraging timely discussion about end-of-life care, patients can also be allowed the opportunity to take an active role in directing their care at a time they are still well enough to do so. Psychological or spiritual/pastoral counseling may help patients and families negotiate these transitions and prepare them for either transplantation or death.
3. Patient/Family Jealousy
During the course of the waiting period, it is not unusual for listed patients to become acquainted with one another during clinic visits and hospital stays. This familiarity can be beneficial to both patients and family members, serving as an additional source of information and support. This can be especially true when the patient is waiting in the ICU and their family members interact with other families in the ICU waiting areas. Nonetheless, this familiarity can become problematic as patients become sicker and the wait for a donor organ more desperate. Inevitably, one patient will undergo transplantation before another, which may raise feelings of jealousy among patients and families still waiting. These feelings may be unexpected, but are understandable in the context of the life-or-death nature of transplantation. In some situations, jealousy may manifest as questions about ranking on the organ wait list, how donor organs are assigned, or body size or blood type requirements. Or it many emerge in the form of renewed frustrations and fears about the ongoing waiting period [8]. Acknowledging these feelings and answering questions can be beneficial for patients and families, though care must be taken not to share confidential information about other patients.
IV. Psychiatric Disorders Affecting Organ Transplant Patients
Similar to other medically ill populations, organ transplant candidates and recipients are at elevated risk for significant psychiatric symptoms and diagnosable psychiatric disorders. The development of psychiatric symptoms in transplant patients can reflect the exacerbation of a pre-existing disorder or the development of a new onset disorder. Mood and anxiety-related disorders are the most common psychiatric illnesses observed both pre- and post-transplant, although delirium and cognitive impairment are also often experienced by many transplant patients in the peri-operative period. In subpopulations of transplant recipients with histories of substance abuse or dependence (e.g. patients with alcoholic liver disease or hepatitis C) the risk for relapse remains a concern both pre- and post-transplant. There has been increased recognition that post-traumatic stress disorder may result from traumatic experiences related to the transplant and/or the ICU stay. There is mounting evidence that each of these classes of psychiatric disorders can affect patient health and psychological outcomes after organ transplantation.
The ICU staff plays an essential role in the identification of psychiatric symptomatology and psychiatric consultants typically rely on the ICU staffs input about these issues. The ICU staffs’ round-the-clock observations of the patient’s behaviors and affective and cognitive states provide the data from which diagnoses can be made and treatment decided. In addition their observations of the patient’s sleep/wake cycles, physical and motoric activity, appetite and eating, provide evidence of important neurovegetative symptoms common to many psychiatric disorders. Patient’s interactions with family and staff are also important to note. Patients and families may voice concerns to the ICU staff that they may feel reluctant to discuss with the transplant team. These concerns may reveal important aspects of their psychological and affective states, sense of hopefulness, and their readiness to either pursue transplantation or engage in the post-transplant recovery/rehabilitation process. The following sections review the prevalence, presentation, and issues relevant to psychiatric disorders in transplant populations. Treatment of these disorders is discussed in a further section below.
A. Mood disorders – Depression and Anxiety
Comorbid psychiatric disorders are common among medically ill transplant candidates: as many as 25% of patients with advanced pulmonary disease, 40% of patients with advanced hepatic disease, and 50% of patients with advanced cardiac disease experience anxiety or depressive disorders [9–11]. Following transplant up to 20% of kidney recipients, 30% of liver recipients and 63% of heart recipients have been found to develop these disorders especially during the first post-transplant year [12–15]. Some anxiety disorders (e.g., panic disorder) appear to be more common both before and after transplantation in patients with end-stage lung disease compared to patients with other types of end-stage organ diseases [14, 16, 17].
In addition to the multiple psychosocial stressors facing these patients (e.g. reduced quality of life, disability, financial pressures), medications and physiologic impairment (e.g. electrolyte imbalance, thyroid disorders, and nutritional deficiencies) can produce secondary psychiatric symptomatology. Among patients evaluated for transplantation, many will be psychologically worn down by the effects of worsening chronic disease. Others with acute failure may be overwhelmed with the suddenness of their disease and its life-or-death implications. Apathy, fatigue, and memory impairment due to depression can interfere with a patient’s ability or motivation to adhere to a post-transplant regimen of medications, self-monitoring, exercise, and clinic appointments. Excessive or irrational fears due to an anxiety disorder can cause patients to avoid tests, treatments, hospitals, and other circumstances that raise their level of distress. Anxiety and depression may therefore negatively impact their adjustment to transplantation and early intervention is recommended [17–21].
Patients with depression may manifest symptoms of depressed mood, irritability, loss of interest in activities, changes in appetite, insomnia or hypersomnia, psychomotor agitation or retardation, poor memory and concentration, thoughts of death or suicidal ideation and feelings of worthlessness or guilt. In a critically ill patient in the ICU, the diagnosis of depression can be challenging as many of these symptoms may also be manifestations of physical illness (see Table 3). The presence of anhedonia, guilt, hopelessness, helplessness and suicidal ideation may be clues that depression is contributing to the clinical presentation [8]. The presence of persistent irritability, rather than sadness or tearfulness, may also suggest depression. Patients may be reticent to complain of depression during this period of time feeling “I should not be depressed” having just received a life-saving procedure. Medical contributions to depression may include medications, rapid taper of steroids, metabolic derangements and central nervous system (CNS) events. Post transplant complications, changes in family and caregiver dynamics and the stress of the illness and hospitalization may all contribute. It is important to carefully evaluate the patient with complaints of depression – residuals of delirium, psychotic symptoms, PTSD, anxiety and cognitive problems may complicate the diagnosis. Families and caregivers may experience symptoms and distress as well as the dynamics in their relationship with the patient undergo changes during the transplant process and the focus shifts from one of caregiving to rehabilitation. [14, 22, 23, 24, 25].
Table 3.
Symptoms/Behaviors of Anxiety and Depression in the Medically Ill
| Somatic symptoms/behaviors 1 | Affective and cognitive symptoms/behaviors2 |
|---|---|
| Fatigue or generalized weakness | Sadness/tearfulness/irritability |
| Appetite disturbances (anorexia or hyperphagia) | Feeling edgy/anxious/overwhelmed |
| Sleep disturbances (insomnia or hypersomnia) | Impaired attention/concentration/memory |
| Increased or excessive physical complaints (out of proportion to the degree of physiologic disturbance) | Loss of interest, pleasure in enjoyable activities(e.g. ability to enjoy visit from family, friends) |
| Psychomotor agitation or retardation | Social withdrawal/apathy |
| Heightened pain perception | Guilt, feeling like a burden to others |
| Jitteriness, tremor, sweating | Feelings of hopelessness/helplessness |
| Nausea, gastrointestinal complaints | Nightmares, flashbacks, avoidance |
| Chest tightness/palpitations | Problems with treatment adherence |
| Shortness of breath/feeling of choking | Heightened vigilance over care needs |
| Dizziness/lightheadedness | Thoughts of death, fears of dying, heightened worries about health |
| Passive wish for death/suicidal ideation |
Somatic symptoms may be attributable to the medical illness or depression/anxiety
Affective/cognitive symptoms may be clues to presence of depression and/or anxiety in the setting of severe medical illness
Anxiety disorders are also common in transplant patients. Patients with pre-existing anxiety disorders frequently have an exacerbation of symptoms in the transplant setting. Pre-operatively patients worry about their health, the outcome of the transplant evaluation and whether the transplant will actually occur. In the perioperative period, patients and families are anxious as to whether the graft will function, whether complications and graft rejection will occur and whether the patient will survive and have an improved quality of life. Pre- transplant, patients are frequently in denial with regard to the rigors and stressors they face post-transplant. These issues become reality in the perioperative period and patients are particularly vulnerable to anxiety at this time. Anxiety symptoms may increase with the stress of the ICU stay, metabolic derangements, sleep deprivation, post-operative complications, episodes of graft rejection and medication side effects. Excessive or irrational fears may cause patients to avoid or refuse tests or treatment and be uncooperative with care. Conflicts over daily care, such as the timing of medications, meals, rehabilitation, and tests, can be related to a patient’s attempt to control rising fears related to worsening health and an uncertain wait for a donor organ [8]. In evaluating the patient it is important to rule out cardiac arrhythmias, angina, electrolyte imbalances, respiratory distress, seizures, CNS infections and other CNS pathology as contributing to or causing anxiety symptoms [14, 22, 23, 24, 25, 26].
B. Post-traumatic stress disorder (PTSD)
With the onset of medical illness and the need for transplantation patients with pre-existing PTSD from combat experiences or other trauma may experience a recurrence or acute increase in PTSD symptoms. In addition, the life-threatening nature of transplant-related events, transplant surgery, and the ICU stay can cause new onset PTSD [27, 28]. There is increasing evidence that the ICU experience can cause PTSD in a significant percentage of general medical patients, with up to 44% experiencing PTSD symptoms [28, 29]. Patients have described vivid flashbacks, severe disturbing nightmares, exaggerated startle responses, and severe anxiety among other symptoms. In a few cases, transplant patients, while delirious, experienced delusions and hallucinations of life threatening events that led to the development of PTSD [28]. In one study PTSD experienced during the first year after heart transplantation (but not non-PTSD anxiety symptoms) predicted mortality during the subsequent 2 year follow-up period [27]. Proactive evaluation, diagnosis, and treatment of anxiety and/or delirium could lessen symptoms and distress but whether these measures would prevent the onset of PTSD or improve outcomes is not known.
C. Psychotic Disorders
While it is rare for transplant candidates to have histories of psychotic disorders (i.e. schizophrenia, schizoaffective disorder, bipolar disorder), such patients can do well post-transplant if their disorders are well-controlled [30]. These patients should undergo an extensive pre-transplant psychiatric evaluation. Information on how they have tolerated prior hospitalizations and specifically ICU stays can help to guide future treatment planning and help the ICU and transplant teams prepare for their ICU stay. Patients with psychotic disorders may experience disturbances in judgment and/or reality testing when faced with new experiences, multiple stresses, or situations where they lack a sense of control. This is especially true in the ICU where stressors related to the severity of their illness, perceived loss of control, and excessive environmental stimulation may precipitate psychotic symptoms. In this context these patients may become agitated, irritable, delusional, or paranoid. They may experience auditory or visual hallucinations or become uncooperative with their medical care. Although the etiology of their symptoms is linked to their underlying psychiatric disorders, the treatment of these symptoms and behaviors is similar to treatments offered for behavioral symptoms caused by medical decompensation related to end-stage organ failure (e.g., hepatic encephalopathy; see section below).
D. Substance Abuse
Whether to offer new organs to patients with substance abuse or dependence disorders has been a source of debate within the transplant community and society at large. While concerns have been raised over post-transplant relapse and its potential to contribute to nonadherence, eventual graft failure and patient death, there is little evidence that carefully selected individuals experience high rates of relapse. In fact a recent meta-analysis demonstrated relapse rates as low as 3–6% of patients per year among individuals transplanted after histories of alcohol and/or illicit drug use [31]. Even those on methadone maintenance do not appear to relapse often while remaining on treatment [32].
Transplant teams often expect patients to achieve a certain duration of pre-transplant abstinence (often at least 6 months) before they are listed for transplant. While this may allow for some demonstration of patients’ commitment to abstinence, stable abstinence is measured in years and patients in end-stage organ failure may not be able to survive the added wait time. More importantly patients must gain understanding of and insight into their addiction and develop healthier coping skills. This often requires participation in formal rehabilitation programs, addiction treatment or 12-step groups, and family education. For patients in the ICU, requirements for addiction rehabilitation and specific periods of abstinence may not be achievable in the time prior to transplantation. These patients may generate a great deal of emotion and even conflict amongst team members as personal opinions on these candidates may be very different. Because there is no national policy for these cases, the decision is left to the treating physicians/clinicians and each transplant team must determine the relative importance of these issues with respect to their own policy and selection criteria. However, teams should apply their criteria consistently in order to prevent disagreements over individual cases.
For ICU staff, the care of patients with addictive disorders who need transplantation requires adoption of a well-informed, non-judgmental stance. Patients with histories of alcohol or benzodiazepine dependence should be monitored for the development of withdrawal symptoms. Many withdrawal syndromes, especially for alcohol, opioids and sedative hypnotics present with symptoms of sympathetic hyperactivity (tachycardia, hypertension, hyperthermia). This may complicate transplant management, confuse the diagnostic picture when evaluating medical complications, and result in increased morbidity. Medications may be required to avoid serious complications of withdrawal such as autonomic instability and seizures. If the patient is alcohol dependent, administration of thiamine may be required to prevent the Wernicke-Korsakoff syndrome. Opioid dependent patients on methadone maintenance will continue to require their outpatient dose of methadone, along with additional opioid medications for treatment of pain [33]. Patients dependent on opioids may have substantial tolerance to these medications and may require higher than expected doses to achieve adequate pain control. While patients with substance abuse may have comorbid anxiety disorders, treatment of their symptoms requires cautious use of benzodiazepines and optimized use of other psychotropic agents. In addition to medication management it is equally important to obtain consultation with specialists in addiction and arrange for the patient to receive treatment for the substance use disorder as soon as practical.
V. Pre-transplant Organ Specific Cognitive Disorders and Encephalopathy
A. Cognitive Disorders and Delirium
Through the pre- to post-transplant phases patients frequently experience reductions in cognitive functioning ranging from subclinical or mild symptoms to frank delirium (see Table 4). Impairment in cognitive function often results from end-stage organ disease and its physiologic sequelae but may also occur due to other co-morbid disease processes (e.g. CNS vascular disease from diabetes or hypertension), damage from prior exposures (e.g. alcohol or drugs) or be the result of prior structural damage (e.g. stroke), medication side effect, or head trauma. Prior to transplantation it is critical to attempt to differentiate between the fluctuating course of a delirium which is potentially reversible and more persistent cognitive deficits that may represent a pre-existing dementia or a static cognitive impairment. The reversibility or even progression of deficits may in part rely on age, the homeostatic reserve of the brain, prior CNS insults, and the ability to withstand future transplant related stresses (e.g. prolonged anesthesia, use of cardiac bypass, hemodynamic fluctuations and post-transplant immunosuppressives). While the restoration of normal organ functioning and physiology post-transplant may be expected to correct the reversible cognitive impairments, deficits may take months to years to resolve [34].
Table 4.
Delirium and other Cognitive Disorders
| SYMPTOMS | ACUTE DELIRIOUS STATE | MILD COGNITIVE DISORDER or EARLY SIGNS OF DELIRIUM |
|---|---|---|
| Onset | Often rapid | Slow, insidious |
| Clouding of consciousness | Yes | No |
| Waxing and waning of alertness | Yes | No or mild |
| Disorientation | Yes | No or mild |
| Fluctuation of symptoms over brief periods of time | Yes, often severe | No |
| Sleep-wake cycle disturbance | Yes, often severe | Possibly present |
| Increased or decreased psychomotor activity | Yes | No or mild |
| Sensory misperceptions ( illusions, hallucinations) | Sometimes | No |
| Tangential, rambling, incoherent thought/speech | Sometimes | No |
| Impaired reality testing/delusions | Sometimes | No |
| Impaired attention/concentration | Yes, often severe | No or mild |
| Memory impairment | Yes, both short and long term memory affected | Yes, mainly short term memory affected |
| Disturbances in executive function- planning, organization, abstraction | Yes, often severe | Yes |
| Mood/personality changes | Yes | Sometimes |
Reference: [90]
In heart failure, low cardiac output and CNS hypoperfusion from reduced cerebral blood flow can contribute to cognitive impairments. Impaired cerebrovascular reactivity and ischemia may result, even in the absence of acute cerebrovascular events. Cardiac medications, including inotropic agents can also contribute to cognitive impairments. CNS microemboli are common in pre-heart transplant patients especially for those on ventricular assist devices (see section on VADs). In end-stage lung disease, hypoxia and hypercapnia may cause mild to severe cognitive deficits in these patients, particularly in the areas of executive functioning, attention and memory [35]. Oxygen therapy may improve cognitive functioning in certain candidates and these patients can benefit from lung transplantation but the extent to which these deficits are reversible is unclear [35]. Hepatic and uremic encephalopathies are two specific areas considered in detail below.
Evaluation of delirium during this period of time must include careful medical examination of the patient and review of the medications and laboratory studies (see Table 5). Brain imaging, EEG recording, and lumbar puncture may also provide important information. The differential diagnosis is broad and includes metabolic derangements, infections and side effects of medications (see Tables 6 and 7) [22]. Environmental attributes can also contribute to the development of delirium. These factors include disruption of the normal day/night cycle with constant stimulation in the ICU, sleep disruption and lack of orienting cues, among others. To the extent possible, normalization of the sleep-wake cycle should be attempted in the ICU, and waking the patient during the night should be avoided unless necessary. Room lights should be off or dimmed during the night unless they are necessary to provide care to the patient. Frequent re-orientation to time and place, and reminding the patient who the staff are who are caring for the patient, and why the patient is hospitalized may also be helpful. Breitbart et. al. noted that delirious patients with perceptual disturbances and severe delusions were more likely to experience later delirium related distress than those without these symptoms [36]. Whether treatment of delirium can prevent future distress or the development of delirium related PTSD symptoms is unknown.
Table 5.
Diagnostic Tools to Identify Cognitive Disorders
|
Reference: [90]
Table 6.
Potential Causes Of Delirium In Transplant Patients
| Metabolic |
| Dehydration |
| Volume Overload |
| Hypoxia |
| Electrolyte imbalances |
| Hyponatremia/hypernatremia |
| Hyperkalemia |
| Hypercalcemia |
| Hypomagnesemia |
| Acidosis |
| Alkalosis |
| Infectious |
| Sepsis |
| Pneumonia |
| Spontaneous Bacterial Peritonitis |
| Abscesses |
| Cellulitis |
| Meningitis/encephalitis |
| Endocarditis |
| Organ Failure |
| Hepatic Encephalopathy |
| Uremic Encephalopathy |
| CNS hypoperfusion |
| Medications – see Table 7 |
| Endocrine |
| Hypothyroidism |
| Hyperthyroidism |
| Cerebrovascular |
| Seizures |
| Cerebral Edema |
| CVA – embolic or hemorrhagic |
| Subdural hemorrhage |
| Hypertensive encephalopathy |
| Miscellaneous |
| Alcohol and/or drug intoxication and withdrawal states |
| Autoimmune disorders – vasculitis |
| Disseminated intravascular coagulation |
| Fever |
| Sensory deprivation |
| Sleep deprivation |
| Neuroleptic malignant syndrome |
| Malignant hyperthermia |
Table 7.
Medications Commonly Used In Transplant Patients That May Cause Delirium
| Immunosuppressants |
| Corticosteroids |
| Calcinurein inhibitors (tacrolimus, cyclosporine) |
| Analgesic pain medications |
| Opioid analgesics |
| Non-steroidal anti-inflammatory medications |
| Antimcrobials |
| Acyclovir, ganciclovir |
| Amphotericins |
| Cephalosporins |
| Interferon-alpha |
| Vancomycin |
| Aminoglycosides |
| Anticholinergics |
| Antihistamines |
| Diphenhydramine |
| Hydroxyzine |
| Benztropine |
| Atropine |
| Scopalamine |
| Tricyclic Antidepressants |
| Amitriptyline |
| Doxepin |
| Phenothiazines |
| Chlorpromazine |
| Anti-emetics and related medications |
| Proclorperazine |
| Promethazine |
| Metoclopramide |
| Cardiac medications |
| Beta-blockers |
| Clonidine |
| Digoxin |
| Sedative-hypnotics |
| Benzodiazepines (e.g. diazepam, lorazepam) |
| Barbiturates |
| Miscellaneous |
| Cimetidine |
| Ranitidine |
| Baclofen |
| Lithium |
| Stimulants |
B. Liver Disease and Hepatic Encephalopathy
Hepatic encephalopathy (HE) is a specific type of delirium commonly experienced by patients with hepatic dysfunction. Symptoms of HE may be considered on a continuum from subclinical or minimal to overt and severe. In addition to the signs and symptoms that characterize delirium (see Table 4) patients can also have affective/emotional dysregulation, psychosis, behavioral disturbances, bioregulatory disturbances, and disturbances of the motor system including asterixis, tremor, increased deep tendon reflexes, increased muscle tone, ataxic gait, bradykinesia, slurred speech, or incoordination. Patients with hepatic encephalopathy associated with acute fulminant hepatic failure are at risk for cerebral edema, increased intracranial pressure, seizures and death pre-transplant [37, 38]. The prognosis for these patients is poor with or without liver transplant particularly if the intracranial pressure is >40mm Hg or cerebral perfusion pressure is <40mm Hg [39]. For patients with acute liver failure who experience an acute change in mental status or progress to advanced stage hepatic encephalopathy, head computed tomography is recommended to evaluate for cerebral edema or intracranial bleed [40]. Persistent HE is rare but can be observed in patients with extensive portocaval collateral circulation or after surgical or transjugular portosystemic stent shunting procedures [41]. An electroencephalogram (EEG) may show common abnormalities such as generalized slowing of dominant rhythm or less commonly non-convulsive seizures; neuropsychological testing assessing psychomotor speed, praxis, concentration and attention is more efficient and perhaps more sensitive in determining minimal HE [38, 40, 42- Weissenborn 2001].
HE most likely has a multifactor pathogenesis. Changes in brain metabolism and disorders of neurotransmission appear to be contributing factors. Although the predominant treatment strategy is to decrease production and absorption of ammonia in the gastrointestinal tract, it is not the only substance implicated in the pathogenesis of HE. HE can be precipitated by significant protein intake, gastrointestinal hemorrhage (causing increased protein load in the intestine), uremia, use of some psychoactive medications or diuretics, dehydration, or electrolyte imbalance [42, 43]. Treatments should be aimed at correcting precipitating factors and should include administration of a non-absorbable disaccharide (e.g. lactulose) which acts as an osmotic laxative to flush out ammonia. Additional treatments include the use of non-absorbable antibiotics to reduce intestinal bacteria that convert protein to ammonia. A protein restricted diet may not be feasible for patients with advanced liver disease with the loss of muscle mass and cachexia. Medications which can contribute to symptoms of HE or slow intestinal motility, such as those with anticholinergic activity and opioid analgesics, should be avoided.
C. Renal Disease and Uremic Encephalopathy
Chronic renal failure results in multiple catabolic, metabolic, and endocrinologic processes that contribute to the development of uremic encephalopathy. The accumulation of neurotoxic substances such as urea, uric acid, guanidine compounds, hippuric acid, indoleacetic acid and others is believed contribute to the encephalopathy; no single metabolite has been identified as the sole cause. Other pathophysiologic changes implicated in uremic encephalopathy include hormonal elevations, and electrolyte imbalances including acidosis, hyponatremia, hyperkalemia, hypocalcemia and hypermagnesemia, anemia, malnutrition, and CNS factors such as increased calcium and decreased GABA and glycine activity.
The symptoms of uremic encephalopathy typically fluctuate and can begin insidiously with patients experiencing mild cognitive impairment, irritability, or insomnia. Physical symptoms (e.g. slurred speech, muscle twitches, or restless legs) can also occur. Symptoms can progress slowly or rapidly to confusion, lethargy, overt delirium, seizures, psychosis, catatonia, and stupor/coma. An EEG can aid in the differential diagnosis of encephalopathy, typically showing generalized slowing of the dominant rhythm, vs. seizures and non-convulsive status epilepticus which can occur in uremia and be mistaken for uremic encephalopathy. Removal of uremic toxins by hemodialysis, correction of electrolyte imbalances and anemia and the treatment of malnutrition can diminish the symptoms of encephalopathy and improve cognition. Seizures may require treatment with anticonvulsants.
Uremic encephalopathy is also associated with a cliniconeuroradiological syndrome termed posterior reversible (leuko)encephalopathy syndrome (PRES – see section on immunosuppressive medications below). Characteristic radiographic findings on CT or MRI are seen in the posterior cortical and subcortical white matter. Risk factors for PRES in renal patients include abrupt changes in blood pressure, autoimmune disorders, thrombotic thrombocytopenic purpura, infections (specifically viral) and sepsis, and nonspecific renal inflammatory conditions (e.g. glomerulonephritis, hepatorenal syndrome) [44]. Early recognition allows corrective action to be taken. Action is especially important with respect to severe/unstable blood pressure, which frequently accompanies the syndrome [44]. Prompt treatment may avoid potentially permanent brain damage.
D. Heart Failure And Ventricular Assist Devices
The extreme shortage of donated hearts and the growing list of heart transplant candidates indicates that ventricular assist device (VAD) therapy will play an increasingly significant role in the treatment of end-stage heart disease. Progress in the development of VADs from external or paracorporeal devices to implantable devices has dramatically improved both the physical and psychological health of patients with end-stage heart failure. While these devices are primarily used as bridges to transplantation, they can also bridge a patient to recovery (e.g. after an acute illness such as fulminant myocarditis) and are now also offered “destination” therapy for some patients ineligible for transplant. The newest VADs now include implantable left ventricular or biventricular versions that have been miniaturized and have improved patient mobility, easy of wearability, and routinely allow discharge from the hospital. Portable pneumatic drivers and battery packs are compact and lightweight and can be worn on a shoulder strap or towed on a luggage-type carrier. Most patients can achieve New York Heart Association functional status I or II while supported on a VAD. Patients can also achieve significant gains in physical and physiological rehabilitation and rebuild muscle mass, potentially stabilizing their cardiac condition [45]. Many patients can engage in light to moderate physical activity (including walking, driving, dancing and even work).
However, despite improvements in quality of life, mobility, and functioning for VAD patients, psychological and cognitive problems are not uncommon. In the first 1–2 weeks post-implant while patients are often in the ICU they report coping well with the VAD and having low symptoms of distress but feel as if they were not doing as well as they had anticipated prior to VAD implantation [46]. Adjusting to the VAD can be psychologically difficult. Incorporating the machinery into their body can evoke feelings of a damaged body image and sense of self and these feelings can be especially traumatic if the VAD implantation is in response to an emergency [47]. Patients can feel vulnerable, apprehensive with the machinery sounds and alarms, and can fear a VAD malfunction [47]. While patients may be too ill before implantation, psychotherapy afterwards to address these issues may ease the transition onto a VAD and help them prepare for eventual transplant.
While patients bridged to transplantation with a VAD have similar post-transplant physical recovery and emotional well-being as patients who never required VAD support, they may have poorer residual cognitive functioning post-transplant [48, 49]. Cognitive impairments may in part be due to the higher risk of thromboembolism while supported on a VAD. While there is a low incidence of thromboembolic complications (0.24 per 100 LVAD days), a high incidence of circulating microemboli on transcranial Doppler ultrasonography has been demonstrated in VAD patients [50]. Using cognitive P300 evoked potentials as a general indicator of neurocognitive functioning, one study showed in the short term VAD implantation could improve neurocognitive impairment by the time patients left the ICU [51]. Nevertheless, while many of the microembolic events are clinically silent [50] the chronic effect of microembolic events (i.e. silent infarctions) on cognitive functioning is speculated to be significant over time. Although it is not feasible to repeatedly perform computed tomography of the brain, transcranial Doppler may be beneficial for predicting the risk and periodic neuropsychologic or cognitive testing may identify silent cerebral infarctions [49].
VI. Treatment Issues – Medications For Psychiatric Disorders
While psychiatric symptoms may seem to be normal reactions to significant stresses of the transplant experience, lack of timely diagnosis and treatment can lead to unneeded suffering, reduced adherence to medical care, heightened physical pain, and greater functional impairment. Nevertheless it is a complex challenge to identify and correct underlying pathophysiologic processes first that could be causing or contributing to psychiatric symptoms. There may be significant overlap in the physical and psychological symptoms of the patient’s medical condition and their psychiatric illness (see Table 3). If medications are needed to treat psychiatric symptoms, careful consideration must be given to the choice of medication, symptoms to be treated, the side effects of the medications, adverse drug interactions and the type and severity of organ failure with respect to alteration in pharmacokinetics. A full discussion of this topic is beyond the scope of this chapter (Table 8 provides some guidelines and suggestions). In these cases psychiatric consultation can assist in the diagnosis and selection and monitoring of psychotropics. Brief psychotherapy, even in the ICU setting may also be beneficial.
Table 8.
Psychotropic Medications in Transplant Patients
| Medication | Most Common Uses in Transplant | Issues |
|---|---|---|
| Antidepressants | ||
| SSRIs (Selective Serotonin Reuptake Inhibitors) | Depression/Anxiety Disorders1,2 | |
| Fluoxetine | Long half-life – takes many days to clear after discontinuation; potential drug interactions | |
| Paroxetine | Mild anticholinergic effects; discontinuation syndrome more problematic than other SSRIs; potential drug interactions | |
| Sertraline | Potential drug interactions | |
| Fluvoxamine | Raises levels of cyclosporine and tacrolimus via inhibition CYP 450 3A4 | |
| Citalopram | Few drug interactions | |
| Escitalopram | Few drug interactions | |
| Tricyclics | Depression/Anxiety Disorders3 | All have cardiac effects – cardiac conduction changes, tachycardia and arrhythmias have been described; QT prolongation |
| Amitriptyline | Significant anticholinergic side effects | |
| Imipramine | Significant anticholinergic side effects | |
| Nortriptyline | Fewer anticholinergic side effects; therapeutic level established (50–150ng/ml) | |
| Doxepin | Moderate anticholinergic side effects | |
| Desipramine | Fewer anticholinergic side effects; may cause anxiety and agitation | |
| Others | ||
| Trazodone | Sleep | Risk of priapism; poor antidepressant efficacy, may help with medication induced sleep disturbances and mightmares due to PTSD |
| Mirtazapine | Depression/Anxiety Disorders | Increased appetitie/weight gain, can reduce nausea, may cause neutropenia |
| Nefazodone | Depression | Potential severe hepatotoxicity; avoid in liver disease Raises levels of cyclosporine and tacrolimis via inhibition of CYP 450 3A4 |
| Bupropion | Depression/Smoking Cessation | Risk of seizure in high doses; dose reduction in hepatic failure |
| Venlafaxine | Depression/Anxiety Disorders/Pain | Dose reductions in hepatic and renal failure; dose- dependent elevations in blood pressure |
| Duloxetine | Depression/Pain | Potential hepatoxicity; avoid in end-stage renal disease and patients with hepatic dysfunction |
| Benzodiazepines | All have abuse potential; risk of withdrawal syndrome with abrupt discontinuation after continued use | |
| Lorazepam | Anxiety Disorders/Alcohol and Drug Withdrawal4 | No active metabolites; may be given po, IM or IV |
| Diazepam | Anxiety Disorders/Alcohol and Drug Withdrawal | Long half-life; active metabolites |
| Clonazepam | Anxiety Disorders | |
| Temazepam | Sleep | No active metabolites |
| Alprazolam | Anxiety Disorders | Short half-life; risk of withdrawal between doses |
| Antipsychotics | Delirium/Hallucinations/Delusions3 | |
| Typical | ||
| Haloperidol | Use lowest possible dose; risk of extrapyramidal symptoms and neuroleptic malignant syndrome is less with IV administraion | |
| Atypical | Delirium/Hallucinations/Delusions3 | Risk of metabolic syndrome in all; |
| Risperidone | Similar to haloperidol in dose >6mg | |
| Olanzapine | Risk of metabolic syndrome –hyperlipidemia hyperglycemia, weight gain | |
| Aripiprazole | Less risk of metabolic syndrome | |
| Ziprasidone | Less risk of metabolic syndrome; risk of QT prolongation | |
| Quetiapine | Moderate risk of metabolic syndrome; some weight gain | |
| Stimulants | Depression/Fatigue/ADHD 3 | All have abuse potential; should see response within several days; may decrease appetite |
| Methylphenidate | Avoid in agitated depression | |
| Dextroamphetamine | Small risk of cardiac side effects | |
| Other Medications | ||
| Lithium | Bipolar Disorder | Potential nephrotoxicity; serious side effects with toxic levels; drug interactions with diuretics, ACE inhibitors and others |
| Clonidine | Post-traumatic stress disorder | Risk of hypotension, sedation |
| Prazosin | Post-traumatic stress disorder | May help nightmares and sleep disturbance related to PTSD |
| Buspirone | Anxiety Disorders | May have respiratory stimulating properties |
Anxiety disorders include: anxiety disorder secondary to medications and medical conditions, Generalized Anxiety Disorder, Post-traumatic Stress Disorder, Phobias including Social Phobia, Panic Disorder, Obsessive Compulsive Disorder And other anxiety disorders
These uses pertain to all drugs in the class
May be used to treat alcohol withdrawal, sedative-hypnotic withdrawal and as adjunctive medication in other withdrawal states
In cases of delirium and other psychotic symptoms it is important to avoid medications that may worsen symptoms. Low doses of typical and atypical antipsychotics may be most appropriate in these circumstances. Haloperidol, risperidone and quetiapine are common choices, depending upon the route of administration available [52]. Haloperidol may be given parenterally or orally. The lowest possible doses of this medication are suggested as it may cause extrapyramidal (parkinsonian) symptoms, akathisia and neuroleptic malignant syndrome. Short-acting risperidone and quetiapine are currently only available in oral forms. Risperidone and olanzepine are available in a quick dissolving tablet that dissolves in seconds when placed on the tongue and may be useful if swallowing pills is a problem. These medications still need to be swallowed after dissolution and require an intact gastrointestinal tract for absorption. Atypical antipsychotics can cause or worsen hyperglycemia and hyperlipidemia (which can also be side effects of immunosuppressive medications) and they also carry a small risk of QT prolongation [52]. When treating delirium regular scheduled doses of medication are preferable to as needed (prn) doses to stabilize symptoms. Delaying treatment until symptoms become problematic and then using prn dosing may create a situation in which higher doses are needed to control behaviors.
Lithium and divalproex (sodium valproate/valproic acid) are commonly used to treat mania, but are complicated to use in the peri-transplant period. Large fluid volume shifts, the combined nephrotoxicity of other medications and frequent use of diuretics make use of lithium potentially dangerous and impractical. Divalproex has many drug interactions and also a small risk of hepatotoxicity. Its use in patients with liver disease is not recommended. Side effects of divalproex include thrombocytopenia, nausea, vomiting and ataxia. Atypical antipsychotics can effectively treat symptoms of mania, psychosis, and mood dysregulation in these patients.
Anxiety symptoms may be safely treated short-term with benzodiazepines; however use of these medications may cause or worsen symptoms of delirium and cognitive impairment. If a benzodiazepine is used, a short-acting medication with no active metabolites is suggested such as lorazepam. The lowest possible dose for the shortest period of time is suggested. As with delirium, treating anxiety with a regularly scheduled medication, rather than prn, may allow more consistent alleviation of symptoms and avoid an escalation of symptoms or a requirement for a higher dose. For those patients with pre-existing alcohol or benzodiazepine addiction, care must be taken with longer-term use of benzodiazepines in order to avoid precipitating a relapse of the addiction. In general, while benzodiazepines are quick acting and effective for immediate treatment of anxiety, for patients with more persisting anxiety consideration of a non-addicting agent for longer term use is suggested.
Both anxiety and depression may be treated with selective serotonin reuptake inhibitors (SSRIs) (e.g. fluoxetine, paroxetine, sertraline and citalopram). These medications are relatively safe in the medically ill patient; however it is important to be aware that fluoxetine has a relatively long half-life and that fluoxetine, paroxetine and sertraline may have cytochrome P450 drug-drug interactions with medications typically administered to these patients. Venlafaxine has relatively few drug-drug interactions but in high doses may worsen hypertension. Fluvoxamine and nefazodone have very significant interactions with calcinurein inhibitors and should be avoided [53]. Bupropion may increase the risk for seizures at higher doses and can cause symptoms of restlessness or tremulousness. It should be used cautiously during the immediate peri-transplant period until the patient is stable
VII. Neuropsychiatric Side Effects of Immunosuppressive Medications
A. Calcineurin-inhibiting immunosuppressive medications (tacrolimus and cyclosporine)
Calcineurin-inhibiting immunosuppressive medications (CIIs) are the mainstay of immunosuppressive medication regimens for most solid organ transplant recipients. Tacrolimus and cyclosporine appear to have similar neurotoxic side effect profiles with up to 40–60% of transplant recipients experiencing mild symptoms including tremulousness, headache, restlessness, insomnia, vivid dreams, photophobia, hyperesthesias and dysasthesias, anxiety, and agitation [54]. Moderate to severe neuropsychiatric side effects (i.e. cognitive impairment, coma, seizures, focal neurological deficits, dysarthria, cortical blindness and delirium) occur less often but can reach 21–32% in the early postoperative period [54]. While there can be many possible etiologies for neuropsychiatric symptoms or mental status in the early post-transplant period (see Tables 6 and 7), the possibility that they reflect CII side effects should always be entertained.
The etiology of CII neurotoxicity is unclear, most likely multifactorial, and may involve biochemical or physiologic derangements or direct or indirect neurotoxic processes (e.g. immune system dysregulation). CII neurotoxicity has been associated with biochemical and electrolyte derangements including higher plasma levels, intravenous administration, hypocholesterolemia and hypomagnesemia [54]. Disruption of the blood-brain barrier—whether structural (e.g. previous strokes, hypertension, ischemia/reperfusion injury) or physiologic (e.g., hepatic encephalopathy)—has also been associated with neurotoxicity and is hypothesized to cause neurotoxicity by allowing higher CII drug levels in the central nervous system [54].
Correcting the metabolic disturbance or decreasing the drug blood level can result in a resolution of symptoms, although for severe symptoms the type of CII may need to be switched (e.g., from tacrolimus to cyclosporine) or discontinued altogether. Anticonvulsants can successfully treat CII-induced seizures and are not required long-term. Seizures may cease if reduction or discontinuation of the drug is possible [54]. Treatment of mild symptoms can include sleep medications for sleep disruption or benzodiazepines or beta-blockers (if the cardiovascular system can tolerate beta blockade) for symptoms of anxiety, tremor, or restlessness. These treatments should be short-termed with the expectation that the majority of symptoms due to CII side effects will spontaneously resolve as the CII blood levels are reduced in the early post-transplant phase. The longer term use of benzodiazepines is not recommend for symptoms of tremor, anxiety, or restlessness as the ability to satisfactorily taper the patients off of these medications at a later point, especially after they develop physiologic and psychologic dependence becomes problematic. However the temporary use of these agents may provide symptom relief as other antidepressants/anxiolytics are being instituted and adjusted to therapeutic doses. Serotonin reuptake inhibiting antidepressants can be more safely used long-term for symptoms of depression/anxiety although these medications can take 3–4 weeks to become effective. Symptoms of cognitive impairment, agitation, and delirium can be treated with haloperidol or atypical antipsychotics. Psychiatric consultation is recommended to assist in the correct diagnosis and choice of appropriate medication therapy (see Table 8).
CIIs have also been associated with a cliniconeuroradiological syndrome termed posterior reversible (leuko) encephalopathy syndrome (PRES). Clinical symptoms can be varied ranging from mental status changes to focal neurological symptoms. Thus moderate to serious symptoms of neurotoxicity warrant a CT or MRI of the brain to evaluate for PRES (also seen in uremic encephalopathy; see above). Characteristic neuro-radiological abnormalities (low attenuation of white matter on CT scan or corresponding hyper-intense lesions on T2 weighted MRI images) are most commonly seen in the cortical and subcortical white matter typically involving the posterior lobes (parietal and/or occipital), although cases have been reported involving in the anterior brain, cerebellum, and brain stem [44]. Specific findings on MRI fluid attenuation inversion recovery (FLAIR) sequences and apparent diffusion coefficient (ADC) mapping (sensitive to water diffusion) provide further evidence towards the theory of neurotoxicity involving a vasogenic edema and may help in comparison to diffusion weighted MRI images (DWI) in distinguishing vasogenic from cytotoxic edema [55]. Although PRES usually occurs in the early post-operative period it can also occur years later. Both symptoms and radiologic findings can resolve with discontinuation of the CII.
Finally, a rare, severe multifocal demyelinating sensorimotor polyneuropathy has been seen in patients treated with CIIs and can occur within weeks post-transplant. Polyneuropathies in general can be severely limiting, may impair physical recovery and could play a role in the liberation from mechanical ventilation. Early recognition of the symptoms is critical to recovery and sensitive electrophysiological testing may be required. Many of these CII polyneuropathies can improve or be reversed following drug discontinuation, plasmapheresis or intravenous immunoglobulin (IVIG), suggesting an immune-mediated cause (e.g. dysimmune neuropathy) [56, 57]
B. Corticosteriods
Although the use of chronic corticosteroid use is becoming less essential in transplant immunosuppression, the use of high dosages are still employed in the early post-operative phase and also as “pulsed” dosages to treat acute rejection. Behavioral and psychiatric side effects of corticosteroids well described but conclusions regarding the incidence, characteristic effects, or the specific dosages required to cause such effects are not well established. The reported incidence of serious psychiatric side effects is low, 5–6 %, and includes a wide range of cognitive (diminished memory, concentration, attention, mental speed, distractibility), affective (depression, anxiety, irritability, emotional lability, hypomania, mania), psychotic (visual and auditory hallucinations, delusions, thought confusion, racing thoughts), and behavioral (restlessness, agitation, hypervigilance, aggression) symptoms [58, 59, 60, 61]. Although dosage is not clearly related to timing, nature, intensity or duration of symptoms [59], the risk of steroid psychosis mainly occurs with dosages of 40 mg/day or more of prednisone or its equivalent [60]. The average length of time from the institution of steroid therapy to the onset of steroid psychosis is 6 days [60]. Pre-existing personality disturbances, psychiatric disorders, or prior history of steroid psychosis does not clearly increase risk [58, 60]. Brain wave slowing, including electroencephalographic increases in central theta activity [62] and decreases in amplitude and frequency of α-rhythm [60], can be seen and normalize following corticosteroid withdrawal.
Similar to the treatment of CII side effects, the treatment of steroid induced symptoms should target specific symptoms with the expectation that therapy will only be required during steroid therapy. For most transplant patients steroids can be dramatically reduced or eliminated which should alleviate the symptoms. The use of sleep medications or benzodiazepines may be effective short-term. Serotonin reuptake inhibiting antidepressants can be more safely used long-term for symptoms of depression, anxiety, or mood dysregulation but may require 3–4 weeks to become effective. The use of haloperidol or atypical antipsychotics can also be effective for mood dysregulation, psychosis, mania, irritability, agitation/aggression or delirium. Psychiatric consultation is recommended to assist in the correct diagnosis and choice of appropriate medication therapy (see Table 8).
VIII. Special Issues
A. Living donation
Living donors constitute 44% of all organ transplant donors in the United States [63]. The vast majority of living donors donate a kidney (95%) or a portion of the liver (4%). The remaining 1% consist of pancreas, intestine, and lung donors. Living donors may be related to the recipient biologically (e.g., siblings) or emotionally (e.g., spouses, close friends), or may have more distant relationships (e.g., acquaintances through an organization such as a faith-based group), or may have no relationship (i.e., anonymous or altruistic donors).
Living donors constitute a unique patient population in that they are healthy individuals who receive a major surgical intervention solely for the benefit of another person. Because it is critical to minimize both the psychological and the physical risks for these individuals, they receive not only careful medical evaluations but careful psychosocial assessments in order to determine their suitability and willingness to donate. However, even the healthiest donors can have medical or psychiatric complications perioperatively or later in their recovery.
Before considering psychiatric sequelae in particular, it is noteworthy that the general medical outcomes of living donor surgery show an increasingly favorable profile, especially in kidney and liver donors [64, 65, 66, 67]. The literature in other types of donors (e.g., lung, intestine) is extremely sparse and thus we focus on kidney and liver donors here. The perioperative mortality rate among kidney donors is 0.03% [65, 66]. Several large patient series (n’s of ~3000–5500) report that perioperative major complications (e.g., re-exploration for bleeding) occur in less than 1% of donors [66]. Minor perioperative complications (e.g., urinary tract infection, wound infection, need for blood transfusion) are more common, occurring in 4–8% of donors [66]. While there are greater risks when donating a portion of the liver [64, 68] the perioperative mortality rate for living liver donors is low - 0.2–0.3% [64, 67]. Recent patient series have shown overall rates of complications to be 14–32%, with the minor complication of biliary leakage being particularly prevalent [67]. While the very long-term medical outcomes of liver donation are not yet known, the liver regenerates and thus the risk of long-term hepatic damage is believed to be low [67]. In kidney donation, the data available to date suggest low risk of renal disease or other organ system impairment even 20–30+ years post-donation [69]. However, long-term followup data remain sparse even for kidney donors.
Reported rates of perioperative psychiatric disturbances in living donors are quite variable, ranging from 0% to 14% [70–74]. These disturbances include delirium, anxiety, depression and—rarely—psychosis. It should be noted that these rates are generally based on referrals for psychiatric evaluation and likely underestimate the actual numbers of donors experiencing psychiatric distress early in their recovery from surgery. Donors frequently comment that their perioperative pain was much greater than they had expected [75, 76]. In addition, due to the perioperative steroids used to reduce inflammation, donors may experience restlessness, agitation, insomnia, and emotional lability (see section on side effects of corticosteroids above). Because donors are healthy before donation, they may be more alert and less impaired post-operatively and can be more observant of the sights and sounds of the ICU environment which can be emotionally disturbing. Thus ICU staff should be attentive to donor psychic and physical discomfort. Donors should be asked about their emotional state and level of pain, and every effort should be made to alleviate pain and psychiatric symptoms or distress. Psychiatric or pain management consultation may be sought to assist in order to assure their comfort.
Despite these stresses the majority of donors return to their former high levels of well-being following the initial recovery period after donation [77, 78, 79], and extremely few donors report that they regret having donated. They frequently report psychological benefits from donation, including the gratification they experience in being able to help another person, and feelings of increased self-esteem [80, 81, 82].
B. Advanced Directives – Chronic rejection in lung transplantation as an example
The emphasis on aggressive if not sometimes heroic treatment of complications following transplantation is understandable given the general goal of medical care to protect and sustain life. This is especially relevant for transplant recipients as early identification and treatment may prevent worse complications or even loss of life. This goal of sustaining and extending life coupled with the tremendous commitment and effort put forth by the team, patient, and caregivers to get to and through transplant creates an environment in which dialogues about advanced directives are not often initiated [83].
While this issue is important for all transplant patients, the development of chronic rejection in lung transplant recipients is a particular opportunity for such discussion. Chronic rejection of the lung transplant occurs in 60–75% of recipients by 5 years post-transplant and is the leading cause of death among recipients [83]. The clinical manifestation of chronic rejection is the bronchiolitis obliterans syndrome and patients with this lesion will have frequent hospitalizations often requiring mechanical ventilation in the ICU [84]. For lung transplant recipients, an ICU admission is associated with significant morbidity and mortality – only 43% will be alive one year later and most will die in the ICU [83, 84].
Once chronic rejection is identified in lung recipients, the overall prognosis is poor but the course of illness can be highly variable. Thus with these patients, as with other transplant recipients, the expected prognosis, their specific clinical course, and the risks of ongoing procedures, treatments, and interventions should be discussed with exploration of the patient’s preferences. Optimally these discussions should be undertaken when the situation is not dire, potentially when the patient is not ill or hospitalized. Unfortunately these opportunities are often missed [83] and the ICU and transplant teams may need to consider discussions of palliative care and end-of-life decisions while the patient is critically ill.
C. Healthcare Provider Stress From Repeated Losses
Healthcare providers who care for patients awaiting transplantation inevitably experience the loss of some of these patients before a donor organ becomes available. Even post-transplant over the course of repeated hospitalizations or a prolonged stay, healthcare providers may become close to certain patients and emotionally invested in their outcome. The deaths of these patients may be especially difficult to accept. With repeated experiences of caregiving and loss, healthcare providers may develop burnout caring for transplant patients. In turn, burnout leads to emotional exhaustion, feeling a lack of personal accomplishment, and negative attitudes towards patients, ultimately compromising caregiver effectiveness [85, 86]. Avoiding burnout requires sensitivity to the impact of end-of-life issues and patient deaths on critical care staff. In addition, temporary reductions in clinical workload, greater attention to patient assignments, individual/group discussions after the loss of a patient, and added support from colleagues help to reduce burnout [4, 87, 88]. Novel approaches to burnout have incorporated additional techniques such as the use of mindfulness meditation [89].
IX. Summary
Transplantation is a challenging process for patients, caregivers, and medical professionals alike. Patients undergo acute and chronic pathophysiologic changes and can experience substantial emotional distress with the tremendous lifestyle changes and psychological stresses they must endure. These stresses are accentuated in the ICU setting where the life-threatening nature of their medical state brings these issues to sharp focus. While the ICU may be only one period of the patients transplant hospital experience it is a critical time and the care provided by the ICU team is essential to their immediate and overall long-term outcomes. In addition to their medical needs the ICU staff must address the psychological and psychiatric needs of the patients. Psychiatric disorders are common in these patients and their identification and prompt treatment are important aspects of the ICU teams care. We have reviewed the essential aspects of the transplant process with specific relevance to the ICU stay. Psychiatric disorders common to transplantation are also described and discussed. This overview should provide ICU staff the information necessary to deal with the psychiatric needs of this unique and complex patient population.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrea DiMartini, Associate, Professor of Psychiatry Associate Professor of Surgery, Consultation liaison to the Liver Transplant Program, Starzl Transplant Institute, University of Pittsburgh Medical Center, 3811 O’Hara Street, Pittsburgh, PA 15213, 412-383-3166, fax: 412-383-4846, email: dimartiniaf@upmc.edu.
Catherine Crone, Associate Professor of Psychiatry, George Washington University Medical Center, Vice Chair Dept of Psychiatry at Inova Fairfax Hospital, Clinical Professor of Psychiatry Virginia Commonwealth University, Inova Fairfax Hospital, 3300 Gallows Road, Falls Church, VA 22042.
Marian Fireman, Associate Professor of Psychiatry, Oregon Health and Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, email: firemanm@ohsu.edu, Phone: 503-494-6250, Fax: 503-220-3499
Mary Amanda Dew, Professor of Psychiatry, Psychology and Epidemiology, Director, Clinical Epidemiology Program, Associate Center Director and Director, Research Methods, and Biostatistics Core, Advanced Center for Interventions and, Services Research in Late Life Mood Disorders, Director, Quality of Life Research, Artificial Heart Program, Adult Cardiothoracic Transplantation, University of Pittsburgh School of Medicine and Medical Center, 3811 O’Hara Street, Pittsburgh, PA 15213, 412-624-3373, fax: 412-383-4846, email: dewma@upmc.edu
References
- 1.U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. [Accessed December 2007]; doi: 10.1111/j.1600-6143.2011.03886.x. http://www.optn.org. [DOI] [PubMed]
- 2.2006 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1996–2005. Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; Rockville, MD: Retrieved online at www.optn.org/AR2006/default.htm. [Google Scholar]
- 3.Russo MW, Galanko JA, Shrestha R, et al. Liver Transplantation for Acute Liver Failure From Drug Induced Liver Injury in the United States. Liver Transpl. 2004;10:1018–1023. doi: 10.1002/lt.20204. [DOI] [PubMed] [Google Scholar]
- 4.Wright L, Pape D, Ross K, et al. Approaching end-of-life care in organ transplantation: the impact of transplant patients’ death and dying. Prog Transplant. 2007;17:57–62. doi: 10.1177/152692480701700109. [DOI] [PubMed] [Google Scholar]
- 5.Larson AM, Curtis JR. Integrating palliative care for liver transplant candidates: “Too well for transplant, too sick for life”. JAMA. 2006;295:2168–76. doi: 10.1001/jama.295.18.2168. [DOI] [PubMed] [Google Scholar]
- 6.Quill TE. Initiating end-of-life discussions with seriously ill patients: addressing the “elephant in the room”. JAMA. 2000;284:2502–7. doi: 10.1001/jama.284.19.2502. [DOI] [PubMed] [Google Scholar]
- 7.Back AL, Arnold RM, Quill TE. Hope for the best, and prepare for the worst. Ann Intern Med. 2003;138:439–43. doi: 10.7326/0003-4819-138-5-200303040-00028. [DOI] [PubMed] [Google Scholar]
- 8.Crone CC, Wise TN. Psychiatric issues in transplantation, II: Preoperative issues. Crit Care Nurse. 1999;19:51–63. [PubMed] [Google Scholar]
- 9.Trumper A, Appleby L. Psychiatric morbidity in patients undergoing heart, heart and lung, or lung transplantation. J Psychosom Res. 2001;50:103–5. doi: 10.1016/s0022-3999(00)00214-2. [DOI] [PubMed] [Google Scholar]
- 10.Parekh PI, Blumenthal JA, Babyak MA, et al. Psychiatric disorder and quality of life in patients awaiting lung transplantation. Chest. 2003;124:1682–88. doi: 10.1378/chest.124.5.1682. [DOI] [PubMed] [Google Scholar]
- 11.Rocca P, Cocuzza E, Rasetti R, et al. Predictors of psychiatric disorders in liver transplantation candidates: logistic regression models. Liver Transpl. 2003:721–26. doi: 10.1053/jlts.2003.50133. [DOI] [PubMed] [Google Scholar]
- 12.DiMartini AF, Dew MA, Trzepacz PT. Organ transplantation. In: Levenson JL, editor. The American Psychiatric Publishing Textbook of Psychosomatic Medicine. Washington, DC: The American Psychiatric Press, Inc; 2005. pp. 675–700. [Google Scholar]
- 13.Dew MA, DiMartini AF. Psychological disorders and distress after adult cardiothoracic transplantation. Journal of Cardiovascular Nursing. 2005;20(5 Suppl):S51–S66. doi: 10.1097/00005082-200509001-00007. [DOI] [PubMed] [Google Scholar]
- 14.Olbrisch ME, Benedict SM, Ashe K, Levenson JL. Psychological assessment and care of organ transplant patients. J Consult Clin Psychol. 2002;70:771–783. doi: 10.1037//0022-006x.70.3.771. [DOI] [PubMed] [Google Scholar]
- 15.Skotzko CE, Strouse TB. Solid Organ Transplantation. In: Wise MG, Rundell JR, editors. The American Psychiatric Publishing Textbook of Consultation-Liaison Psychiatry: Psychiatry in the Medically Ill. Washington, DC: The American Psychiatric Press, Inc; 2002. pp. 623–655. [Google Scholar]
- 16.Dew MA, Kormos RL, Winowich S, Harris RC, Stanford EA, Carozza L, Griffith BP. Quality of life outcomes after heart transplantation in individuals bridged to transplant with ventricular assist devices. J Heart Lung Transplant. 2001;20(11):1199–212. doi: 10.1016/s1053-2498(01)00333-3. [DOI] [PubMed] [Google Scholar]
- 17.Dobbels F, Verleden G, Dupont L, Vanhaecke J, De Geest S. To transplant or not? The importance of psychosocial and behavioural factors before lung transplantation. Chronic Respiratory Disease. 2006;3(1):39–47. doi: 10.1191/1479972306cd082ra. [DOI] [PubMed] [Google Scholar]
- 18.Levenson JL, Olbrisch ME. Psychosocial screening and selection of candidates for organ transplantation. In: Trzepacz P, DiMartini A, editors. The Transplant Patient: biological, psychiatric and ethical issues in organ transplantation. Cambridge University Press; UK: 2000. pp. 21–41. [Google Scholar]
- 19.Dew MA, Myaskovsky L, Switzer GE, et al. Profiles and predictors of the course of psychological distress across four years after heart transplantation. Psychol Med. 2005;35:1215–27. doi: 10.1017/s0033291705004563. [DOI] [PubMed] [Google Scholar]
- 20.DeVito Dabbs A, Dew MA, Stilley CS, et al. Psychosocial vulnerability, physical symptoms and physical impairment after lung and heart-lung transplantation. J Heart Lung Transplant. 2003;22(22):1268–75. doi: 10.1016/s1053-2498(02)01227-5. [DOI] [PubMed] [Google Scholar]
- 21.Bunzel B, Laederach-Hoffman K. Solid organ transplantation: are there predictors for posttransplant noncompliance? A literature overview. Transplantation. 2000;70:711–16. doi: 10.1097/00007890-200009150-00001. [DOI] [PubMed] [Google Scholar]
- 22.Trzepacz PT, Levenson JL, Tringali R. Psychopharmacology and neuropsychiatric syndromes in organ transplantation. Gen Hosp Psychiatry. 1991;13:233–45. doi: 10.1016/0163-8343(91)90124-f. [DOI] [PubMed] [Google Scholar]
- 23.House R, Trzepacz PT, Thompson TL. Psychiatric consultation to organ transplant services. In: Tasman A, Goldfinger SM, Kaufman CA, editors. Review of psychiatry volume 9. Washington, DC: American Psychiatric Press; 1990. pp. 515–535. [Google Scholar]
- 24.House RM, Thompson TL. Psychiatric aspects of organ transplantion. JAMA. 1988;260(4):535–39. [PubMed] [Google Scholar]
- 25.Levenson JL, Olbrisch ME. Psychiatric aspects of heart transplantation. Psychosomatics. 1993;34(2):114–123. doi: 10.1016/S0033-3182(93)71901-5. [DOI] [PubMed] [Google Scholar]
- 26.Dew MA, Kormos RL, DiMartini AF, et al. Prevalence and risk of depression and anxiety-related disorders during the first three years after heart transplantation. Psychosomatics. 2001;42:300–312. doi: 10.1176/appi.psy.42.4.300. [DOI] [PubMed] [Google Scholar]
- 27.Dew MA, Kormos RL, Roth LH, Murali S, DiMartini A, Griffith BP. Early post-transplant medical compliance and mental health predict physical morbidity and mortality 1–3 years after heart transplantation. J Heart Lung Transplant. 1999;18:549–562. doi: 10.1016/s1053-2498(98)00044-8. [DOI] [PubMed] [Google Scholar]
- 28.DiMartini A, Dew MA, Kormos R, et al. Post-Traumatic Stress Disorder Caused by Hallucinations and Delusions Experienced While Delirious. Psychosomatics. 2007;48(5):436–9. doi: 10.1176/appi.psy.48.5.436. [DOI] [PubMed] [Google Scholar]
- 29.Cuthbertson BH, Hull A, Strachan M, et al. Intensive Care Med. 2004;30:450–455. doi: 10.1007/s00134-003-2004-8. [DOI] [PubMed] [Google Scholar]
- 30.Coffman K, Crone C. Rational guidelines for transplantation in patients with psychotic disorders. Current Opinions in Organ Transplantation. 2002;7:385–88. [Google Scholar]
- 31.Dew MA, Dimartini AF, Steel J, et al. Meta-analysis of risk for relapse to substance use after transplantation of the liver and other organs. Liver Transpl. 2008;14:159–72. doi: 10.1002/lt.21278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu LU, Schiano TD, Lau N, et al. Survival and risk of recidivism in methadone-dependent patients undergoing liver transplantation. Am J Transplant. 2003;3:1273–7. doi: 10.1046/j.1600-6143.2003.00199.x. [DOI] [PubMed] [Google Scholar]
- 33.Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144:127–34. doi: 10.7326/0003-4819-144-2-200601170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarter RE, Switala JA, Arria A, Plail J, Van Thiel DH. Subclinical hepatic encephalopathy: comparison before and after orthotopic liver transplantation. Transplantation. 1990;50:632–637. [PubMed] [Google Scholar]
- 35.Parekh PI, Blumenthal JA, Babyak MA, et al. Gas exchange and exercise capacity affect neurocognitive performance in patients with lung disease. Psychosom Med. 2005;67:425–32. doi: 10.1097/01.psy.0000160479.99765.18. [DOI] [PubMed] [Google Scholar]
- 36.Breitbart W, Gibson C, Tremblay A. The delirium experience: Delirium recall and delirium related stress in hospitalized patients with cancer, their spouses/caregivers and their nurses. Psychosomatics. 2002;43(3):183–194. doi: 10.1176/appi.psy.43.3.183. [DOI] [PubMed] [Google Scholar]
- 37.Bajaj JS, Saeian K, Verber MD, et al. Inhibitory Control Test Is a Simple Method to Diagnose Minimal Hepatic Encephalopathy and Predict Development of Overt Hepatic Encephalopathy. Am J Gastroenterol. 2007;102:754–760. doi: 10.1111/j.1572-0241.2007.01048.x. [DOI] [PubMed] [Google Scholar]
- 38.Ferenci P, Lockwood A, Mullen K, et al. Hepatic Encephalopathy—Definition, Nomenclature, Diagnosis, and Quantification: Final Report of the Working Party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–721. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 39.David A Sass, Shakil A Obaid. Fulminant Hepatic Failure. Liver Transpl. 2005;11(6):594–605. doi: 10.1002/lt.20435. [DOI] [PubMed] [Google Scholar]
- 40.Stravitz RT, Kramer AH, Davern T, Shaikh OS, Caldwell SH, Mehta RL, Blei AT, Fontana RJ, McGuire BM, Rossaro L, Smith AD, Lee WM., MD Acute Intensive care of patients with acute liver failure:Recommendations of the U.S. Acute Liver Failure Study Group. Crit Care Med. 2007;35:2498–2508. doi: 10.1097/01.CCM.0000287592.94554.5F. [DOI] [PubMed] [Google Scholar]
- 41.Weissenborn K, Bokemeyer M, Krause J, Ennen J, Ahl B. Neurological and neuropsychiatric syndromes associated with liver disease. AIDS. 2005;19(suppl 3):S93–S98. doi: 10.1097/01.aids.0000192076.03443.6d. [DOI] [PubMed] [Google Scholar]
- 42.Chung RT, Podolsky DK. In: Cirrhosis and its complications, in Harrison’s Principles of Internal Medicine. Braunwald E, Fauci AS, Isselbacher KJ, et al., editors. New York: McGraw-Hill; 2003. [Google Scholar]
- 43.Dejong CHC, van de Poll MCG, Soeters PB, et al. Aromatic Amino Acid Metabolism during Liver Failure. J Nutr. 2007;137:1579S–1585S. doi: 10.1093/jn/137.6.1579S. [DOI] [PubMed] [Google Scholar]
- 44.Bartynski WS, Boardman JF. Distinct Imaging Patterns and Lesion Distribution in Posterior Reversible Encephalopathy Syndrome. Am J Neuroradiol. 2007;28:1320–27. doi: 10.3174/ajnr.A0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCarthy PM. Implantable left ventricular assist device bridge to transplantation: natural selection, or is this the natural selection? J Am Coll Cardiol. 2002;39:1255–1237. doi: 10.1016/s0735-1097(02)01764-3. [DOI] [PubMed] [Google Scholar]
- 46.Grady KL, Meyer P, Mattea A, et al. Improvement in Quality of Life Outcomes 2 Weeks After Left Ventricular Assist Device Implantation. J Heart Lung Transplant. 2001;20:657–669. doi: 10.1016/s1053-2498(01)00253-4. [DOI] [PubMed] [Google Scholar]
- 47.Chapman E, Parameshwar J, Jenkins D, Large S, Tsui S. Psychosocial issues for patients with ventricular assist devices: a qualitative pilot study. A J Critical Care. 2007;16(1):72–81. [PubMed] [Google Scholar]
- 48.Dew MA, Kormos RL, Winowich S, Harris RC, Stanford EA, Carozza L, Griffith BP. Quality of life outcomes after heart transplantation in individuals bridged to transplant with ventricular assist devices. Journal of Heart & Lung Transplantation. 2001;20(11):1199–212. doi: 10.1016/s1053-2498(01)00333-3. [DOI] [PubMed] [Google Scholar]
- 49.Komoda T, Drews T, Sakuraba S, et al. Executive Cognitive Dysfunction without Stroke after Long-Term Mechanical Circulatory Support. ASAIO Journal. 2005;51:764–768. doi: 10.1097/01.mat.0000183685.81983.5d. [DOI] [PubMed] [Google Scholar]
- 50.Thoennissen NH, Schneider M, Allroggen A, et al. High level of cerebral microembolization in patients supported with the DeBakey left ventricular assist device. J Thorac Cardiovasc Surg. 2005;130:1159–66. doi: 10.1016/j.jtcvs.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 51.Zimpfer D, Wieselthaler G, Czerny M, et al. Neurocognitive Function in Patients with Ventricular Assist Devices: A Comparison of Pulsatile and Continuous Blood Flow Devices. ASAIO Journal. 2006;52:24–27. doi: 10.1097/01.mat.0000191334.51375.7e. [DOI] [PubMed] [Google Scholar]
- 52.Han C, Kim Y. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45(4):297–301. doi: 10.1016/S0033-3182(04)70170-X. [DOI] [PubMed] [Google Scholar]
- 53.Fireman M, DiMartini AF, Armstrong SC, Cozza KL. Med-psych drug interactions update: immunosuppressants. Psychosomatics. 2004;45(4):p354–360. [Google Scholar]
- 54.Bechstein WO. Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transplant International. 2000;13(5):313–26. doi: 10.1007/s001470050708. [DOI] [PubMed] [Google Scholar]
- 55.Ahn K, Lee JW, Hahn ST, Yang DW, Kim PS, Kim HJ, Kim CC. Diffusion-weighted MRI and ADC mapping in FK506 neurotoxicity. British Journal of Radiology. 2003;76:916–919. doi: 10.1259/bjr/77297900. [DOI] [PubMed] [Google Scholar]
- 56.Echaniz-Laguna Battaglia F, Ellero B, et al. Chroinc inflammatory demyelinating polyradiculoneuropathy in patients with liver transplantation. Muscle Nerve. 2004;30:501–504. doi: 10.1002/mus.20086. [DOI] [PubMed] [Google Scholar]
- 57.Wilson JR, Conwit RA, Eidelmann BH, et al. Sensorimotor neuropathy resembling CIDP in patients receiving FK506. Muscle Nerve. 1994;17:528–532. doi: 10.1002/mus.880170510. [DOI] [PubMed] [Google Scholar]
- 58.Kershner P, Wang-Cheng R. Psychiatric side effects of steroid therapy. Psychosomatics. 1989;30(2):135–9. doi: 10.1016/S0033-3182(89)72293-3. [DOI] [PubMed] [Google Scholar]
- 59.Lewis DA, Smith RE. Steroid-induced psychiatric syndromes: A report of 14 cases and a review of the literature. Journal of Affective Disorders. 1983;5(4):319–32. doi: 10.1016/0165-0327(83)90022-8. [DOI] [PubMed] [Google Scholar]
- 60.Hall RC, Popkin MK, Stickney SK, Gardner ER. Presentation of the steroid psychoses. Journal of Nervous & Mental Disease. 1979;167(4):229–36. doi: 10.1097/00005053-197904000-00006. [DOI] [PubMed] [Google Scholar]
- 61.Varney NR, Alexander B, MacIndoe JH. Reversible steroid dementia in patients without steroid psychosis A. J Psych. 1984;141(3):369–72. doi: 10.1176/ajp.141.3.369. [DOI] [PubMed] [Google Scholar]
- 62.Wolkowitz OM, Reus VI. Treatment of depression with antiglucocorticoid drugs. Psychosomatic Medicine. 1999;61:698–711. doi: 10.1097/00006842-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 63.OPTN/UNOS Bylaws, Appendix B, Attachment I, Section XIII, C(2) and (4), Designated Transplant Program Criteria, Programs performing living donor kidney and liver transplants approved, September 17, 2007 (www.unos.org). www.unos.org/news/newsdetail.asp?id-946
- 64.Middleton PF, Duffield M, Lynch SV, et al. Living donor liver transplantation--adult donor outcomes: a systematic review. Liver Transplant. 2006;12(1):24–30. doi: 10.1002/lt.20663. [DOI] [PubMed] [Google Scholar]
- 65.Matas AJ, Bartlett ST, Leichtman AB, Delmonico FL. Morbidity and mortality after living kidney donation, 1999–2001: survey of United States transplant centers. Am J Transplant. 2003;3(7):830–4. [PubMed] [Google Scholar]
- 66.Tan HP, Spector ZZ, Shapiro R. Perioperative donor risk [kidney] In: Tan HP, Marcos A, Shapiro R, editors. Living donor transplantation. New York: Informa Healthcare; 2007a. pp. 69–79. [Google Scholar]
- 67.Tan HP, Martin AE, Kilac A, Lopez R, Marcos A. Donor outcomes [liver] In: Tan HP, Marcos A, Shapiro R, editors. Living donor transplantation. New York: Informa Healthcare; 2007b. pp. 185–195. [Google Scholar]
- 68.Brown RS, Jr, Russo MW, Lai M, Shiffman ML, Richardson MC, Everhart JE, Hoofnagle JH. A survey of liver transplantation from living adult donors in the United States. New England J Med. 2003;348:818–25. doi: 10.1056/NEJMsa021345. [DOI] [PubMed] [Google Scholar]
- 69.Davis CL. Long-term risks of living donation. In: Tan HP, Marcos A, Shapiro R, editors. Living donor transplantation. New York: Informa Healthcare; 2007. pp. 77–85. [Google Scholar]
- 70.Erim Y, Beckmann M, Valentin-Gamazo C, Malago M, Frilling A, Schlaak JF, Gerken G, Broelsch CE, Senf W. Quality of life and psychiatric complications after adult living donor liver transplantation. Liver Transplant. 2006;12:1782–90. doi: 10.1002/lt.20907. [DOI] [PubMed] [Google Scholar]
- 71.Jacobs CL, Roman D, Garvey C, Kahn J, Matas AJ. Twenty-two nondirected kidney donors: an update on a single center’s experience. Am J Transplant. 2004;4:1110–6. doi: 10.1111/j.1600-6143.2004.00478.x. [DOI] [PubMed] [Google Scholar]
- 72.Johnson EM, Remucal MJ, Gillingham KJ, Dahms RA, Najarian JS, Matas AJ. Complications and risks of living donor nephrectomy. Transplantation. 1997;64:1124–8. doi: 10.1097/00007890-199710270-00007. [DOI] [PubMed] [Google Scholar]
- 73.Moss J, Lapointe-Rudow D, Renz JF, Kinkhabwala M, Dove LM, Gaglio PJ, Emond JC, Brown RS., Jr Select utilization of obese donors in living donor liver transplantation: implications for the donor pool. Am J Transplant. 2005;5:2974–81. doi: 10.1111/j.1600-6143.2005.01124.x. [DOI] [PubMed] [Google Scholar]
- 74.Verbesey JE, Simpson MA, Pomposelli JJ, Richman E, Bracken AM, Garrigan K, Chang H, Jenkins RL, Pomfret EA. Living donor adult liver transplantation: a longitudinal study of the donor’s quality of life. Am J Transplant. 2005;5:2770–7. doi: 10.1111/j.1600-6143.2005.01092.x. [DOI] [PubMed] [Google Scholar]
- 75.Diaz GC, Renz JF, Mudge C, et al. Donor health assessment after living-donor liver transplantation. Ann Surg. 2002;236:120–6. doi: 10.1097/00000658-200207000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim-Schluger L, Florman SS, Schiano T, et al. Quality of life after lobectomy for adult liver transplantation. Transplantation. 2002;73:1593–97. doi: 10.1097/00007890-200205270-00012. [DOI] [PubMed] [Google Scholar]
- 77.Bergman S, Feldman LS, Mayo NE, Carli F, Anidjar M, Klassen DR, Andrew CG, Vassiliou MC, Stanbridge DD, Fried GM. Measuring surgical recovery: the study of laparoscopic live donor nephrectomy. Am J Transplant. 2005;5:2489–95. doi: 10.1111/j.1600-6143.2005.01054.x. [DOI] [PubMed] [Google Scholar]
- 78.Kok NF, Alwayn IP, Tran KT, Hop WC, Weimar W, Ijzermans JN. Psychosocial and physical impairment after mini-incision open and laparoscopic donor nephrectomy: A prospective study. Transplantation. 2006;82:1291–7. doi: 10.1097/01.tp.0000239312.45050.05. [DOI] [PubMed] [Google Scholar]
- 79.Lumsdaine JA, Wray A, Power MJ, Jamieson NV, Akyol M, Andrew Bradley J, Forsythe JL, Wigmore SJ. Higher quality of life in living donor kidney transplantation: prospective cohort study. Transplant International. 2005;18:975–80. doi: 10.1111/j.1432-2277.2005.00175.x. [DOI] [PubMed] [Google Scholar]
- 80.Clemens KK, Thiessen-Philbrook H, Parikh CR, et al. Psychosocial health of living kidney donors: A systematic review. Am J Transplant. 2006;6(12):2965–277. doi: 10.1111/j.1600-6143.2006.01567.x. [DOI] [PubMed] [Google Scholar]
- 81.Dew MA, Switzer GE, DiMartini AF, Myaskovsky L, Crowley-Matoka M. Psychosocial aspects of living organ donation. In: Tan HP, Marcos A, Shapiro R, editors. Living Donor Organ Transplantation. NY: Taylor and Francis; 2007. pp. 7–26. [Google Scholar]
- 82.Simmons RG, Klein SD, Simmons RL. Gift of life: The social and psychological impact of organ transplantation. NY: Wiley; 1977. Reprinted with additions, Brunswick, NJ: Transaction Books, 1987. [Google Scholar]
- 83.Song MK, De Vito Dabbs AJ. Advance care planning after lung transplantation: a case of missed opportunities. Progress in Transplantation. 2006;16(3):222–5. doi: 10.1177/152692480601600306. [DOI] [PubMed] [Google Scholar]
- 84.Hadjiliadis D, Steele MP, Govert JA, Davis RD, Palmer SM. Outcome of lung transplant patients admitted to the medical ICU. Chest. 2004;125(3):1040–5. doi: 10.1378/chest.125.3.1040. [DOI] [PubMed] [Google Scholar]
- 85.Poncet MC, Toullic P, Papazian L, et al. Burnout syndrome in crtical care nursing staff. Am J Respir Crit Care Med. 2007;175:698–704. doi: 10.1164/rccm.200606-806OC. [DOI] [PubMed] [Google Scholar]
- 86.Embriaco N, Papazian L, Kentish-Barnes N, et al. Burnout syndrome among critical care healthcare workers. Curr Opin Crit Care. 2007;13:482–8. doi: 10.1097/MCC.0b013e3282efd28a. [DOI] [PubMed] [Google Scholar]
- 87.Levy MM. Caring for the caregiver. Crit Care Clin. 2004;20:541–7. doi: 10.1016/j.ccc.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 88.Espeland KE. Overcoming burnout: how to revitalize your career. Journal of Continuing Education in Nursing. 2006;37:178–184. doi: 10.3928/00220124-20060701-04. [DOI] [PubMed] [Google Scholar]
- 89.Davies WR. Mindful meditation: healing burnout in critical care nursing. Holist Nurs Pract. 2008;22:32–6. doi: 10.1097/01.HNP.0000306326.56955.14. [DOI] [PubMed] [Google Scholar]
- 90.Trzepacz PT. Delirium. In: Levenson J, editor. Textbook of psychosomatic medicine. Washington, DC: American Psychiatric Publishing; 2005. pp. 91–130. [Google Scholar]