Abstract
AMPA receptors are glutamate-gated ion channels that are essential mediators of synaptic signals in the central nervous system. They form tetramers that are assembled as combinations of the subunits GluR1-4, each of which contains a ligand-binding domain (LBD). Crystal structures of the GluR2 LBD have revealed an agonist-binding cleft, which is located between two lobes and which acts like a Venus flytrap. In general, agonist efficacy is correlated with the extent of cleft closure. However, recent observations show that cleft closure is not the sole determinant of relative efficacy for glutamate receptors. In addition, these studies have focused on the GluR2 subunit, which is the specific target of a physiologically important RNA-editing modification in vivo. We therefore wished to test the generality of the cleft closure:efficacy correlation for other AMPA-R subunits. Here, we present crystal structures of the GluR4flip LBD in complex with both full and partial agonists. As for GluR2, both agonists stabilize a closed-cleft conformation, and the partial agonist induces a smaller cleft closure than the full agonist. However, a detailed analysis of LBD:kainate interactions reveals the importance of subtle backbone conformational changes in the ligand-binding pocket in determining the magnitude of agonist-associated conformational changes. Furthermore, the GluR4 subunit exhibits a different correlation between receptor activation and LBD cleft closure than does GluR2.
Keywords: ionotropic glutamate receptor, AMPA receptor ion channel, GluR4 ligand-binding domain, X-ray crystallography, electrophysiology, subunit dimerization, relative agonist efficacy, conformational change
Within the central nervous system, most fast excitatory synaptic signals are mediated by ionotropic glutamate receptors (iGluRs) that are selective for the synthetic agonist AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) (1). In addition to their role in neurotransmission, the AMPA receptors (AMPA-Rs) contribute to synaptic plasticity and are thus thought to play important roles in learning and memory (2). AMPA-Rs have also been implicated in neuropathological conditions such as epilepsy, ischemia, neurodegeneration, glioma, and multiple sclerosis (3–5).
AMPA-Rs are assembled as tetramers of subunits known as GluR1-GluR4. The composition of the receptors in a given neuron depends on which subunits are expressed, and on a variety of post-transcriptional modifications, including alternative splicing and RNA editing (reviewed in ref. 1). A particularly important modification involves the selective RNA editing of the mRNA encoding the GluR2 subunit, which converts a genetically encoded Gln codon common to all subunits to an Arg codon at position 586 in the mature sequence of GluR2. This editing is nearly 100% efficient in adults. Heteromeric channels that include edited GluR2-R subunits are the most common form of AMPA-R in vivo. They are non-rectifying and conduct predominantly monovalent cations. In contrast, AMPA-Rs that do not include GluR2-R subunits display inward rectification and are permeable to both monovalent ions and Ca2+. Although less widespread, there is increasing evidence that such Ca2+-permeable AMPA-Rs play important roles in both physiological and pathophysiological processes (reviewed in refs. 6, 7).
The GluR2 ligand-binding domain (LBD) forms a bilobate structure that binds ligands in a deep cleft located between the two lobes. High-resolution X-ray structures of multiple LBD:ligand complexes have shown that full agonists stabilize a fully closed cleft, while antagonists stabilize an open cleft, and partial agonists induce intermediate degrees of cleft closure (8). Thus, although the ligand-free form of the core LBD exhibits some conformational flexibility (9), the ligand-bound GluR2 structures generally show a direct correlation between the degree of cleft closure and the extent of channel activation (10). Recent LRET-reporter assays suggest that the conformational changes seen in the isolated LBD are reflective of changes that take place in functional receptors (11). However, the same correlation does not hold in the case of the N-methyl-D-aspartate (NMDA)-selective family of ionotropic glutamate receptors. Instead, it has been observed that both full and partial agonists induce a full cleft closure of the NR1 LBD (12). Furthermore, a GluR2 mutation (T686A) has been identified that differentially affects the efficacies of glutamate and quisqualate without altering the extent of cleft closure seen in the isolated LBD (13). To account for this observation, it has been proposed that the T686A mutation destabilizes the closed versus the open cleft conformation.
While the fully edited GluR2 subunit plays an important role in the regulation of the Ca2+ permeability of iGluRs (14), the edited GluR2 subunit is poorly expressed and forms only weakly conducting ion channels by itself (15, 16). As a result, functional studies involving GluR2 homomers have been carried out on the GluR2-Q form, taking advantage of the fact that the electrophysiological behavior of the unedited GluR2 subunit is similar to that of the non-GluR2 subunits. To date, our understanding of the role of cleft closure in AMPA-R activation is based on this work, coupled with crystal structures of the GluR2-LBD. In contrast, structures are available for multiple members of the other major families of iGluRs (selective for NMDA and kainate, respectively) (12, 17, 18). As a result, it is important to assess the extent to which the relationship between cleft closure and relative efficacy is a general characteristic of AMPA-R LBDs. To address this question, we have pursued the crystallization and structure determination of the GluR4flip subunit.
GluR4 plays a crucial role during the early postnatal phase in the proper development of synaptic circuitry by forming functional channels in response to weak synaptic activity (19). GluR4 subunits are co-expressed with GluR2 in the rat subthalamic cells (20) and in granule cells of the cerebellum and cochlear nucleus (21), and GluR2 and GluR4 are known to form functional heteromeric channels (6, 22). Furthermore, during the development of cerebellar granule cells, expression of the GluR4flip isoform predominates during early development, but remains relatively constant, whereas expression of the GluR4flop isoform, which has different biophysical characteristics, increases with age (23, 24). Studies carried out in cultured hippocampal neurons subjected to sublethal ischemic conditions have shown a shift in AMPA-R expression patterns, characterized by increased GluR4 expression levels, and a greater permeability to Ca2+ ions which could lead to post-ischemic neuronal damage (25). Ultimately, GluR4 may therefore be an important therapeutic target.
Here, we present a structural analysis of the GluR4flip LBD core in complexes with the physiological full agonist glutamate and the partial agonist kainate (KA), together with an electrophysiological analysis of the GluR4 channel responses to these agonists. Our studies reveal a dependence of relative agonist efficacy on cleft closure that is qualitatively similar to, but quantitatively distinct from that seen for GluR2. In addition, these structures allow us to analyze the compatibility of trans-subunit dimerization interactions and to investigate stereochemical differences in LBD:ligand interactions. These results emphasize the three-dimensional nature of the activation process in ligand-gated ion channels and the resulting need for high-resolution structural information on fully assembled receptors.
EXPERIMENTAL PROCEDURES
Expression constructs
Full-length constructs for electrophysiological studies were derived from the pRK plasmid encoding the full-length GluR4flip glutamate-receptor subunit from R. norvegicus (provided by Dr. P. Seeburg, MPI, Heidelberg, Germany). The coding sequence, which contained Ile residues at positions 713 and 834, was modified to match the published GluR4flip sequence (NP_058959, ref. 26), which contains Thr residues at these positions, using the QuikChange (Stratagene, CA) mutagenesis kit. The L484Y mutation was then introduced using the QuikChange kit. Preliminary analysis showed no significant effect of the substitutions (I713T and I834T) on the relative efficacy of kainate. The LBD construct used in crystallization studies has been described elsewhere (27). It contains the GluR4flip S1 and S2 sequences (residues 393–507 and 633–775 of the mature sequence, respectively) joined by a GT linker and sub-cloned into the pET16b vector (Novagen) with a thrombin cleavable polyhistidine tag. The accuracy of all constructs was verified by DNA sequencing.
LBD purification, crystallization, and data collection
Detailed expression, purification, and crystallization conditions for the GluR4 LBD construct have been reported (27). Briefly, proteins were expressed in XJB(DE3) cells (Novagen), purified by immobilized metal affinity chromatography, and treated with thrombin to release the polyhistidine tag. The cleaved protein was collected from the flow-through of a Ni-NTA column, dialyzed extensively in crystallization buffer (10 mM HEPES-NaOH, pH 7.0, 30 mM NaCl, 1 mM EDTA), mixed with ligand, and crystallized by vapor diffusion against 25% (w/v) PEG 4000, 0.1 M sodium acetate pH 4.6, 0.2 M ammonium acetate (glutamate complex) or 25.5% (w/v) PEG 1500 and 50 mM sodium acetate pH 4.5 (kainate complex). A complete X-ray dataset was collected for a GluR4 LBD-Glu crystal (at 100K) and for a GluR4 LBD-KA crystal (at RT) on a MAR345dtb image plate system, using Cu Kα radiation produced by a rotating anode (Rigaku) equipped with focusing mirrors (Genova) and a Cryostream 700 (Oxford Cryosystems).
Structure refinement
All datasets were indexed, integrated and scaled using programs of the XDS package (28). Since the GluR4-LBD:Glu crystal exhibited non-crystallographic symmetry (NCS), the Rfree test set was selected in thin shells using the program SFTOOLS. In the case of the GluR4-LBD:KA model, the Rfree test set was picked by random selection using the program SFTOOLS. In each case, the same Rfree set was used throughout refinement.
We first determined the structure of the GluR4-LBD:Glu co-crystal, for which the highest resolution data were available. Initial phases were obtained by molecular replacement using CNS (29), with the GluR2-LBD:Glu structure (PDB code: 1FTJ, ref. 30) as a search model. Cross rotation and translation functions yielded a final correlation coefficient of 0.67 (12 – 3 Å resolution) and a calculated solvent content of 54%. In order to minimize model bias, following rigid-body refinement, simulated annealing and composite omit-map calculations were performed, together with interative cycles of solvent flattening, histogram matching, and two-fold NCS averaging, using the program DM (31).
An initial model was manually built into this iteratively averaged omit map using the program COOT (32). Refinement was carried out using a combination of the CNS and CCP4 (33) programs. Following manual model building, rigid-body refinement, conjugate-gradient minimization, and individual and grouped B-factor refinement were performed in CNS, applying strict two-fold NCS constraints. After several rounds of refinement, a second protein molecule was generated and two-fold NCS restraints were applied. At the stage of refinement where clear and unambiguous density was seen for the ligand in FO-FC maps, it was added to the model (Figure 1B). Once the model was well-refined, water molecules were picked from 2FO-FC maps using PEAKMAX and WATPEAK (>4σ, <4 Å from nearest atom), and were refined after placement in the electron density map. Waters were discarded if they were unstable during refinement, exhibited weak density, or if they were <3.5 Å from a hydrogen-bonding partner. The final stages of refinement were carried out using TLS refinement in REFMAC5 (34).
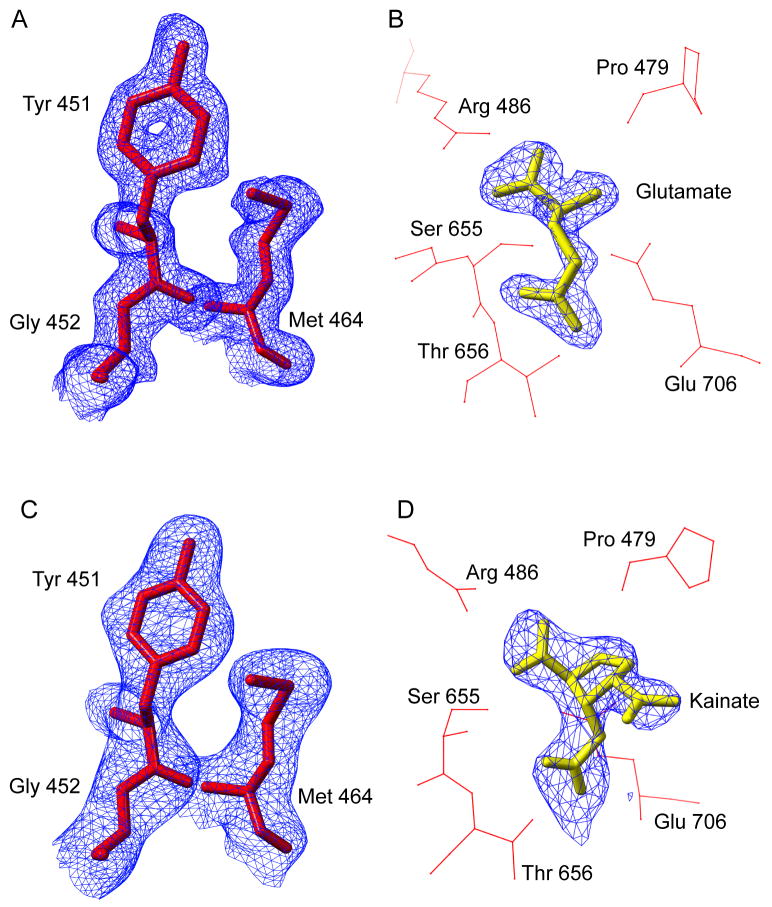
Figure 1. Experimental electron density for the GluR4-LBD:Glu complex.
The final refined GluR4-Glu (A) and GluR4-KA (C) structures (red stick figures) are shown together with the respective density-modified, composite omit maps (blue) used for initial model building. The glutamate (B) and kainate (D) ligands (yellow stick figures) were added to the models only after clear electron density (blue) was observed at the corresponding ligand location, following several rounds of refinement in the absence of any ligand molecule. Several of the residues surrounding the ligand molecule are shown (red stick figures). This figure was prepared using CHIMERA (36).
To determine the structure of the GluR4-LBD:KA complex, the refined GluR4-LBD:Glu structure was used as a search model for molecular replacement calculations. Cross rotation and translation functions yielded a final correlation coefficient of 0.71 (12 – 3 Å resolution) and a calculated solvent content of 50%. Initial density modified composite omit calculations, model building and refinement of the GluR4-LBD:KA structure were carried out using the same strategy as for the GluR4-LBD:Glu model, with the exception that NCS was not present.
Domain superpositions
The program LSQKAB (35) was used to perform superposition of the GluR4 and GluR2 LBDs. The GluR2 LBD structures used for the comparison were: GluR2-LBD:KA (PDB entry 1FWO) and GluR2-LBD:Glu (1FTJ). Residues that composed the core secondary structure elements comprising the proteins were used to define the regions of superposition. In lobe 1, GluR4 residues were matched to GluR2 residues as follows: 396–400 to 395–399, 411–415 to 410–414, 418–421 to 417–420, 424–438 to 423–437, 441–445 to 440–444, 463–469 to 462–468, 484–488 to 483–487, 489–492 to 488–491, 496–499 to 495–498, 731–739 to 730–738 and 743–756 to 742–755. For lobe 2, superpositions were based on the matching of GluR4 residues to GluR2 residues in the following order: 501–507 to 500–506, 636–642 to 635–641, 647–649 to 646–648, 655–662 to 654–661, 666–677 to 665–676, 686–696 to 685–695, 701–706 to 700–705, 707–710 to 706–714, 721–724 to 720–723 and 758–770 to 757–769. All reported cleft closure angles were calculated using LSQKAB (35). Linker distances were measured in the program CHIMERA (36).
tsA201 Cell culture and transfection
The human embryonic kidney 293 derivative cell line tsA201 (kindly provided by Dr. Derek Bowie, McGill University, Montreal, Canada) was grown on tissue culture dishes (Falcon) and maintained in Dulbecco’s modified Eagle’s medium (Mediatech) supplemented with 10% fetal bovine serum (Invitrogen), 2 mM L-glutamine, 50 IU/mL penicillin, and 50 μg/mL streptomycin (Mediatech) at 37° and 5% CO2. tsA201 cells were transiently transfected with 2μg AMPA receptor cDNA construct and 0.8 μg pGreenLantern plasmid (Invitrogen) using Lipofectamine 2000. Whole-cell measurements were conducted approximately 24 hours after transfection. Outside-out patches were recorded after 48 hours. The pGreenLantern plasmid was used as a positive transfection indicator.
Electrophysiology
L-glutamic acid Na-hydrate (Sigma) and kainate from Digenea simplex (Sigma) stock solutions were prepared from solids and used within 48 hours of dilution. Cyclothiazide (CTZ, Tocris) was dissolved in DMSO to form a 50 mM stock solution. CTZ was used at a final concentration of 100 μM for recordings.
Borosilicate glass pipettes with filament (Sutter Instrument Co.) were pulled and fire-polished to a final resistance, when filled, of 5–8 MΩ. All recordings were performed at room temperature (~22°C). Whole cells and outside-out patches were voltage-clamped at −60 mV. Agonist solutions were applied as described previously (37) using the LSS-3100 High Speed Positioning System (Burleigh Instruments Inc., Fishers, NY), Teflon tubing and a double-barreled theta outlet pipe with a tip diameter of 100–120 μm (Sutter Instrument Co.). Open-tip, whole-cell and patch currents showed that 10–90% maximal on and off responses were attained in less than 2 ms. During recordings, the culture dish was perfused with a solution composed of 145 mM NaCl, 1.8 mM MgCl2 1 mM CaCl2, 3 mM KCl, 10 mM Glucose and 10 mM HEPES (pH to 7.4 using NaOH). The internal pipette solution was composed of the following: 140 mM CsCl, 2 mM MgCl2, 1 mM CaCl2, 10 mM EGTA, 2 mM Na2ATP and 10 mM HEPES (pH to 7.35 using KOH). Data were acquired using an EPC-9 amplifier and were low-pass filtered at 10 kHz. Between two and five current responses of 100 ms duration each were averaged for subsequent analysis of peak current (Ipeak) using HEKA Pulse and PulseFit software (Instrutech Corp.).
RESULTS AND DISCUSSION
In this study, crystal structures have been determined for the GluR4 LBD in complex with the physiological agonist glutamate and in complex with the partial agonist kainate (Table 1). The presence of non-crystallographic symmetry in the GluR4-LBD:Glu crystals provided a strong basis for reducing model bias following phase determination by molecular replacement. The electron density in iteratively averaged composite omit maps was of excellent quality and permitted direct modeling of the GluR4-LBD core structure (Figure 1A). After several rounds of refinement at 1.85 Å resolution, the density for the bound glutamate ligand was also of excellent quality (Figure 1B), and ligand was incorporated into the model. The final model consists of 514 residues in two molecules, with a bound glutamate ligand for each, and a total of 310 water molecules (Table 1). The rms difference between the two chains in the asymmetric unit is 0.18 Å, consistent with a highly conserved, stable structure. The refined GluR4-LBD:Glu model was used without ligand as a molecular replacement search model for the GluR4-LBD:KA complex. Density-modified composite omit maps were again unambiguous (Figure 1C). The structure was refined at 2.43 Å resolution. As was the case for the glutamate complex, clear density for the bound kainate ligand emerged during refinement (Figure 1D), and was fit accordingly. The final kainate model consists of 257 residues, a bound kainate ligand, and 97 water molecules (Table 1). The resulting models provide insights into the structure of an additional AMPA receptor subunit and permit comparison with the previously determined GluR2-LBD structures. Coordinates of the GluR4-LBD:Glu and GluR4-LBD:KA models have been deposited in the Protein Data Bank, with PDB codes are 3EPE and 3EN3, respectively.
Table 1.
Data collection and refinement statistics
| Protein | GluR4 LBD | |
|---|---|---|
| Ligand | L-Glutamate | Kainate |
| Conditions | 100 K, flash-cooled | RT, capillary mounted |
| Space Group | P21 | C2 |
| Unit Cell Parameters (Å, °) | a=47.4 b=105.1 c=66.6 β=97.3 |
a=125.9 b=48.8 c=47.8 β=109.1 |
| Matthews coefficient (Å3/Da) | 2.67 | 2.32 |
| Solvent content (%) | 53.6 | 49.4 |
| Resolutiona | 19.51 – 1.85 (1.93 – 1.85) | 11.95 – 2.43 (2.49 – 2.43) |
| Total reflections a | 202326 (23356) | 38423 (2663) |
| Unique reflections a | 52938 (6140) | 10025 (681) |
| Rsym (%) ab | 8.9 (17.5) | 10.9 (35.7) |
| Completeness (%) a | 96.3 (94.1) | 95.3 (94.1) |
| Refinement statistics | ||
| # protein atoms (non-H) | 4028 | 2014 |
| # waters | 310 | 97 |
| Rwork (%) a | 19.1 (18.6) | 16.2 (21.6) |
| Rfree (%) a | 22.0 (24.0) | 21.9 (29.0) |
| I/σIa | 11.5 (7.1) | 9.2 (4.1) |
| Ramachandran plot c (%) | 93.3/6.7/0/0 | 94.2/5.8/0/0 |
| RMSD (Bonds/Angles) (Å, °) | 0.009/1.125 | 0.008/1.167 |
| Protein Bav (Å2) d (chain A/B) | 12.45 (12.48/12.42) | 43.58 (N/A) |
| Ligand Bav (Å2) d (chain A/B) | 5.7 (5.9/5.6) | 30.91 (N/A) |
| Water Bav (Å2) d | 14.42 | 46.37 |
Values in parentheses indicate statistics for the highest resolution shell of data;
Rsym = Σhj|Îh−Ihj|/ΣhjIhj;
Core/allowed/generously allowed/disallowed;
Residual B factors calculated by REFMAC (does not include the contribution to atomic displacements from translation, libration and screw-rotation displacement).
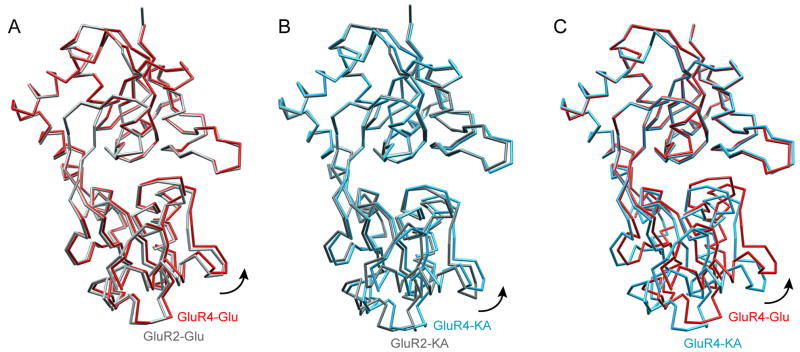
In complex with either glutamate (Figure 2A and 2C, red) or kainate (Figure 2B and 2C, blue), the GluR4 LBD exhibits a bilobate structure with a deep ligand binding cleft between the two lobes, similar to that observed in other members of the iGluR family, and in the homologous bacterial glutamine binding protein (reviewed in ref. 10). Superposition of the glutamate-bound forms of the GluR4 and GluR2 LBD showed that they are very similar (Figure 2A, red and gray, respectively), in terms of both the individual lobes and the overall structure, with rms differences ranging from 0.2–0.3 Å. The kainate-bound structures of the GluR4 and GluR2 LBD (Figure 2B, blue and gray, respectively) show slightly higher rms differences (0.3–0.5 Å), but remain very similar overall.
Figure 2. Comparison of cleft closure in GluR4 and GluR2 ligand-bound structures.
(A) Superposition of glutamate-bound GluR4 LBD (red) and GluR2 LBD (gray) structures. (B) Superposition of the kainate-bound GluR4 LBD (blue) and GluR2 LBD (gray) structures. (C) Superposition of GluR4 LBD structures bound to glutamate (red) and kainate (blue). The Glu-bound structure shows a greater degree of cleft closure than the kainate-bound structure (difference ~6.7°). In each case, the core residues of lobe 1 were superimposed. This figure was prepared using CHIMERA (36)
Trans-subunit compatibility of dimerization interfaces
The protein chains in GluR4-LBD agonist co-crystals pack in a back-to-back fashion to form a 2-fold symmetric dimer (Figure 3A). The dimerization interface consists of hydrogen bonds, hydrophobic interactions, and van der Waals interactions and is mediated between opposing faces of the membrane-distal, i.e. N-terminal, lobes of each LBD (lobe 1). A similar arrangement has been seen in several of the GluR2-LBD:ligand co-crystals (Figure 3B) and has been shown to play an important role in the gating and desensitization of the receptors (38).
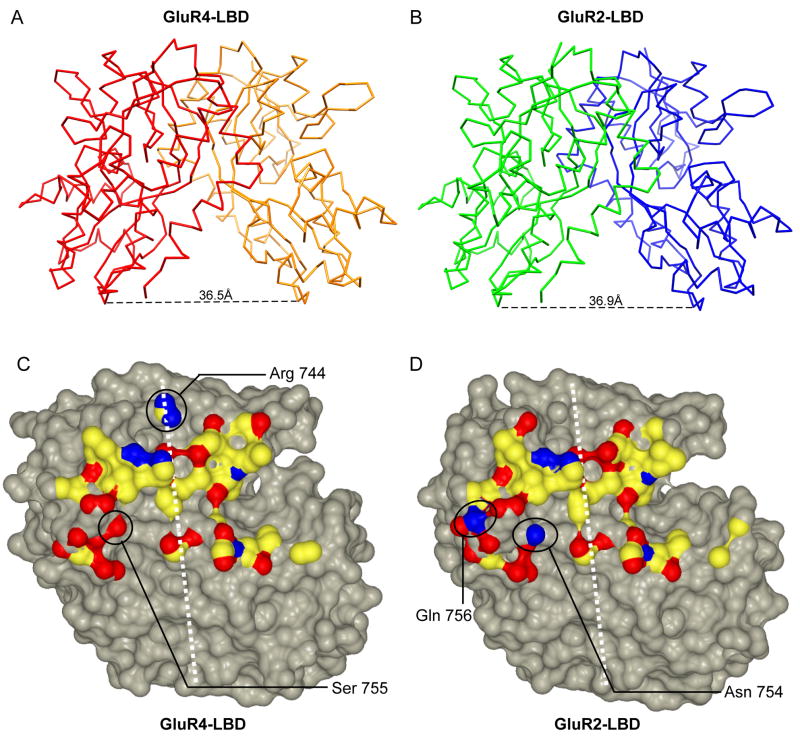
Figure 3. Conservation of the GluR2 and GluR4 dimerization interfaces.
Cα traces of the GluR4-LBD:Glu (A) and GluR2-LBD:Glu (B) dimers are shown with the distances between Gly Cα atoms in the Gly-Thr linker depicted at the bottom. The buried surfaces involved in the homodimer interfaces of the GluR4 LBD (C) and the GluR2 LBD (D) are also shown for a single subunit. In each case, the dimer two-fold axis is shown as a white dashed line. The surface of the residues buried in the interface is colored by atom type (carbon, yellow; oxygen, red; nitrogen, blue). The surfaces of atoms not involved in the dimer interface are colored gray. The footprint of the dimer interface is similar for GluR2 and GluR4, as is the stereochemistry, with the exception of the solvent exposed residue Arg 744 and the GluR4flip/R residues Ser 755 and Ala 757, which replace the GluR2flop/G residues Gly 743, Asn 754, and Gln 756, respectively (circled). This figure was prepared using CCP4MG (55).
The LBD dimer is thought to be incorporated within the framework of a dimer-of-dimers assembly of intact iGluR subunits (reviewed in ref. 10). However, in the absence of structural information on other subunits that comprise physiological, heteromeric AMPA-Rs, it has not been possible to assess directly the compatibility of the interfaces. In addition, alternative splicing of GluR1-4 subunit mRNAs produces either “flip” or “flop” versions of the subunits, which exhibit distinct activation and desensitization characteristics (26). Finally, in GluR2, GluR3, and GluR4, an RNA editing site is located just before the flip/flop cassette (39). The presence of either a Gly or an Arg at this “R/G” site also determines key biophysical properties of receptors containing these subunits (40). Despite this diversity, with the exception of PDB entry 2UXA (41), all published crystal structures have been determined for the “flop” version of GluR2, with a Gly at the R/G site.
The form of GluR4 that has been crystallized in this study is the “flip” variant, with an Arg at the R/G site. A superposition of the glutamate-bound GluR4 and GluR2 LBD dimers reveals an rms difference of only 0.4 Å (Figures 3A and 3B). This value is comparable to the 0.2–0.3 Å rms differences between LBD monomers, suggesting a highly similar dimerization interaction. A comparison of the buried surface of the GluR4flip/R dimer interface (Figure 3C) with that of the GluR2flop/G interface (Figure 3D) reveals a high level of stereochemical compatibility. Since these domains each can form homodimers (two-fold axis shown in white, Figures 3C and 3D), their similarity is also consistent with the ability of the two different subunits and splice variants to form heteromeric channels in vivo (20, 21). In particular, there is a pair of conserved salt bridges between Glu487 on one chain and Lys594 on the opposite chain, as well as a conserved hydrogen bond between Asn748 and Glu487 (GluR4 numbering). Leu484, a residue which blocks desensitization when mutated to a Tyr in GluR3 (42), maintains a set of hydrophobic contacts similar to those seen in GluR2 (38). Even where stereochemical differences are observed (Figures 3C and 3D, circled), interface compatibility is generally preserved. For example, in GluR2flop, Asn754 directly makes a hydrogen bond with Ser729 on the opposite chain. In the case of GluR4flip, the corresponding Ser755 makes a water-mediated hydrogen bond with Ser730 on the protein chain across the dimer interface. At the edge of the interface, Gln 756 in GluR2flop is replaced by Ala 757 in GluR4flip.
The other major stereochemical difference involves Arg744 at the R/G editing site (Figure 3C, circled). Surprisingly, the terminal guanido groups of the two Arg side chains point towards each other, with Nη groups only 3.1 Å apart. A similar arrangement has been observed in the GluR2flip structure (41, 43). There are no stabilizing lattice contacts in the vicinity in either the GluR4flip structures reported here nor in the GluR2flip structure (PDB entry 2UXA). A possible explanation involves the presence of a set of conserved negatively charged or polar residues in the vicinity (41), as well as the fact that the side chains are partially solvent exposed. In the case of GluR2, Arg743 promotes homotetramerization, efficient folding and ER budding (41). However, it remains possible that Arg/Gly interactions may also act to favor heterotetramerization across subunits.
Stereochemistry of ligand binding
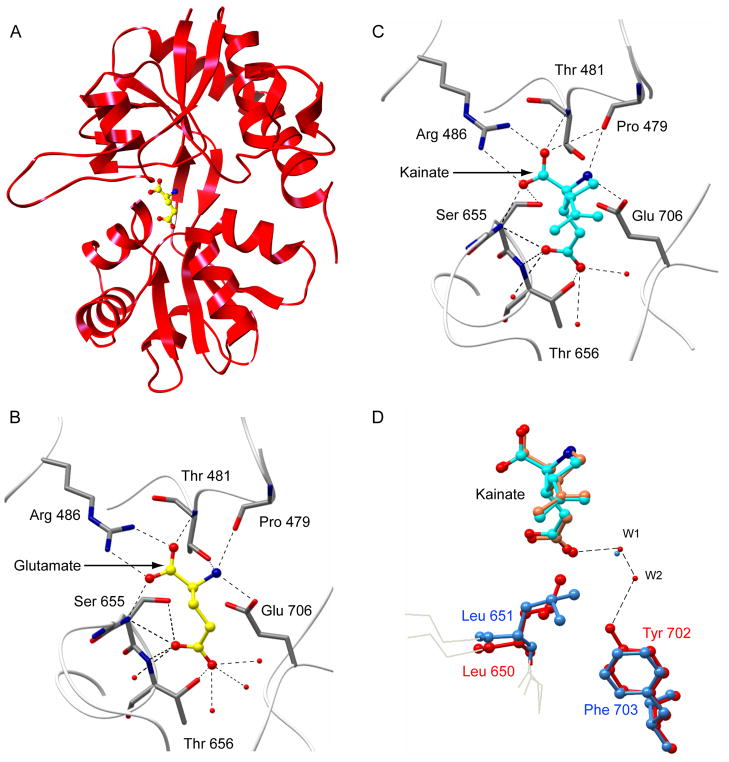
The GluR4 LBD binds to its full agonist glutamate (Figures 4A and 4B) and partial agonist kainate (Figure 4C) in a manner similar to that seen for the GluR2-ligand co-crystal structures (30). In particular, in both of the GluR4 LBD structures, the α-carboxyl and α-amino groups of glutamate and kainate interact with side chains of conserved residues surrounding the cleft: Arg486, Thr481, and Pro479 in lobe 1, as well as Ser655 and Glu706 in lobe 2, in a manner that agrees very well with that seen previously for other iGluR family members (Figures 4B and 4C). This observation is consistent with the 89% sequence identity between the domains. It validates the assumptions underlying our previous homology-based analysis of GluR4:ligand binding energetics using FTIR spectroscopy (44–46). It also confirms the structural basis of the analysis of GluR4 LBD:agonist interaction kinetics, which revealed a two-step binding process (47).
Figure 4. GluR4-LBD:ligand interactions.
(A) Ribbon diagram is shown for the GluR4 LBD bound to glutamate. Close-up views of the ligand-binding pockets show contacts between the glutamate (B, yellow) or kainate (C, cyan) ligands and the surrounding amino acids in each binding cleft. This view of the ligand binding cleft is in the same orientation of the model as presented in (A). Ligands are depicted in ball-and-stick representation, colored by atom type (carbon, white or yellow; oxygen, red; nitrogen, blue). Water molecules are shown as red spheres. Hydrogen bonds are illustrated with dashed lines. Tyr451 is located in front of the plane of the figure in this orientation and is omitted for clarity. (D) A close-up view of the ligand binding pocket shown following superimposition of the GluR2 and GluR4 LBD structures in complex with kainate. Protein residues (stick figures) and water molecules (spheres) belonging to the GluR4 chain are colored blue, and those belonging to the GluR2 chain are red. Kainate bound to GluR4 is colored cyan, while the GluR2-bound kainate is colored coral. The dashed lines depict water-mediated hydrogen bonds formed between Tyr 702 and kainate in the GluR2 LBD structure. This figure was prepared using CHIMERA (36) and CCP4MG (55)
Interactions of the LBD binding pocket with agonist side-chain moieties are also strongly conserved. Ser655 and Thr656 both form hydrogen bonds (Figures 4B and 4C), while Leu651 acts as a “foot in the door” for the kainate isopropenyl group (Figure 4D). In addition, as seen in GluR2 LBD:ligand complexes (30), a number of water-molecules are observed to interact with the agonist side chains in the binding cleft, providing stereochemical flexibility for the accommodation of different agonists and antagonists (Figures 4B and 4C). However, this water network is generally conserved as well. Within a hydrogen-bonding distance of the bound kainate ligand, there are three water molecules in both GluR2 and GluR4 structures, all of which overlap.
Comparison of the glutamate- and kainate-bound structures reveals a different degree of cleft closure for each complex (Figure 2C). As seen with the binding of partial and full agonists to GluR2, GluR5 and GluR6 (17, 18, 30) but not to NR1 (12) LBD, the full agonist glutamate induces a more compact structure of the GluR4 LBD than does the partial agonist kainate (Figure 2C). Even though an apo or antagonist-bound crystal structure is not available for the GluR4 LBD, small-angle X-ray scattering studies have previously shown that the full-length GluR4 LBD is more compact in the presence of the full agonists glutamate or AMPA than it is in the apo state or in complexes formed with competitive quinoxaline antagonists (9). Thus, given that kainate is a partial agonist for GluR4 (48), the qualitative correlation between cleft closure and relative agonist efficacy, originally identified for the GluR2 LBD, appears to be conserved at least for the non-NMDA iGluRs.
Differential relationship between relative efficacy and cleft closure
Despite this qualitative similarity, there are differences in the relative degrees of cleft closure seen in GluR2 vs. GluR4. Specifically, while the GluR2 LBD cleft is 8.9° more closed in the presence of glutamate than in the presence of kainate, for the GluR4 LBD, the difference is only 6.7°. In other words, in GluR4, the kainate-bound LBD structure is ~2° closer to the glutamate-bound structure than is the case for GluR2. Previously, an increase of only 3° in GluR2 LBD cleft closure was proposed to account for an increase in the relative efficacy of kainate from 2% to 24% (49). As a result, we wished to test whether the greater relative cleft closure associated with kainate binding to the GluR4 LBD corresponds to a similarly enhanced relative efficacy.
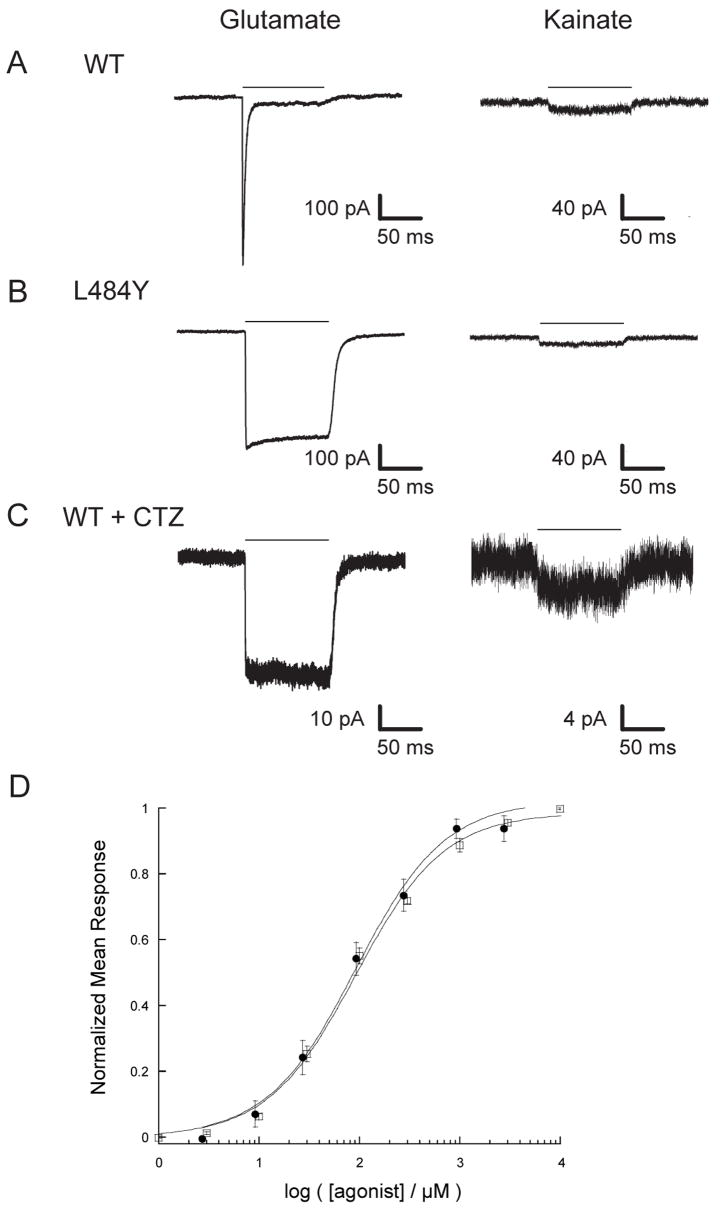
Like other AMPA-Rs, wild-type (WT) GluR4 homomeric receptors exhibit fast desensitization kinetics, making it difficult to measure peak currents accurately (Figure 5A). A point mutation in the dimer interface that converts a Leu (483 in GluR2) to a Tyr is known to inhibit desensitization in the GluR1, GluR2, and GluR3 subunits (38, 42). In order to test whether the homologus mutation would have a similar effect in GluR4, we generated an expression vector encoding the full-length GluR4 subunit with the L484Y mutation and measured the responses to glutamate and to kainate in GluR4-L484Y homotetramers. As shown in Figure 5B, the GluR4-L484Y mutation has the expected effect, dramatically decreasing the extent of desensitization and permitting more reliable assessment of agonist-induced peak currents.
Figure 5. Kainate is a weak partial agonist of GluR4.
(A) The response of a representative outside-out patch to a 100 ms pulse of 10 mM glutamate (left) or kainate (right) is shown for GluR4-WT (A, C) and GluR4-L484Y (B) receptors in the absence (A,B) or presence (C) of 100 μM cyclothiazide. The horizontal bar represents the time of agonist application. Traces for kainate (right) are scaled up 2.5× relative to traces for glutamate (left). (D) Normalized dose-response curves are shown for GluR4-L484Y using kainate (●) and glutamate (□). Curves were acquired using whole-cell voltage-clamp recordings. Data were fit using the Hill equation y=Imax* xn/( kn + xn ), where y = I/Imax, Imax = maximum current amplitude, x = [agonist], n = Hill coefficient, and k = EC50.
A dose-response analysis of currents elicited by 100 ms pulses of kainate or glutamate acting on the L484Y receptor showed similar EC50 values for kainate and glutamate (92 ± 14 μM and 92 ± 10 μM, respectively; Figure 5D). Hill coefficients were close to unity for both kainate (.98 ± .12) and glutamate (.99 ± .09). To determine the relative peak current of kainate to glutamate, outside-out patches of tsA201 cells expressing GluR4-L484Y subunits were exposed to 100 ms pulses of buffer containing 10 mM glutamate or 10 mM kainate (i.e. ~100 × EC50). For each patch, 2–5 individual responses were averaged, and the ratio of Ipeak in response to kainate versus Ipeak in response to glutamate was determined. This ratio was 2.7 ± 0.4% (n=5) for cells expressing GluR4-L484Y subunits, which is very similar to the value of 2% reported for GluR2-L483Y receptors (49). CTZ is a pharmacological agent that provides an alternative means of suppressing AMPA-R desensitization and has also been used in studies of GluR2 agonist responses (49). As seen with GluR2 receptors, CTZ suppresses desensitization of GluR4 receptors more strongly than does the L484Y mutation (Figure 5C). In the presence of CTZ, the ratio of kainate- to glutamate-induced peak currents was 7.8 ± 0.1% (n=8), well below the value of 17% observed for GluR2 receptors under comparable conditions (49).
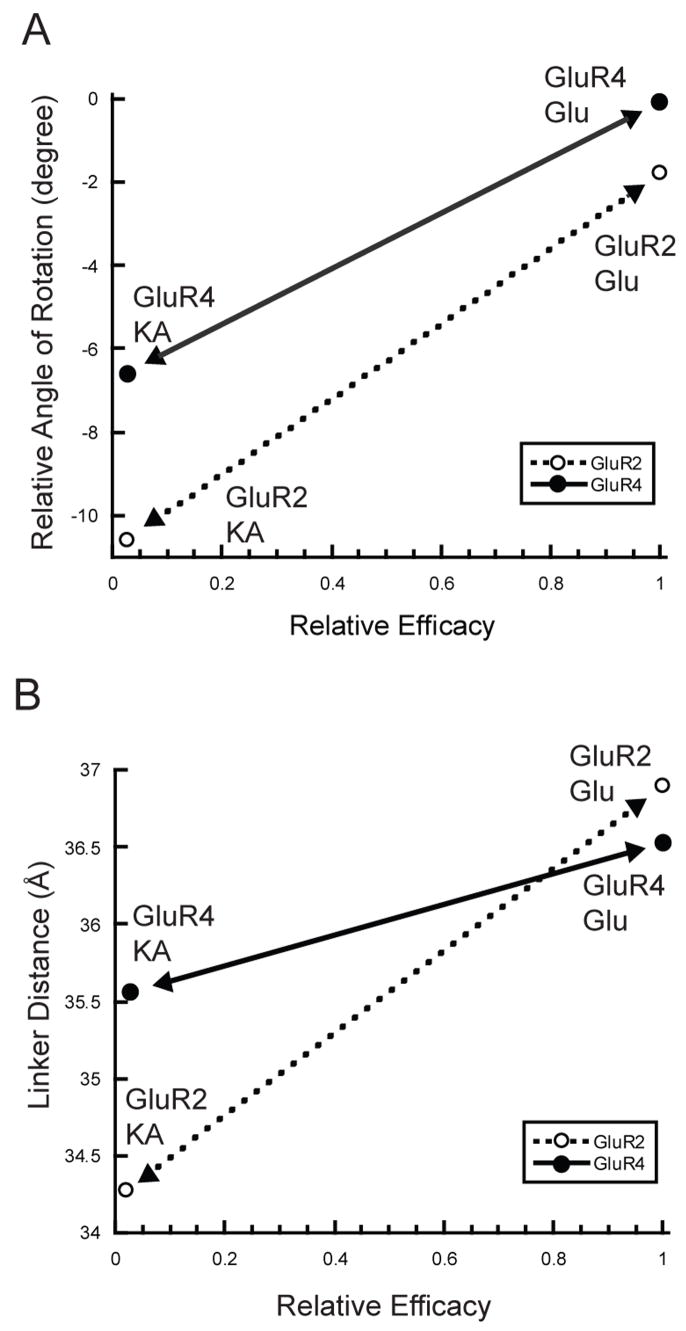
Thus, despite the greater degree of relative cleft closure in GluR4, kainate remains a very weak partial agonist in comparison to glutamate, with relative efficacy less than or equal to that seen with GluR2 channels. These data stand in contrast to the observations made with the GluR2-L650T channels, which indicated that a few additional degrees of cleft closure can significantly enhance efficacy (49). As a result, the slope of the line relating efficacy to the rotation angle of cleft closure appears to be different for the GluR2 and GluR4 subunits. Indeed, as shown in Figure 6A, the line is shallower for GluR4 than it is for GluR2, and is thus intermediate between GluR2 and NR1, for which the slope is close to zero (12). An alternative representation for the extent of cleft closure is the separation between the two linker peptides in the LBD dimer. This corresponds roughly to the separation between the attachment points that connect to the first two transmembrane domains of a pair of subunits in the receptor. When plotted for the GluR2 and GluR4 glutamate and kainate structures (Figure 6B), the difference in slope is even more dramatic. Glutamate binding to GluR2 drives an increase in linker separation of 2.6 Å more than kainate. In contrast, glutamate binding to GluR4 drives an increase in linker separation of only 0.9 Å compared to kainate. Nevertheless, the relative efficacy difference between kainate and glutamate is very similar for homomeric receptors composed of either subunit.
Figure 6. Differential relationships between relative agonist efficacy and LBD conformational changes in GluR4 and GluR2.
(A) Plot of the degree of cleft closure versus agonist efficacy for kainate- and glutamate-bound forms of both LBD. Following superimposition of lobe 1 of GluR4-LBD:KA, GluR2-LBD:KA(1FWO), or GluR2-LBD:Glu(1FTJ) onto lobe 1 of GluR4-LBD:Glu, the relative angle of rotation required to superimpose lobe 2 of the structures onto lobe 2 of GluR4-LBD:Glu was determined. Negative values indicate a more open cleft. (B) Graph plotting values of linker distance (as measured between Glycine Cα atoms in the GT linker) versus agonist efficacy for the same set of structures. To illustrate the relative slope, lines with arrows are shown connecting the kainate- and glutamate-bound points for GluR4 (solid) and GluR2 (dashed).
One interpretation of our data is that different subunits exhibit a different sensitivity or “gain” when converting cleft closure or linker separation into channel activation. Such differences could reflect the three-dimensional structure of the coupling and gating mechanisms of intact subunits or receptors. Indeed, the fact that changes in linker separation do not strictly track cleft closure (Figures 6A and 6B) suggests that the relationship between lobe closure and transmembrane connector spacing is itself complex. Another interpretation is that relative destabilization of the kainate-bound GluR4-LBD reduces the net efficacy of the partial agonist in a way that compensates for enhanced activation via greater cleft closure. Finally, as discussed below, the stereochemistry of the GluR4 ligand-binding site accommodates the greater degree of kainate-associated cleft closure observed. Nevertheless, although analysis of lattice effects on GluR2 LBD cleft closure suggest that they are modest (50) and our crystallographically independent Glu-bound protomers are extremely similar, it remains a formal possibility that crystal contacts may also contribute to the precise conformations observed. In any case, it is clear that there is not a single parameter governing the conversion of crystallographically observed LBD cleft-closure to channel activation across AMPA-R subunits. Ultimately, these differences may be important in determining the physiological effects of AMPA-R modulatory compounds as a function of subunit composition.
The structural basis of differential cleft closure
In principle, the difference in relative cleft closure between kainate and glutamate for GluR2 vs. GluR4 could reflect differences in either the kainate- or glutamate-bound states, or both. As shown in Figure 6A, it is clear that both ligand complexes are more closed for the GluR4 LBD than are the corresponding complexes with the GluR2 LBD. However, the difference between the glutamate-bound GluR4 and GluR2 structures (1.8°, Figure 2A) is smaller than that between the kainate bound forms (4.2°, Figure 2B). Remarkably, even though the GluR4-LBD:Glu structure is slightly more closed than the GluR2-LBD:Glu structure, the relationship between the linker distance is inverted, with the GluR2-LBD exhibiting a greater linker separation (36.9 Å, vs. 36.5 Å for GluR4). In contrast, the GluR4-LBD:KA linker separation (35.6 Å) is significantly larger than that of the GluR2-LBD:KA complex (34.3 Å, Figure 6A) even though kainate is a similarly weak partial agonist for both subunits. In fact, the GluR4-LBD:KA linker separation is greater than the GluR2-L650:KA separation (35.3 Å), which is associated with a significantly higher relative kainate efficacy of 24% (49). Such differences between GluR2 and GluR4 again underscore the danger of an overly linear interpretation of three-dimensional conformational transitions. The variability in cleft closure and linker separations also suggests that additional flexibility may be observed in other subunits and in complexes with other ligands.
What accounts for the nearly 4° difference in cleft closure between the kainate-bound forms of GluR2 and GluR4? A key determinant of the extent of cleft closure is the side chain at position 651 (650 in GluR2) (49). The isopropenyl group of the kainate side chain would clash sterically with Leu651 if the GluR4 LBD were in the glutamate-bound conformation with kainate in the binding pocket. In order to avoid this clash, when kainate is bound, the cleft closes less tightly. However, GluR2 and GluR4 both have a Leu side chain at the 650/651 position. The essential difference in cleft closure is thus accommodated not by a change in the covalent structure of the LBD, but rather by a reorientation of the terminal methyl groups of the Leu side chain. Whereas these point towards the ligand in GluR2, and thus impose a more open structure, in GluR4 they are oriented away from the ligand, allowing a greater degree of closure (Figure 4D).
This reorientation is at least in part facilitated by the replacement of Tyr702 in GluR2 by Phe703 in GluR4. In GluR2, the hydroxyl group of Tyr702 makes contact with kainate via water-mediated hydrogen bonds (Figure 4D, W1 and W2, red spheres), one of which (W2) is found only in the GluR2 structure (30). At the same time, the hydroxyl group prevents the more streamlined orientation of Leu650. In contrast, in the GluR4-KA structure, replacement of Tyr702 with Phe703 creates a hydrophobic surface against which the methyl groups of Leu651 can pack. While lattice contacts may influence the exact extent of cleft closure observed (50), this ability of the Leu side chain to reorient ultimately permits the GluR4 LBD to close more than the GluR2 LBD, an absolute prerequisite for the smaller conformational difference between these full and partial agonist-bound forms of GluR4.
Interestingly, the replacement of Tyr702 with a Phe is not by itself sufficient to allow the reorientation of the Leu650 side chain, as shown by Frandsen et al. (51). While the GluR2-Y702F mutation did increase cleft closure slightly, it did not cause a reorientation of the Leu650 terminal methyl groups. Instead, the increase was attributed to the creation of a more hydrophobic region surrounding residue 702, leading to a shift of bound water molecules and a tighter interaction between residues that provided the interdomain lock, as well as to the different conformation of Ser654 (51). Analysis of the GluR2 Y702F LBD structure in complex with KA shows that if GluR2-Leu650 were oriented away from kainate, as Leu651 is in GluR4, a modest steric clash could still occur between Leu650 and Phe702, due to differences in the backbone orientation between the two subunits. And, on the other hand, if the terminal methyl groups of Leu651 were to be oriented in the same manner in GluR4 as they are in GluR2-Leu650, then a steric clash between the isoproprenyl group of kainate and Leu651 would occur, indicating that the side chain at Leu651 is not free to reorient itself away from Phe703. Our results highlight the potential limitations of focusing exclusively on the residues that directly interact with a ligand. Instead, in this case, it is a combination of side-chain substitutions and backbone rearrangements that permit the differential cleft closure. Both of these differences involve “second-shell” residues, i.e. the residues that do not directly interact with the ligand, but affect the stereochemistry of the binding site.
Such second-shell residues could potentially also play a role in the binding of GluR4-selective pharmacological agents. Agonists such as 6-aza substituted willardiine derivatives display an increased potency for GluR4 receptors (52, 53), and others like 2-Bn-Tet-AMPA exhibit a 40-fold increased potency at GluR4 receptors compared to GluR1/2 receptors (54). An examination of the ligand-binding sub-pockets in the vicinity of the functional groups that are changed did not show any obvious stereochemical differences between GluR4 and GluR2 structures that would account for these results. Instead, it is likely that small sequence differences in second-shell or other residues might provide some torsional flexibility that help to better accommodate these subtype-selective agonists. A fuller understanding of the subtype selectivity of such pharmacological compounds is therefore likely to require co-crystal structures for these agonists with both GluR4 and GluR2.
CONCLUSION
The structures of GluR4flip LBD in complex with agonist and partial agonist permit a comparison of conformational changes across subunits of the AMPA-R family of ion channels. The electrophysiological behavior of GluR4 follows the trend that has been observed for the GluR2 subunit, showing a positive correlation between cleft closure and agonist efficacy. On the other hand, comparative analysis of GluR4 structures with previously determined GluR2 structures shows that the slope of this correlation is different, and has distinct consequences in terms of the potential separation of transmembrane domains within a subunit pair. It is likely that activation is dictated not simply by a set of linear extrapolations, but by a complex, three-dimensional conformational rearrangement that will require detailed structural analysis of intact iGluR. Our findings also emphasize the potential contributions of subtle ligand-binding interactions that govern subunit-selective interactions with ligands or modulators. Ultimately, it will be important to focus on the structural analysis of heteromeric subunit combinations that more accurately reflect the composition of physiological AMPA-Rs. In the meantime, comparative analysis of multiple individual subunits provides an opportunity to distinguish subunit-specific vs. general characteristics of this important family of neurotransmitter receptors.
Acknowledgments
We thank Dr. P. Seeburg (MPI, Heidelberg) for the initial GluR4 WT expression vector, Dr. D. Bowie (McGill University, Montréal) for the tsA201 cell line, and Dr. C. Panetti, Dr. R. Maue, and Dr. J. Kull (DMS) for helpful discussions.
ABBREVIATIONS
- iGluR
ionotropic glutamate receptor
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- AMPA-R
AMPA receptor
- LBD
ligand-binding domain
- NMDA
N-methyl-D-aspartic acid
- KA
kainic acid (2-carboxy-3-carboxymethyl-4-isopropenyl-pyrrolidine)
- PEG
polyethylene glycol
- NCS
non-crystallographic symmetry
- CTZ
cyclothiazide
Footnotes
A.G. was supported in part by the John H. Copenhaver, Jr., and William H. Thomas, M.D., 1952 Fellowship Fund and by the Rosaline Borison Memorial Fund. A.B.-B. was supported by an NIH Training Grant (T32 DK07301). Financial support was provided in part by grants from the Hitchcock Foundation (to D.R.M.) and the NIH (R01-DA014137, to L.P.H.).
References
- 1.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharm Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 2.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 3.Newcombe J, Uddin A, Dove R, Patel B, Turski L, Nishizawa Y, Smith T. Glutamate Receptor Expression in Multiple Sclerosis Lesions. Brain Pathology. 2008;18:52–61. doi: 10.1111/j.1750-3639.2007.00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipton SA, Rosenberg PA. Excitatory Amino Acids as a Final Common Pathway for Neurologic Disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 5.Sontheimer H. Malignant gliomas: perverting glutamate and ion homeostasis for selective advantage. Trends Neurosci. 2003;26:543–549. doi: 10.1016/j.tins.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobio. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Oswald RE. Ionotropic glutamate receptor recognition and activation. Advances in Protein Chemistry. 2004;68:313–349. doi: 10.1016/S0065-3233(04)68009-0. [DOI] [PubMed] [Google Scholar]
- 9.Madden DR, Armstrong N, Svergun D, Perez J, Vachette P. Solution X-ray scattering evidence for agonist- and antagonist-induced modulation of cleft closure in a glutamate receptor ligand-binding domain. J Biol Chem. 2005;280:23637–23642. doi: 10.1074/jbc.M414523200. [DOI] [PubMed] [Google Scholar]
- 10.Madden DR. The structure and function of glutamate receptor ion channels. Nat Rev Neurosci. 2002;3:91–101. doi: 10.1038/nrn725. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez J, Rambhadran A, Du M, Jayaraman V. LRET investigations of conformational changes in the ligand binding domain of a functional AMPA receptor. Biochemistry. 2008;47:10027–10032. doi: 10.1021/bi800690b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inanobe A, Furukawa H, Gouaux E. Mechanism of Partial Agonist Action at the NR1 Subunit of NMDA Receptors. Neuron. 2005;47:71–84. doi: 10.1016/j.neuron.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Cho Y, Lolis E, Howe JR. Structural and Single-Channel Results Indicate That the Rates of Ligand Binding Domain Closing and Opening Directly Impact AMPA Receptor Gating. J Neurosci. 2008;28:932–943. doi: 10.1523/JNEUROSCI.3309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- 15.Greger IH, Khatri L, Kong X, Ziff EB. AMPA receptor tetramerization is mediated by Q/R editing. Neuron. 2003;40:763–774. doi: 10.1016/s0896-6273(03)00668-8. [DOI] [PubMed] [Google Scholar]
- 16.Swanson GT, Kamboj SK, Cull-Candy SG. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J Neurosci. 1997;17:58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nanao MH, Green T, Stern-Bach Y, Heinemann SF, Choe S. Structure of the kainate receptor subunit GluR6 agonist-binding domain complexed with domoic acid. Proc Natl Acad Sci U S A. 2005;102:1708–1713. doi: 10.1073/pnas.0409573102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer ML. Crystal structures of the GluR5 and GluR6 ligand binding cores: molecular mechanisms underlying kainate receptor selectivity. Neuron. 2005;45:539–552. doi: 10.1016/j.neuron.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: Delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3:1098–1106. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]
- 20.Tai LS, Ng TKY, Mak NK, Yung KKL. Co-localization of AMPA-type glutamate receptor immunoreactivity in neurons of the rat subthalamic nucleus. Brain Research. 2001;895:95–103. doi: 10.1016/s0006-8993(01)02036-4. [DOI] [PubMed] [Google Scholar]
- 21.Hunter C, Petralia RS, Vu T, Wenthold RJ. Expression of AMPA-selective glutamate receptor subunits in morphologically defined neurons of the mammalian cochlear nucleus. J Neurosci. 1993;13:1932–1946. doi: 10.1523/JNEUROSCI.13-05-01932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wyllie DJ, Traynelis SF, Cull-Candy SG. Evidence for more than one type of non-NMDA receptor in outside-out patches from cerebellar granule cells of the rat. J Physiol. 1993;463:193–226. doi: 10.1113/jphysiol.1993.sp019591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monyer H, Seeburg PH, Wisden W. Glutamate-operated channels: developmentally early and mature forms arise by alternative splicing. Neuron. 1991;6:799–810. doi: 10.1016/0896-6273(91)90176-z. [DOI] [PubMed] [Google Scholar]
- 24.Mosbacher J, Schoepfer R, Monyer H, Burnashev N, Seeburg PH, Ruppersberg JP. A molecular determinant for submillisecond desensitization in glutamate receptors. Science. 1994;266:1059–1062. doi: 10.1126/science.7973663. [DOI] [PubMed] [Google Scholar]
- 25.Ying HS, Weishaupt JH, Grabb M, Canzoniero LMT, Sensi SL, Sheline CT, Monyer H, Choi DW. Sublethal Oxygen-Glucose Deprivation Alters Hippocampal Neuronal AMPA Receptor Expression and Vulnerability to Kainate-Induced Death. J Neurosci. 1997;17:9536–9544. doi: 10.1523/JNEUROSCI.17-24-09536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sommer B, Keinänen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Köhler M, Takagi T, Sakmann B, Seeburg PH. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990;249:1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- 27.Gill A, Madden DR. Purification and crystallization of a non-GluR2 AMPA-receptor ligand-binding domain: a case of cryo-incompatibility addressed by room-temperature data collection. Acta Crystallogr. 2008;F64:831–835. doi: 10.1107/S1744309108025426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kabsch W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J Appl Crystallogr. 1993;26:795–800. [Google Scholar]
- 29.Brünger AT, Adams PD, Clore GM, Delano WL, Gros P, Grosse-Kunstleve RW, Jiang J-S, Kuszewski J, Nilges N, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography and NMR system (CNS): a new software system for macromolecular structure determination. Acta Crystallogr. 1998;D54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong NA, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–181. doi: 10.1016/s0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- 31.Cowtan K. ‘dm’: An automated procedure for phase improvement by density modification. Joint CCP4 and ESF-EACBM Newsletter on Protein Crystallography. 1994;31:34–38. [Google Scholar]
- 32.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. 2004;D60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 33.Collaborative Computational Project, N. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. 1994;D50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 34.Winn MD, Isupov MN, Murshudov GN. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr. 2001;D57:122–133. doi: 10.1107/s0907444900014736. [DOI] [PubMed] [Google Scholar]
- 35.Kabsch W. A solution for the best rotation to relate two sets of vectors. Acta Crystallogr. 1976;A32:922–923. [Google Scholar]
- 36.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera - A Visualization System for Exploratory Research and Analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 37.Yang P, Jones BL, Henderson LP. Role of the α subunit in the modulation of GABA(A) receptors by anabolic androgenic steroids. Neuropharm. 2005;49:300–316. doi: 10.1016/j.neuropharm.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Sun Y, Olson R, Horning M, Armstrong N, Mayer M, Gouaux E. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- 39.Lomeli H, Mosbacher J, Melcher T, Hoger T, Geiger JR, Kuner T, Monyer H, Higuchi M, Bach A, Seeburg PH. Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science. 1994;266:1709–1713. doi: 10.1126/science.7992055. [DOI] [PubMed] [Google Scholar]
- 40.Krampfl K, Schlesinger F, Zorner A, Kappler M, Dengler R, Bufler J. Control of kinetic properties of GluR2 flop AMPA-type channels: impact of R/G nuclear editing. Eur J Neurosci. 2002;15:51–62. doi: 10.1046/j.0953-816x.2001.01841.x. [DOI] [PubMed] [Google Scholar]
- 41.Greger IH, Akamine P, Khatri L, Ziff EB. Developmentally regulated, combinatorial RNA processing modulates AMPA receptor biogenesis. Neuron. 2006;51:85–97. doi: 10.1016/j.neuron.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 42.Stern-Bach Y, Russo S, Neuman M, Rosenmund C. A point mutation in the glutamate binding site blocks desensitization of AMPA receptors. Neuron. 1998;21:907–918. doi: 10.1016/s0896-6273(00)80605-4. [DOI] [PubMed] [Google Scholar]
- 43.Greger IH, Ziff EB, Penn AC. Molecular determinants of AMPA receptor subunit assembly. TINS. 2007;30:407–416. doi: 10.1016/j.tins.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Jayaraman V, Keesey R, Madden DR. Ligand-protein interactions in the glutamate receptor. Biochemistry. 2000;39:8693–8697. doi: 10.1021/bi000892f. [DOI] [PubMed] [Google Scholar]
- 45.Madden DR, Cheng Q, Thiran S, Rajan S, Rigo F, Keinänen K, Reinelt S, Zimmermann H, Jayaraman V. The stereochemistry of glutamate receptor agonist efficacy: engineering a dual-specificity AMPA/kainate receptor. Biochemistry. 2004;43:15838–15844. doi: 10.1021/bi048447y. [DOI] [PubMed] [Google Scholar]
- 46.Madden DR, Thiran S, Zimmermann H, Romm J, Jayaraman V. Stereochemistry of quinoxaline antagonist binding to a glutamate receptor investigated by Fourier transform infrared spectroscopy. J Biol Chem. 2001;276:37821–37826. doi: 10.1074/jbc.M106171200. [DOI] [PubMed] [Google Scholar]
- 47.Abele R, Keinänen K, Madden DR. Agonist-induced isomerization in a glutamate receptor ligand-binding domain: a kinetic and mutagenetic analysis. J Biol Chem. 2000;275:21355–21363. doi: 10.1074/jbc.M909883199. [DOI] [PubMed] [Google Scholar]
- 48.Keinänen K, Wisden W, Sommer B, Werner P, Herb A, Verdoorn TA, Sakmann B, Seeburg PH. A family of AMPA-selective glutamate receptors. Science. 1990;249:556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- 49.Armstrong N, Mayer M, Gouaux E. Tuning activation of the AMPA-sensitive GluR2 ion channel by genetic adjustment of agonist-induced conformational changes. Proc Natl Acad Sci USA. 2003;100:5736–5741. doi: 10.1073/pnas.1037393100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin R, Gouaux E. Probing the function, confomational plasticity, and dimer-dimer contacts of the GluR2 ligand-binding core: studies of 5-substituted willardiines and GluR2 S1S2 in the crystal. Biochemistry. 2003;42:5201–5213. doi: 10.1021/bi020632t. [DOI] [PubMed] [Google Scholar]
- 51.Frandsen A, Pickering DS, Vestergaard B, Kasper C, Nielsen BB, Greenwood JR, Campiani G, Fattorusso C, Gajhede M, Schousboe A, Kastrup JS. Tyr702 Is an Important Determinant of Agonist Binding and Domain Closure of the Ligand-Binding Core of GluR2. Mol Pharmacol. 2005;67:703–713. doi: 10.1124/mol.104.002931. [DOI] [PubMed] [Google Scholar]
- 52.Jane DE, Hoo K, Kamboj R, Deverill M, Bleakman D, Mandelzys A. Synthesis of Willardiine and 6-Azawillardiine Analogs: Pharmacological Characterization on Cloned Homomeric Human AMPA and Kainate Receptor Subtypes. J Med Chem. 1997;40:3645–3650. doi: 10.1021/jm9702387. [DOI] [PubMed] [Google Scholar]
- 53.Stensbøl TB, Madsen U, Krogsgaard-Larsen P. The AMPA Receptor Binding Site: Focus on Agonists and Competitive Antagonists. Curr Pharm Design. 2002;8:857–872. doi: 10.2174/1381612024607090. [DOI] [PubMed] [Google Scholar]
- 54.Jensen AA, Christesen T, Bolcho U, Greenwood JR, Postorino G, Vogensen SB, Johansen TN, Egebjerg J, Brauner-Osborne H, Clausen RP. Functional Characterization of Tet-AMPA [Tetrazolyl-2-amino-3-(3-hydroxy-5-methyl- 4-isoxazolyl)propionic Acid] Analogues at Ionotropic Glutamate Receptors GluR1-GluR4. The Molecular Basis for the Functional Selectivity Profile of 2-Bn-Tet-AMPA. J Med Chem. 2007;50:4177–4185. doi: 10.1021/jm070532r. [DOI] [PubMed] [Google Scholar]
- 55.Potterton L, McNicholas S, Krissinel E, Gruber J, Cowtan K, Emsley P, Murshudov GN, Cohen S, Perrakis A, Noble M. Developments in the CCP4 molecular-graphics project. Acta Crystallogr. 2004;D60:2288–2294. doi: 10.1107/S0907444904023716. [DOI] [PubMed] [Google Scholar]