Abstract
Background & Aims
Nonalcoholic fatty liver disease is associated with insulin resistance and diabetes. The purpose of this study was to determine the relationship between intrahepatic triglyceride (IHTG) content and insulin action in liver (suppression of glucose dioduction), skeletal muscle (stimulation of glucose uptake) and adipose tissue (suppression of lipolysis) in non-diabetic, obese subjects.
Methods
A euglycemic-hyperinsulinemic clamp procedure and stable isotopically labeled tracer infusions were used to assess insulin action, and magnetic resonance spectroscopy was used to determine IHTG content, in 42 non-diabetic, obese subjects (BMI 36±4 kg/m2) who had a wide range of IHTG content (1%−46%).
Results
Hepatic insulin sensitivity, assessed as a function of glucose production rate and plasma insulin concentration, was inversely correlated with IHTG content (r=−0.599; P<0.001). The ability of insulin to suppress the release of fatty acids from adipose tissue and to stimulate glucose uptake by skeletal muscle were also inversely correlated with IHTG content (adipose tissue: r=−0.590; P<0.001; skeletal muscle: r=−0.656; P<0.001). Multivariate linear regression analyses found that IHTG content was the best predictor of insulin action in liver, skeletal muscle and adipose tissue, independent of BMI and percent body fat, and accounted for 34%, 42%, and 44% of the variability in these tissues, respectively (P< 0.001 for each model).
Conclusions
These results demonstrate that progressive increases in IHTG content are associated with progressive impairment of insulin action in liver, skeletal muscle and adipose tissue in non-diabetic, obese subjects. Therefore, NAFLD should be considered part of a multi-organ system derangement in insulin sensitivity.
Introduction
Nonalcoholic fatty liver disease (NAFLD) has become an important public health problem in many industrialized countries because of its high prevalence, potential progression to severe liver disease,1 and association with cardiometabolic abnormalities, including diabetes, the metabolic syndrome and coronary heart disease (CHD).2–4 Obesity is an important risk factor for NAFLD, and the prevalence of NAFLD is linearly associated with body mass index (BMI).5 The precise mechanism(s) responsible for the link between obesity and its metabolic complications is not known, but likely involves insulin resistance, which is a common feature of obesity and NAFLD, and is an important risk factor for cardiometabolic disease.6, 7
The term “insulin resistance” is most commonly used to describe impaired insulin-mediated glucose uptake in skeletal muscle. However, insulin also has important metabolic effects in other organ systems. Insulin resistance associated with obesity often involves the liver (impaired insulin-mediated suppression of glucose production) and adipose tissue (impaired insulin-mediated suppression of lipolysis).8 Recently, Gastaldelli et al. found that intrahepatic triglyceride (IHTG) content in lean, overweight and obese subjects with type 2 diabetes mellitus was directly correlated with the severity of insulin resistance in both liver and skeletal muscle.9 However, the relationship between IHTG content and insulin action in subjects who do not have diabetes is unclear, because of conflicting results from different studies, which have reported insulin resistance in liver but not muscle,10 insulin resistance in muscle but not liver,11 and insulin resistance in both liver and muscle12, 13 in subjects with NAFLD. The reason(s) for these apparent discrepancies is not clear, but could be related to differences among studies in body mass index and IHTG content in the study subjects and in plasma insulin concentration achieved during the clamp procedure used to evaluate insulin sensitivity.
The purpose of the present study was to determine the relationship between IHTG content and insulin action in liver (glucose production), skeletal muscle (glucose uptake) and adipose tissue (lipolysis) in non-diabetic, obese subjects who had a wide range of hepatic fat accumulation. A euglycemic-hyperinsulinemic clamp procedure, in conjunction with stable isotopically labeled tracer infusions, was used to assess insulin action, and magnetic resonance spectroscopy was used to determine IHTG content. We hypothesized that IHTG content would be inversely correlated with insulin sensitivity in all tissues, demonstrating that NAFLD is part of a multi-organ metabolic disease complex.
Materials and Methods
Subjects
Forty-two obese subjects (11 men, 31 women; 41±11 years old) participated in this study (Table 1). All subjects completed a comprehensive medical evaluation, which included a history and physical examination, blood tests, the Michigan Alcohol Screening Test (MAST),14 and a 2-h oral glucose tolerance test (OGTT). Fourteen (33%) subjects had impaired glucose tolerance, based on the results of the 2-h OGTT. Subjects who had diabetes, chronic liver disease other than NAFLD, a MAST score ≥4, and those who had been taking medications known to cause liver abnormalities or affect metabolism were excluded. All subjects were sedentary (i.e., participated in regular exercise <1 hour/wk and ≤1 time/wk) and weight stable (i.e., <2% weight change) for at least 3 months before the study. All subjects provided written, informed consent before participating in the study, which was approved by the Human Studies Committee and the General Clinical Research Center (GCRC) Advisory Committee of Washington University School of Medicine in St. Louis.
Table 1.
Body composition of the study subjects
| Median (interquartile range) | |
|---|---|
| Body mass index (kg/m2) | 35 (32–40) |
| Body fat mass (%) | 41 (37–45) |
| Fat-free mass (kg) | 58 (52–67) |
| Abdominal subcutaneous adipose tissue (cm3) | 3542 (2663–4280) |
| Intra-abdominal adipose tissue (cm3)* | 1443 (857–1792) |
| Intrahepatic triglyceride content (%) | 12.1 (3.9–23.3) |
Intra-abdominal adipose tissue volume values available for 39 of 42 subjects because of technical problems in 3 subjects.
Experimental Protocol
Body composition analyses
Fat mass (FM) and fat-free mass (FFM) were determined by using dual-energy X-ray absorptiometry (Hologic QDR 4500, Waltham, MA). Abdominal subcutaneous and intra-abdominal fat volumes were determined by using magnetic resonance imaging (MRI) (Siemens, Iselin, NJ); the sum of 8 axial images of 1 cm thickness, beginning from the L4–L5 interspace and extending proximally were used to determine abdominal subcutaneous and intra-abdominal adipose tissue volumes. Magnetic resonance spectroscopy (MRS) (Siemens, Erlanger, Germany) was used to determine IHTG content; three 2 × 2 × 2 cm voxels were examined in each subject and the values were averaged to provide an estimate of the percent of total liver volume comprised of fat.15
Hyperinsulinemic-euglycemic clamp procedure
Subjects were admitted to the inpatient unit of the GCRC at Washington University School of Medicine on the evening before the clamp procedure. At 1900 h, subjects consumed a standard meal, which provided 12 kcal/kg adjusted body weight and contained 55% of total energy as carbohydrate, 30% as fat and 15% as protein. Adjusted body weight was calculated as ideal body weight (based on the midpoint of the medium frame of the Metropolitan Life Insurance Tables) plus 0.25 × (actual body weight - ideal body weight). A 240-kcal liquid snack (Ensure, Ross Laboratories) was consumed at 2000 h. Subjects then fasted until completion of the clamp procedure the next day.
At 0500 h the following morning, one catheter was inserted into a forearm vein to infuse stable isotopically labeled tracers (purchased from Cambridge Isotope Laboratories, Andover, MA), dextrose and insulin, and a second catheter was inserted into a radial artery in the contralateral hand to obtain blood samples. Radial artery cannulation was not successful in 5 subjects, so a catheter was inserted into a hand vein, which was heated to 55°C by using a thermostatically controlled box, to obtain arterialized blood samples16. At 0600 h, a primed, continuous infusion of [6,6-2H2]glucose (priming dose: 22.5 umol·kg−1; infusion rate: 0.25 umol·kg−1 min−1), dissolved in 0.9% NaCl solution, was started and maintained for 5.5 h, until the end of stage 1 of the euglycemic-hyperinsulinemic clamp procedure. At 0800 h, a continuous infusion of [2,2-2H2]palmitate (infusion rate: 0.035 umol·kg−1·min−1), bound to human albumin, was started and maintained for 3.5 h (through the end of stage 1 of the euglycemic-hyperinsulinemic clamp procedure). At 0930 h (3.5 h after starting the infusion of glucose tracer), a two-stage euglycemic-hyperinsulinemic clamp procedure was started and continued for 6 h. During stage 1 of the clamp procedure (3.5 to 5.5 h), insulin was infused at a rate of 20 mU·m−2 body surface area (BSA)·min−1 (initiated with a priming dose of 80 mU·m−2 BSA·min−1 for 5 min and then 40 mU·m−2 BSA·min−1 for 5 min). During stage 2 of the clamp procedure (5.5 to 9.5 h), insulin was infused at a rate of 50 mU·m−2 BSA·min−1 (initiated with a priming dose of 200 mU·m−2 BSA·min−1 for 5 min and then 100 mU·m−2 BSA·min−1 for 5 min). These two insulin infusion rates were chosen to evaluate adipose tissue insulin sensitivity (low dose insulin infusion to submaximally suppress lipolysis of adipose tissue triglycerides) and skeletal muscle insulin sensitivity (high-dose insulin infusion to adequately stimulate muscle glucose uptake).17 Euglycemia was maintained at a blood glucose concentration of approximately 5.6 mmol/L (100 mg/dL) throughout stages 1 and 2 by infusing 20% dextrose enriched to 2.5% with [6,6-2H2]glucose at variable rates. The infusion rates of [6,6-2H2]glucose and [2,2-2H2]palmitate were reduced by 50% during stage 1, and [6,6-2H2]glucose infusion was reduced by 75% during stage 2 of the clamp procedure to account for changes in hepatic glucose production and lipolytic rates.
Blood samples were obtained before beginning the tracer infusion to determine background glucose and palmitate tracer-to-tracee ratios (TTRs), and every 10 min during the final 30 min of the basal period and stages 1 and 2 of the clamp procedure to determine glucose, free fatty acid (FFA) and insulin concentrations and substrate kinetics. These blood samples were collected in chilled tubes containing sodium EDTA. Samples were placed on ice, plasma was separated by centrifugation within 30 min of collection and then stored at −70 ºC until final analyses were performed. Blood was also obtained every 10 min during insulin infusion to monitor plasma glucose concentrations. These samples were collected in tubes containing heparin, and analyzed immediately by using an automated glucose analyzer (Yellow Spring Instruments Co, Yellow Springs, OH).
Sample analyses
Plasma FFA concentrations were quantified by using gas chromatography (Hewlett-Packard 5890-II, Palo Alto, CA) after adding heptadecanoic acid to plasma as an internal standard.18 Plasma insulin concentration was measured by using radioimmunoassay (Linco Research, St. Charles, MO). Plasma glucose and palmitate tracer to tracee ratios (TTRs) were determined by using electron impact ionization gas chromatography-mass spectroscopy (GC-MS; MSD 5973 system with capillary column; Hewlett-Packard; Palo Alto, CA), as previously described.19
Calculations
Glucose and palmitate kinetics
Isotopic steady state conditions were achieved during the final 30 min of the basal period and stages 1 and 2 of the clamp procedure. Basal endogenous glucose rate of appearance (Ra) in plasma was calculated by dividing the glucose tracer infusion rate by the average plasma glucose TTR during the last 30 min of the basal period. It was assumed that glucose rate of disappearance (Rd) was equal to glucose Ra during basal conditions; during the clamp procedure glucose Rd was assumed to be equal to the sum of endogenous glucose Ra and the rate of infused glucose. Palmitate Ra, an index of adipose tissue lipolysis was calculated by dividing the palmitate tracer infusion rate by the average plasma palmitate TTR obtained during the final 30 min of the basal period and stage 1 of the clamp procedure.20
Insulin sensitivity
Hepatic insulin sensitivity was determined by the reciprocal of the Hepatic Insulin Resistance Index, which was calculated as the product of the basal endogenous glucose production rate (in μmol·kg FFM−1·min−1) and fasting plasma insulin concentration (in mU/L).21, 22 Adipose tissue insulin resistance was assessed by calculating the relative decrease from basal in palmitate Ra during stage 1 of the clamp procedure. Skeletal muscle insulin resistance was assessed by calculating the relative increase from basal in glucose Rd during stage 2 of the clamp procedure.
Statistical analyses
The statistical significance of the effect of insulin infusion on substrate metabolism was analyzed by using the Student’s t-test for paired samples. The statistical significance of relationships between variables was evaluated by using the Pearson product moment correlation coefficient. Multiple step-wise linear regression analyses (forward and backward with age, sex, BMI, percent body fat, FFM, IHTG content, and IAAT volume as independent variables) were performed to identify predictors of insulin action in liver, skeletal muscle, and adipose tissue. All reported p-values are two-sided, and a p-value of <0.05 was considered to be statistically significant. Statistical analyses were performed by using SPSS (Windows) v14.0. Results are expressed as means±standard deviations, unless otherwise stated.
Results
Body composition
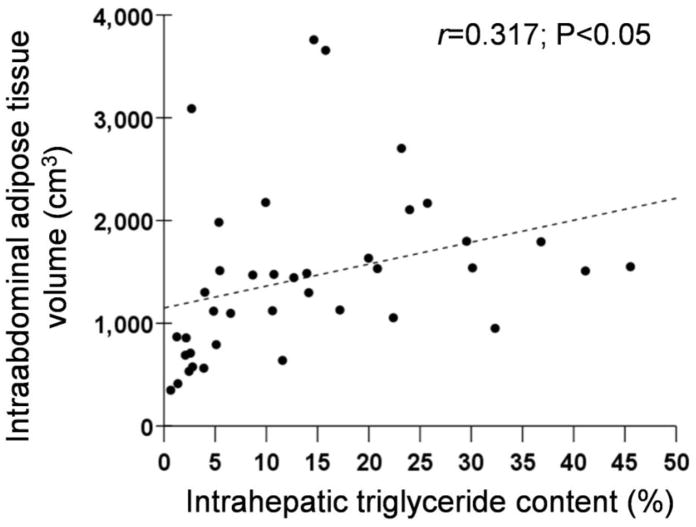
There was a 65-fold range in IHTG content (from 0.7% to 45.5%) and a 10-fold range in intra-abdominal adipose tissue (IAAT) volume (from 349 cm3 to 3759 cm3), but only a 2-fold range in percent body fat (from 28% to 52%) and a 1.5-fold range in BMI (from 30 kg/m2 to 46 kg/m2) (Table 1). No significant relationships were detected between IHTG content and BMI (r=0.258, P=0.10) or percent body fat (r=−0.063, P=0.69). In contrast, IAAT volume correlated directly with IHTG content (Figure 1; r=0.317, P<0.05). Three subjects had very high values for IAAT volume; eliminating these outliers improved the correlation between IHTG and IAAT (r=0.543, P=0.001).
Figure 1.
Relationship between intra-abdominal adipose tissue volume and intrahepatic triglyceride content.
Liver biochemistry
Eight of the 42 subjects (19%) had elevated serum alanine aminotransferase (ALT) concentration (ALT > 53 IU/mL). There was a direct correlation between IHTG content and serum ALT (r=0.647; p<0.001). Serum ALT was also inversely correlated with hepatic insulin sensitivity (r=−0.340; p=0.03), adipose tissue insulin sensitivity (r=−0.493; p=0.001) and skeletal muscle insulin sensitivity (r=−0.379; p=0.01).
Basal metabolic variables
Basal glucose Ra ranged from 10.2 to 17.6 μmol· kg FFM−1·min−1 and basal palmitate Ra ranged from 1.16 to 3.88 μmol· kg FFM−1·min−1 (Table 2). Basal glucose Ra and palmitate Ra did not correlate with IHTG content (r=0.141, P=0.37 and r=0.130, P=0.41, respectively).
Table 2.
Metabolic characteristics of the study subjects
| Median (interquartile range) | |
|---|---|
| Plasma glucose (mg/dL) | 94 (90–102) |
| Plasma insulin (μU/mL) | 15.0 (10.7–21.3) |
| Plasma triglycerides (mg/dL) | 118 (88–173) |
| Plasma HDL-cholesterol (mg/dl) | 44 (36–56) |
| Plasma LDL-cholesterol (mg/dL) | 95 (76–108) |
| Serum alanine aminotransferase (U/L) | 30 (23–45) |
| Basal glucose Ra (μmol· kg FFM−1·min−1) | 13.86 (12.52–15.04) |
| Basal palmitate Ra (μmol· kg FFM−1·min−1) | 1.99 (1.63–2.56) |
Ra=rate of appearance into the bloodstream;
FFM=fat-free mass
Insulin sensitivity
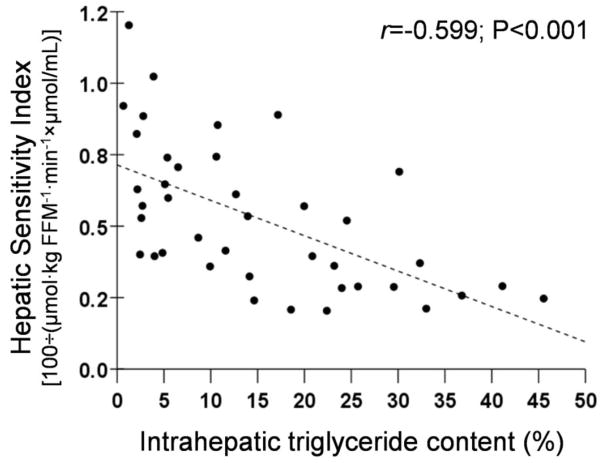
Intrahepatic triglyceride content was directly correlated with basal plasma insulin concentrations (r=0.598, P<0.001) and inversely correlated with hepatic insulin sensitivity (r=−0.599, P<0.001) (Figure 2, top panel).
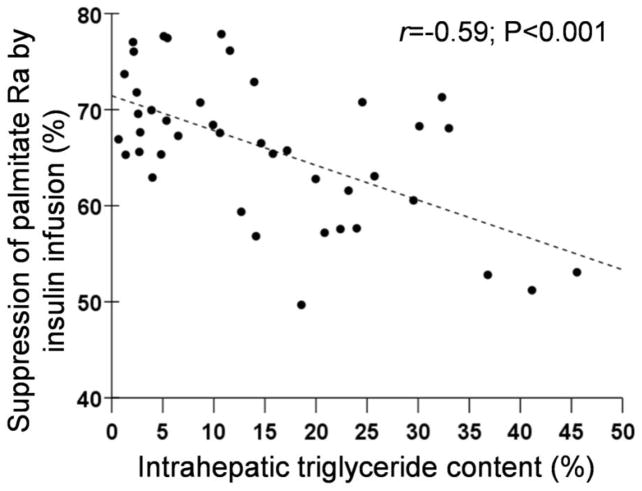
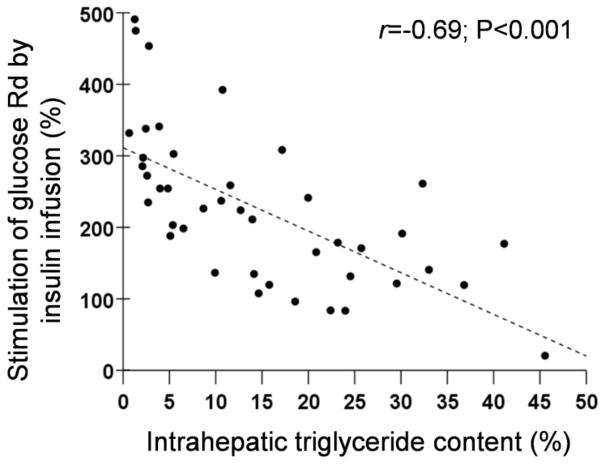
Figure 2.
Relationships between: 1) hepatic insulin sensitivity and intrahepatic triglyceride content (Top Panel); 2) insulin-mediated suppression of palmitate Ra during stage 1 of the euglycemic-hyperinsulinemic clamp procedure and intrahepatic triglyceride content (Middle Panel); and 3) insulin-mediated stimulation of glucose uptake (glucose Rd) during stage 2 of the euglycemic-hyperinsulinemic clamp procedure and intrahepatic triglyceride content (Bottom Panel).
The euglycemic-hyperinsulinemic clamp procedure was used to assess adipose tissue (data from stage 1) and skeletal muscle (data from stage 2) insulin sensitivity. Mean plasma insulin concentrations increased from 17±8 μU/mL at baseline to 48±14 μU/mL during stage 1 and to 106±26 μU/mL during stage 2 of the euglycemic-hyperinsulinemic clamp procedure. Palmitate Ra decreased by 66±8% during stage 1 of the euglycemic-hyperinsulinemic clamp procedure (from 2.1±0.6 μmol·kg FFM−1·min−1 during basal conditions to 0.7±0.3 μmol·kg FFM−1·min−1, during stage 1) (P<0.001). The relative suppression of palmitate Ra by low-dose insulin infusion during stage 1 was inversely correlated with IHTG content (r=−0.590, P<0.001) (Figure 2, middle panel). Glucose Rd increased 3-fold above baseline during stage 2 of the euglycemic-hyperinsulinemic clamp procedure (from 14.4±1.8 μmol·kg FFM −1·min−1 during basal conditions to 46.7±16.1 μmol·kg FFM −1·min−1 during stage 2) (P<0.001). The relative increase in glucose Rd by high-dose insulin infusion during stage 2 was inversely correlated with hepatic fat content (r=−0.656, P<0.001) (Figure 2, bottom panel).
Multivariate linear regression analyses, which included the age, sex, BMI, percent body fat, FFM, IHTG, and IAAT volume as independent variables, found that IHTG content was the best predictor of insulin action in liver, skeletal muscle and adipose tissue, accounting for 34%, 42%, and 44% of the variability in these tissues, respectively (P< 0.001 for each model). In addition, IAAT volume was also identified as an independent predictor of insulin action in liver and skeletal muscle, so IHTG and IAAT accounted for 42% and 57%, respectively (P< 0.001 for each model). None of the other measures of body composition or fat distribution (FFM, percent body fat, and BMI) were independent predictors of insulin action in any tissue.
Discussion
Obesity is an important risk factor for NAFLD and insulin resistance. In the present study, we evaluated the relationship between IHTG content and insulin sensitivity in obese subjects who did not have type 2 diabetes, to avoid the potential confounding influence of advanced insulin resistance and beta cell failure on our measures of insulin action. In addition, we studied subjects who had a large range in IHTG content (1%–46% of liver volume) but a small range in percent body fat, to increase our ability to determine the relationship between hepatic fat and insulin action, independent of adiposity. Insulin action was assessed in liver, skeletal muscle and adipose tissue, because these are the major organs involved in the metabolic pathophysiology of obesity. Our data demonstrate that the amount of IHTG in obese persons without diabetes is directly correlated with impaired insulin action in liver (suppression of glucose production), skeletal muscle (stimulation of glucose uptake) and adipose tissue (suppression of lipolysis), independent of percent body fat and IAAT volume. These results suggest that NAFLD should be considered part of a multi-organ system derangement in insulin sensitivity, and help explain why NAFLD is so closely linked with diabetes23 and the metabolic syndrome,2 and is an important risk factor for coronary heart disease.4, 24
Our study cannot determine whether NAFLD actually causes or is simply a consequence of insulin resistance. In fact, it is possible that excessive IHTG is both a cause and a manifestation of insulin resistance, resulting from a sequence of events initiated by adipose tissue insulin resistance and propagated by excessive IHTG, whereby: 1) adipose tissue insulin resistance increases the rate of release of FFA into the bloodstream and increases FFA delivery to the liver,25 2) inadequate hepatic oxidization and/or secretion (as VLDL-triglyceride) of the increased fatty acid load results in fatty acid esterification and IHTG accumulation,26 3) hyperinsulinemia and skeletal muscle insulin resistance also increase IHTG content by stimulating hepatic de novo lipogenesis and hepatic triglyceride synthesis,27 4) excessive IHTG release fatty acids into the cytoplasm, which can cause hepatic insulin resistance and inflammation,28 and 5) localized intrahepatic inflammation can contribute to peripheral insulin resistance.29 It is also possible that a fatty liver secretes cytokines, α −2-Heremans-Schmid glycoprotein and other, as yet unknown, products into the systemic circulation that can cause peripheral insulin resistance.30
Traditionally, excessive IHTG, or steatosis, has been defined chemically when IHTG content exceeds 5% of liver volume or liver weight, or histologically when 5% of hepatocytes contain visible intracellular triglycerides.31, 32 Recently, data obtained from two studies, which evaluated IHTG content by using magnetic resonance spectroscopy in large numbers of subjects, provide additional insights into defining “normal” IHTG content.33, 34 The results from one study, conducted in a cohort of Hispanic and non-Hispanic Caucasians and African American subjects, who were considered to be at low-risk for NAFLD (i.e. BMI<25 kg/m2, no diabetes, and normal fasting serum glucose and alanine aminotransferase concentrations), suggest the threshold for a normal amount of IHTG should be 5.6% of liver volume, because this value represented the 95th percentile for this “normal” population.33 Data from the second study, found the 95th percentile for IHTG content was 3% in lean, young adult, and Caucasian men and women who had normal oral glucose tolerance.34 None of the cutpoints that have been proposed for diagnosing steatosis are based on the relationship between IHTG and a rigorous assessment of either metabolic or clinical outcome. The results from our study demonstrate that the relationship between insulin sensitivity and IHTG content is monotonic, without evidence of an obvious threshold that can be used to define normality.
The data from our study demonstrate that IHTG content is a marker of insulin sensitivity in multiple tissues, independent of BMI and percent body fat. In addition, IHTG content correlated directly with IAAT volume, which has also been observed in other populations, including patients with type 2 diabetes,9, 35, 36 non-diabetic overweight and obese adults37 and overweight adolescents (S. Klein unpublished observations),38 and helps explain the metabolic interrelationships often observed between subjects with abdominal obesity and NAFLD.2–5 However, IHTG content was a better predictor of insulin action in liver, skeletal muscle and adipose tissue than was IAAT volume, and accounted for 34%–44% of the variability in insulin sensitivity in these tissues. The detection of insulin resistance in our subjects was not simple, and required the use of isotope tracer infusion to assess substrate kinetics and determining the metabolic response to a physiological challenge of insulin infusion. Therefore, these data suggest that quantification of IHTG content by using MRS, could become a useful diagnostic test by identifying insulin-resistant patients who might not be detected by a standard clinical evaluation.
Although we found a relationship between hepatic insulin sensitivity, measured by using the hepatic insulin sensitivity index, and IHTG content, we did not observe a significant correlation between the relative suppression of endogenous glucose production during step 1 (low dose insulin infusion) of the hyperinsulinemic-euglycemic clamp procedure and IHTG. However, stage 1 of our clamp was designed to evaluate adipose tissue, not hepatic, insulin sensitivity. The short duration of stage 1 (2 hours) is not long enough to reliably achieve near steady-state conditions needed for accurate assessment of insulin-mediated suppression of glucose production at low plasma insulin concentrations.39
In summary, the findings from this study demonstrate that NAFLD in non-diabetic, obese persons is part of a multi-organ disease complex, manifested by insulin resistance in the liver, skeletal muscle and adipose tissue. Moreover, there was a direct monotonic relationship between IHTG content and insulin resistance in all three tissues, across a large range of percent liver fat. Therefore, even small amounts of IHTG were associated with metabolic dysfunction, without an obvious threshold effect. Even though IHTG content correlated with IAAT volume, IHTG content was a better predictor of insulin resistance, independent of percent body fat and IAAT volume. These results suggest that measurement of IHTG content could be a useful clinical tool to identify patients at increased risk of cardiometabolic disease.
Table 3.
Substrate kinetics during basal conditions and during stage 1 (low-dose insulin) and stage 2 (high-dose insulin) of the hyperinsulinemic-euglycemic clamp procedure in the entire cohort of study subjects.
| Basal | Stage 1 | Stage 2 | |
|---|---|---|---|
| Endogenous Glucose Ra (μmol·kg FFM−1 · min−1) | 14.0 ± 1.8 | 4.2 ± 1.7 | 1.6 ± 1.9 |
| Glucose Rd (μmol·· kg FFM−1·· min−1) | 14.4 ± 1.8 | 21.0 ± 7.3 | 46.7 ± 16.1 |
| Palmitate Ra) (μmol·· kg FFM−1·· min−1) | 2.1 ± 0.6 | 0.7 ± 0.3 | 0.4 ± 0.2 |
Values are means±SD
Ra=rate of appearance
Acknowledgments
This research was supported by National Institutes of Health grants DK 37948, DK 56341 (Clinical Nutrition Research Unit), RR-00036 (General Clinical Research Center), and RR-00954 (Biomedical Mass Spectrometry Resource) and by DK 52574 (Washington University DDRCC) and an AGA Roche Junior Faculty Clinical Research Award in Hepatology (KMK). No conflicts of interests exist.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 3.Ioannou GN, Weiss NS, Boyko EJ, Mozaffarian D, Lee SP. Elevated serum alanine aminotransferase activity and calculated risk of coronary heart disease in the United States. Hepatology. 2006;43:1145–1151. doi: 10.1002/hep.21171. [DOI] [PubMed] [Google Scholar]
- 4.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71–79. doi: 10.1053/gast.2003.50004. [DOI] [PubMed] [Google Scholar]
- 6.Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, Karim R, Lin R, Samarasinghe D, Liddle C, Weltman M, George J. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35:373–379. doi: 10.1053/jhep.2002.30692. [DOI] [PubMed] [Google Scholar]
- 7.Pagano G, Pacini G, Musso G, Gambino R, Mecca F, Depetris N, Cassader M, David E, Cavallo-Perin P, Rizzetto M. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35:367–372. doi: 10.1053/jhep.2002.30690. [DOI] [PubMed] [Google Scholar]
- 8.Groop LC, Saloranta C, Shank M, Bonadonna RC, Ferrannini E, DeFronzo RA. The role of free fatty acid metabolism in the pathogenesis of insulin resistance in obesity and noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1991;72:96–107. doi: 10.1210/jcem-72-1-96. [DOI] [PubMed] [Google Scholar]
- 9.Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R, Buzzigoli E, Sironi AM, Cersosimo E, Ferrannini E, DeFronzo RA. Relationship Between Hepatic/Visceral Fat and Hepatic Insulin Resistance in Nondiabetics and Type 2 Diabetic Subjects. Gastroenterology. 2007:133. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 10.Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A, Halavaara J, Yki-Jarvinen H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 11.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 12.Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E, Rizzetto M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–642. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 13.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 14.Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- 15.Frimel TN, Deivanayagam S, Bashir A, O’Connor R, Klein S. Assessment of intrahepatic triglyceride content using magnetic resonance spectroscopy. J Cardiometab Syndr. 2007;2:136–138. doi: 10.1111/j.1559-4564.2007.07168.x. [DOI] [PubMed] [Google Scholar]
- 16.Jensen MD, Heiling VJ. Heated hand vein blood is satisfactory for measurements during free fatty acid kinetic studies. Metabolism. 1991;40:406–409. doi: 10.1016/0026-0495(91)90152-m. [DOI] [PubMed] [Google Scholar]
- 17.Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest. 1989;84:205–213. doi: 10.1172/JCI114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patterson BW, Zhao G, Klein S. Improved accuracy and precision of gas chromatography/mass spectrometry measurements for metabolic tracers. Metabolism. 1998;47:706–712. doi: 10.1016/s0026-0495(98)90035-x. [DOI] [PubMed] [Google Scholar]
- 19.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res. 1999;40:2118–2124. [PubMed] [Google Scholar]
- 20.Mittendorfer B, Liem O, Patterson BW, Miles JM, Klein S. What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes. 2003;52:1641–1648. doi: 10.2337/diabetes.52.7.1641. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 22.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA. Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia. 2004;47:31–39. doi: 10.1007/s00125-003-1263-9. [DOI] [PubMed] [Google Scholar]
- 23.Amarapurka DN, Amarapurkar AD, Patel ND, Agal S, Baigal R, Gupte P, Pramanik S. Nonalcoholic steatohepatitis (NASH) with diabetes: predictors of liver fibrosis. Ann Hepatol. 2006;5:30–33. [PubMed] [Google Scholar]
- 24.Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, Zoli M, Marchesini G. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473–480. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- 25.Horowitz JF, Klein S. Whole body and abdominal lipolytic sensitivity to epinephrine is suppressed in upper body obese women. Am J Physiol Endocrinol Metab. 2000;278:E1144–1152. doi: 10.1152/ajpendo.2000.278.6.E1144. [DOI] [PubMed] [Google Scholar]
- 26.Cassader M, Gambino R, Musso G, Depetris N, Mecca F, Cavallo-Perin P, Pacini G, Rizzetto M, Pagano G. Postprandial triglyceride-rich lipoprotein metabolism and insulin sensitivity in nonalcoholic steatohepatitis patients. Lipids. 2001;36:1117–1124. doi: 10.1007/s11745-001-0822-5. [DOI] [PubMed] [Google Scholar]
- 27.Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB, Papademetris X, Rothman DL, Shulman GI. Inaugural Article: The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Krober SM, Machicao F, Fritsche A, Haring HU. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care. 2006;29:853–857. doi: 10.2337/diacare.29.04.06.dc05-1938. [DOI] [PubMed] [Google Scholar]
- 31.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 32.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008–1017. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- 33.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 34.Petersen KF, Dufour S, Feng J, Befroy D, Dziura J, Dalla Man C, Cobelli C, Shulman GI. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci U S A. 2006;103:18273–18277. doi: 10.1073/pnas.0608537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adiels M, Taskinen MR, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, Vehkavaara S, Hakkinen A, Olofsson SO, Yki-Jarvinen H, Boren J. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49:755–765. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- 36.Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab. 2003;285:E906–916. doi: 10.1152/ajpendo.00117.2003. [DOI] [PubMed] [Google Scholar]
- 37.Westerbacka J, Corner A, Tiikkainen M, Tamminen M, Vehkavaara S, Hakkinen AM, Fredriksson J, Yki-Jarvinen H. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia. 2004;47:1360–1369. doi: 10.1007/s00125-004-1460-1. [DOI] [PubMed] [Google Scholar]
- 38.Fishbein MH, Mogren C, Gleason T, Stevens WR. Relationship of hepatic steatosis to adipose tissue distribution in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2006;42:83–88. [PubMed] [Google Scholar]
- 39.Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev. 1985;6:45–86. doi: 10.1210/edrv-6-1-45. [DOI] [PubMed] [Google Scholar]