Abstract
Background
Although highly protective, cardiac surgery using cardioplegia and cardiopulmonary bypass (CP/CPB) subjects myocardium to hypothermic reversible ischemic injury that can impair cardiac function which results in a greatly enhanced risk of mortality. Acute changes in myocardial contractile activity are likely regulated via protein modifications. We performed the following study to determine changes in the protein profile of human myocardium following CP/CPB.
Methods and Results
Right atrial appendage was collected from 8 male patients pre and post-CP/CPB. Atrial tissue lysates were subjected to 2-dimensional electrophoresis, total protein staining, gel averaging, and quantitative densitometry. Ten prominent spots regulated in response to CP/CPB were identified using mass spectrometry. Two hundred twenty-five and 256 protein spots were reliably detected in 2D-gels from pre- and post-CP/CPB patients, respectively. Five unique (ie, not detected post-CP/CPB) and 17 significantly increased spots were detected pre-CP/CPB. Thirty-four unique and 25 significantly increased spots were detected in the post-CP/CPB group. Identified proteins that changed after CP/CPB included: MLC-2a, ATP-synthase delta chain and Enoyl-CoenzymeA hydratase, glutathione-s-transferase omega, α-1-acid-glycoprotein, and phosphatidylethanolamine-binding protein.
Conclusions
Cardiac surgery results in multiple consistent changes in the human myocardial protein profile. CP/CPB modifies specific cytoskeletal, metabolic, and inflammatory proteins potentially involved in deleterious effects of CP/CPB.
Keywords: cardioplegia, cardiopulmonary bypass, proteomics
Cardioplegic arrest (CP) is highly protective to cardiac tissue subjected to ischemic insults. The main protective benefit of CP is mediated through myocardial hypothermia and diastolic arrest which preserves myocardial energy reserves relative to unprotected ischemia/reperfusion (I/R) injury. Although protective, CP remains associated with prolonged ischemic insults including myocyte hypoxia, acidosis, oxidant dependent damage, metabolic and structural alterations, and reduced cardiac function1-4 Despite significant improvements in myocardial protection, ischemic insults associated with cardiac surgery persist and remain a significant cause of mortality for high risk populations. Classically, investigations into mechanisms of CP/CPB-induced injury or improved cardioprotection strategies have focused on single targets or individual manipulations. However, given the complexity of the stimulus associated with myocardial I/R injury, a more complete picture of the molecular basis of functional changes is required to design more informed and multifaceted cardiprotection strategies. The majority of deleterious effects associated with CP/CPB are likely regulated via acute protein modifications.5,6 Proteomic analysis allows the simultaneous detection of changes in potentially hundreds of proteins, and can provide invaluable insight to mechanisms of disease. The purpose of the following study was to determine changes in the myocardial protein profile in patients undergoing cardiac surgery using CP/CPB. Human atrial samples, pre- and post-CP/CPB, were used in 2-dimensional electrophoresis, total protein staining, and mass spectrometry analysis to identify specific proteins regulated in response to CP/CPB. The identification of specific protein alterations associated with deleterious effects of CP/CPB may guide future cardioprotection strategies.
Methods
Tissue Collection Pre- and Post-CP/CPB
Samples were obtained from 8 male patients undergoing cardiac surgery with cardioplegic arrest (CP) and moderately hypothermic (32°C to 34°C) CPB for CABG or valve repair/replacement as described previously.7 The Clinical Research Committee of the Beth Israel Deaconess Medical Center approved this study.
Lysate Preparation and 2-D Gel Electrophoresis
Proteins were extracted in RIPA buffer with protease and phosphatase inhibitors using Pressure Cycling Technology (Pressure Biosciences). The samples were placed in a NEP3229 Barocycler subjected to 40 cycles of high pressure for 30 seconds (35 000 psi) followed by atmospheric pressure for 10 seconds as previously described.8 After tissue disruption and cellular lysis, samples were centrifuged at 10 000 RCF for 10 minutes, and protein concentration of the supernatant was determined by BCA protein assay (Pierce). 100 μg of total protein per sample was used to hydrate immobilized pH gradients, pH 3 to 10 for 6 hours. Isoelectric focusing and 2-dimensional electrophoresis were performed as previously described.9 2D gels were stained for total proteins with SYPRO Ruby (Invitrogen) or Coomassie Brilliant Blue (CCB; Proteome Systems) according to the manufacturers instructions. Gels were imaged using a BioRad FX laser scanning densitometer or a Licor Infrared gel scanner for Sypro Ruby and CBB respectively.10
Two-Dimensional Gel Analysis and Quantitative Densitometry
Protein spot detection was performed on all gels using Phoretix 2-D gel analysis software (Nonlinear Dynamics). Briefly automated spot detection was based on initial manual user supervised matches between prominent spots in the same location and within the same protein patterns between all gels. Automated spot matching was performed with the phoretix 2-D software. All matches were reviewed and approved via manual inspection of the matched spots. Densitometry values for spots matched between gels were determined after background subtraction and normalization by total spot intensity of each gel. Changes in the number of pre versus post samples are the result of normalized percent change (calculated relative to both pre- and post-CP/CPB) data of individual patients. Total gel counts performed represent the spots detected in either the pre or post group that were common to at least 75% of the gels per group (minimum 6 of 8). Counted unique spots represent proteins detected in at least 75% of gels in the respective group and with a frequency <50% in the matched pre/post sample. Significant increases/decreases in spot intensity were calculated for those proteins detected and matched in at least 75% of the 8 gels in either the pre or post group (see statistics). To quantify relative differences in expression, % change from the matched sample in the corresponding group was calculated for both the post versus pre and pre versus post comparisons.
Tandem Mass Spectrometry and Protein Identification
Ten spots which appeared consistently up/downregulated or present only in pre or post samples were excised with a sterile cut pipette tip and washed. Cysteine residues were reduced with 10 mmol/L dithiothreitol for 30 minutes at 56°C and alkylated with 10 mmol/L iodoacetamide in the dark for 45 minutes. Gel bands were washed and dried in a Speedvac concentrator. 250 ng of Promega modified trypsin in 50 mmol/L ammonium bicarbonate (pH=8.3) was added, and the protein bands were digested overnight at 37°C. Peptides were extracted from the gel pieces with 20 mmol/L ammonium bicarbonate followed by 40% acetonitrile/2% formic acid to a final elution volume of 65 μL. The elution was dried in a Speedvac to a 10 μL final volume in a 200 μL autosampler vial.
A 4-μL aliquot of peptide mixture was injected onto a microcapillary reversed-phase liquid chromatography tandem mass spectrometry system (μRP-LC/MS/MS) using a self-packed 75 μm id×10 cm length C18 column at a flow rate of ≈300 nL/min. Data dependent MS/MS spectra were collected using a Thermo Scientific LTQ 2D linear ion trap mass spectrometer operated in the positive ion mode. MS/MS spectra were searched versus a reversed (decoy) nonredundant protein database from UniProtKB using the Sequest; algorithm. Proteins that matched the target database were identified if the consensus score was greater than 1.0 and at least 2 unique peptides were identified per protein. Peptide sequences were validated using score cutoffs (1+ and 2+ ions: XCorr 2.0, 3+ ions: XCorr 2.5, 4+ ions: Xcorr 3.25; Sf=0.35) as well as manual inspection to insure that the sequences were consistent with the typical b- and y- series fragment ions. Relative protein abundance and ranking of positive identification within samples was quantified using two measures, spectral counting and spectral TIC.11
1D Electrophoresis and Immunoblot Analysis
Sodium dodecylsulfate-polyacrylamide gel electrophoresis and immunoblot analyses were performed as previously described.7 A bicinchroninic acid protein assay was performed to allow equal gel loading; 10 to 30 μg of lysates were loaded on tris-glycine gradient gels and electrophoresed for approximately 1 hour at 150 volts. Gels were transferred to polyvinylidene difluoride membranes for 1 hour at 100 volts. Gels were blocked in 3% nonfat dry milk in trisbuffered saline (TBS) for 1 hour, followed by incubation in primary antibodies in 3% TBS milk or 3% bovine serum albumin according to the manufacturer’s recommendation. Blots were washed 3 times in TBS and incubated with the appropriate horseradish peroxidase-conjugated secondary antibody for 1 hour, washed 3 times in TBS, and detected using chemiluminescent detection (Pierce, Rockford, Ill). Antibodies for immunoblot were as follows: anti GSTO1 and A1AGP: Abcam, anti-MLC-2a: Synaptic Systems, anti-PEBP: Zymed, anti HSP27: Stressgen. Antibody dilutions were according to the manufacturer’s instructions.
Statistics
Statistical significance of densitometry values between groups for known proteins was determined first by computing the paired t statistic and its corresponding probability value for each protein (significance P<0.05). These individual probability values were used to determine the significant increases/decreases controlling for the false discovery rate (FDR) attributable to multiple comparison as described by Benjamini and Hochberg.12 The FDR was set at 0.2. In choosing this we considered the following (1) conventional practice, (2) in this pilot study we did not want to miss too many real difference so that some important proteins are not ignored in a more rigorous future study, (3) we did not want to select too many false-positives.
Results
Characteristics of patients used in the study are presented in Table 1. Female patients were excluded to minimize variability. There were 2 noninsulin-dependent diabetic patients included in this study. HbA1c levels for the 2 diabetic patients were 6.7 and 5.4.
Table 1. Characteristics of Patients Used in the 2D Gel Analysis.
| Age, mean±SEM (range) | 60.9±2.1 (54, 71) |
| Sex | 8 male, 0 female |
| Surgery data | |
| Procedure | 6 CABG, 2 CABG/AVR |
| No. of vessels, mean±SEM | 2.5±0.5 |
| X-clamp time, mean±SEM | 65.3±9.0 minutes |
| Bypass time, mean±SEM | 81.6±10.7 minutes |
| Risk factors | |
| Hypertension | 7/8 |
| History of tobacco | 4/8 |
| Diabetes (NIDDM) | 2/8 |
| HbA1c (mean (range)) | 5.93 (5.4, 6.7) |
| Hypercholesterolemia | 8/8 |
CP/CPB resulted in multiple changes in the myocardial protein profile of patients during cardiac surgery. The protein expression patterns of patient right atrial appendage pre-(Figure 1A) and post- CP/CPB (Figure 1B), were compared by 2D-PAGE and total protein staining. Numbered spots in Figure 1A and 1B were identified using mass spectrometry analysis (subsequent figure). Approximately 250 protein spots were reliably detected in both the pre- and post-CP/CPB samples (Table 2). Spot matching between the pre- and post-CP/CPB samples demonstrated 5 and 34 reliably detected unique spots in the pre- and post-CP/CPB samples, respectively. Quantitative densitometry of the matched spots demonstrated 25 proteins significantly higher in the post-CP/CPB sample normalized to 100% corresponding pre-CP/CPB values, and 17 spots significantly higher in the Pre-CP/CPB group normalized to 100% post-CP/CPB values (Table 2). A representative expression difference map of the averaged densitometry values from post-CP/CPB patients (expression relative to pre-CP/CPB samples) is shown in Figure 2. The densitometry values and spot indications are overlaid on a representative post-CP/CPB gel.
Figure 1.
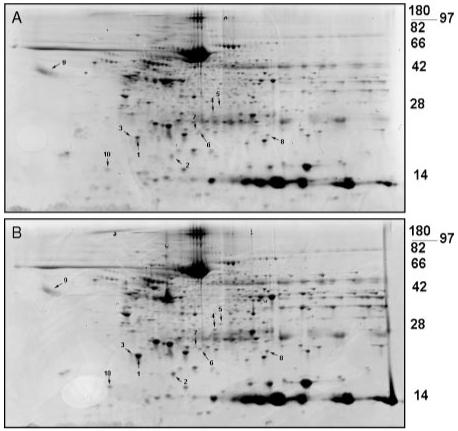
Two-dimensional electrophoresis of human atrial samples before and after cardiac surgery using cardioplegia and cardiopulmonary bypass. Total protein stained gels from pre- (A) and post-CP/CPB (B). Representative blots from 1 patient are shown (total n=8).
Table 2. Protein Spots Reliably Detected in the Pre- and Post-CP/CPB Samples.
| Pre-CP/CPB | Post-CPCPB | |
|---|---|---|
| Total protein spots | 225 | 256 |
| Unique spots | 5 | 34 |
| Significantly increased | 17 | 25 |
Figure 2.
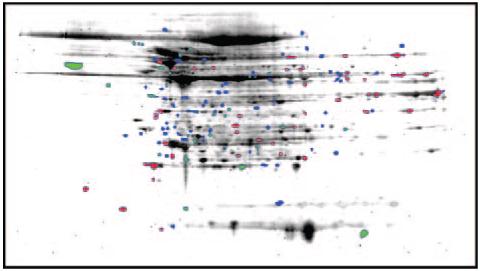
Changes in the myocardial protein profile of patients after CP/CPB. Difference map of post-CP/CPB changes relative to pre-CP/CPB samples. Red spots indicate >1.5-fold increase over pre-CP/CPB, green spots >1.5-fold decrease over post-CP/CPB, and blue indicates spots not matched to pre-CP/CPB. Averaged expression analysis data are overlaid on a representative post-CP/CPB gel.
Proteins prominently altered by CP/CPB are listed in Table 3 and are involved in various aspects of perturbed myocardial function after I/R injury. These include metabolism, proteolysis, contractility, inflammatory responses, and oxidative regulation. As the majority of spots picked contained more than 1 protein, the second ranked protein is included in the table as the second entry for each picked spot. Ranking and identity of the highest abundance protein in each spot that most likely reflects the changes in densitometry were determined by the peptide # and average TIC. TIC was not determined (N.D.) with 2 or fewer identified spectra. Relative densitometry of the differentially expressed proteins is shown in Figure 3A. Statistically significant increases were detected post-CP/CPB for 3 variants of myosin light chain 2a (MLC-2a), glutathione-s-transferase omega 1 (GSTO1), enoyl-coA hydratase (ECHS1), phosphatidylethanolamine binding protein (PEBP), and ATP synthase delta subunit (ATP5D) (Table 3 and Figure 3a). Significant decreases post-CP/CPB were detected for α-1-acid glycoprotein (A1AGP) (Table 3 and Figure 3a). Representative images pre/post CP/CPB of multiple patients demonstrate reliable detection and differences in the identified protein spots including MLC-2a (Figure 3B through 3D arrows, spots 1 to 3 in Table 3, respectively) and PEBP (Figure 3E, arrows). In Figure 3B, additional gels either sequentially stained for phosphoproteins and total proteins (top panels), as well as immunoblot analysis with specific MLC-2a antibody (bottom panel), demonstrated spot 2 (Table 3, and Figure 3B, dashed arrow) is likely phosphorylated MLC-2a.
Table 3. Proteins Regulated During CP/CPB Identified by LC-MS/MS.
| Spot # | Name | Abbr. | UniProt No. |
UniProt Name |
Synonyms | Frequency Pre/Post |
Calculated pI |
Calculated MW |
Estimated pI |
Estimated MW |
No. of Identified Spectra |
Unique Peptides |
Average TIC |
Function |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Myosin Light Chain 2a | MLC-2a | Q01449 | MLRA_HUMAN | MYL7, MYLC2A |
8/8 | 4.83 | 19 448 | 4.75 | 20 | 24 | 9 | 350 095 | Contraction |
| Myosin Light Chain 1 - atrial/embryonic muscle | MYL4 | P12829 | MYL4_HUMAN | MLC-1 | 4.98 | 21 565 | 2 | 2 | N.D. | Muscle; Structural |
||||
| 2 | Myosin Light Chain 2a | MLC-2a | Q01449 | MLRA_HUMAN | MYL7, MYLC2A |
4/6 | 4.83 | 19 448 | 5.8 | 16 | 5 | 4 | 9568 | Inflamamtion |
| Ig Kappa Chain C region | IGKC | P01834 | KAC_HUMAN | ... | 5.58 | 11 608 | 2 | 1 | N.D. | Contraction | ||||
| 3 | Myosin Light Chain 2a | MLC-2a | Q01449 | MLRA_HUMAN | MYL7, MYLC2A |
7/8 | 4.83 | 19 448 | 4.5 | 20 | 14 | 8 | 191 032 | Contraction |
| Atrial Myosin Light Chain 1 | MYL4 | P12829 | MYL4_HUMAN | MLC-1 | 4.98 | 21 565 | 5 | 4 | 8567 | Muscle; Structural |
||||
| 4 | Glutathione-S-transferase omega 1 | GSTO1 | P78417 | GSTO1_HUMAN | GSTTLP28 | 6/6 | 6.24 | 27 566 | 6.5 | 30 | 16 | 11 | 99 832 | GST activity/ ascorbate reductase activity |
| Dodecenoyl-Coenzyme A delta isomerase | DCI | Q96DC0 | D3D2_HUMAN | ... | 6.33 | 26 982 | 6 | 6 | 38 302 | Beta - Oxidation |
||||
| 5 | Enoyl-Coenzyme A Hydratase 1 | ECHS1 | P30084 | ECHM_HUMAN | SCEH | 7/7 | 8.34 | 31 387 | 6.5 | 32 | 17 | 8 | 75 369 | Beta - Oxidation |
| Proteosome component c2 | PSMA1 | P25786 | PSA1_HUMAN | PROS-30 | 6.15 | 29 556 | 7 | 4 | 17 963 | Proteolysis | ||||
| 6 | S100A8 Ca+ + binding protein A8 | S100A8 | P05109 | S10A8_HUMAN | CAGA, CFAG |
8/8 | 6.51 | 10 834 | 6 | 24 | 6 | 3 | 164 435 | Inflammation |
| Heat shock Protein 27 | HSPB1 | P04792 | HSPB1_HUMAN | HSP27 | 5.98 | 22 782 | 6 | 5 | 80 923 | Chaperone/ anti-apoptotic |
||||
| 7 | Proteosome Subunit beta Type 6 | PSB6 | P28072 | PSB6_HUMAN | macropain | 7/8 | 4.8 | 25 358 | 4.75 | 24 | 6 | 5 | 57 300 | Proteolysis |
| Tumor Protein Translationally controlled 1 | TPT1 | Q5W0H4 | Q5W0H4_HUMAN | ... | 5.34 | 21 526 | 6 | 5 | 35 413 | Unknown | ||||
| 8 | Phosphatidylethanolamine Binding Protein 1 | PEBP1 P30086 |
PEBP1_HUMAN | HCNPpp, RKIP |
6/7 | 7.01 | 21 057 | 7 | 21 | 4 | 2 | 221 443 | Inhibits Raf-1, PE binding, protease inhibition |
|
| Superoxide Dismutase [Mn], mitochondrial precursor |
SOD2 | P04179 | SODM_HUMAN | ... | 8.35 | 24 722 | 5 | 3 | 28 400 | Converts superoxide to H202 |
||||
| 9 | Alpha - 1 - Acid Glycoprotein isoform1 | ORM1 | P02763 | A1AG1_HUMAN | AGP1, orosomucoid1 |
6/6 | 4.93 | 23 512 | 3.5 | 60 | 14 | 5 | 264 247 | Acute phase response, inflammation |
| 9 | Alpha - 1 - Acid Glycoprotein isoform2 | ORM2 P19652 |
A1AG2_HUMAN | AGP2, orosomucoid2 |
5.03 | 23 602 | 9 | 3 | 215 530 | Acute phase response, inflammation |
||||
| 10 | ATP Synthase Delta chain | ATP5D | P30049 | ATPD_HUMAN | ... | 8/8 | 5.38 | 17 489 | 3.75 | 143 | 112 886 | ATP synthesis | ||
| Myosin Light Chain 6 - smooth and non-muscle | MYL6 | P60660 | MYL6_HUMAN | MLC3, LC17 |
4.5 | 16 930 | 6 | 3 | 33 648 | Contraction |
Figure 3.

Quantification and representative images of CP/CPB-regulated proteins identified in Table 1. A, Data are presented as the % of the maximal response (pre- or post-CP/CPB). Statistical significance (P<0.05) determined using paired t test. *P<0.05. B, Representative total protein stained gels of MLC2a (solid arrow, spot 1 in Table 1, dashed arrow, spot 587). C, Phosphoprotein gel staining of MLC-2a post-CP/CPB (upper panels, spot 3, dashed arrow; Red: phosphorylated proteins, Green: Total Proteins), and an additional gel immunostained with anti-MLC-2a (lower panel). D, Representative images of truncated MLC-2a pre- and post-CP/CPB. (Arrow: MLC2a, spot 2 in Table 2). E, Representative total protein-stained gels of PEBP (solid arrow, spot 8 in Table 3).
In a distinct set of similar patients, abundance differences in proteins identified by mass spectrometry in the unbiased 2D analysis were verified by immunoblot with specific antibodies (Figure 4). Densitometry changes in A1AGP, PEBP, GSTO1, and truncated MLC-2a displayed significant changes similar to Figure 3A (Figure 4A through 4E). Total levels of MLC2a displayed an insignificant trend for increased levels post CP/CPB (Figure 4F). All proteins were normalized to total levels of HSP27, abundance levels of which do not change after CP/CPB.7
Figure 4.
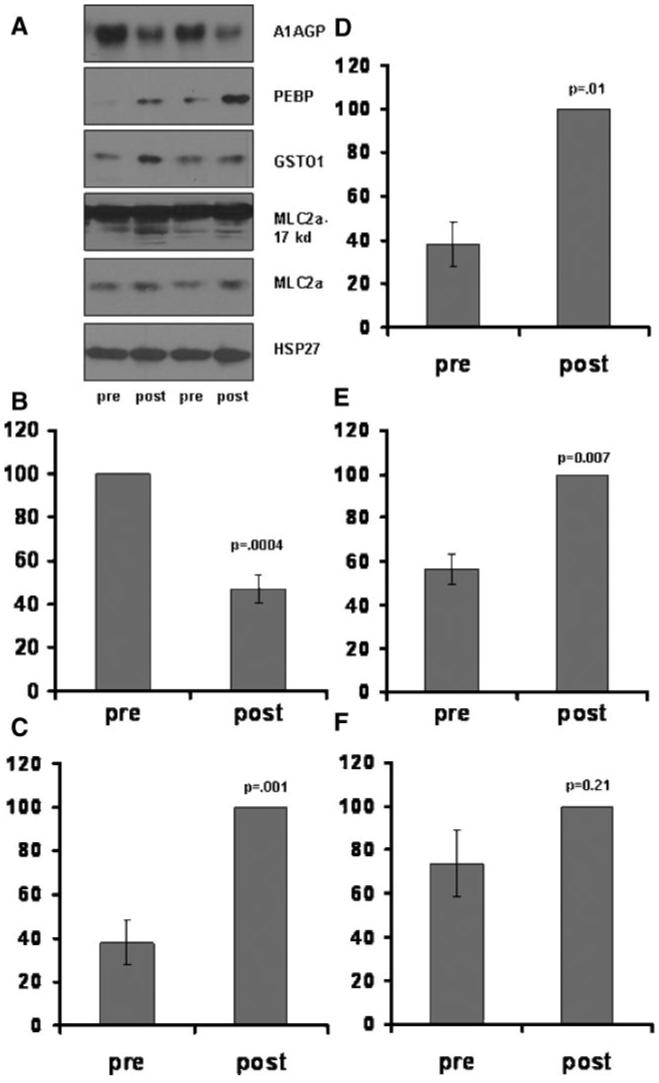
Verification of abundance changes in identified proteins with specific immunoblot analysis. A, Representative immunoblots using specific antibodies to A1AGP, PEBP, GSTO1, MLC2a, and HSP27. Two representative patient samples are shown. Quantitave analysis of the data shown in A for: A1AGP (B), PEBP (C), GSTO1 (D), truncated MLC2a (E) (lower band/s, upper dark band is full length MLC2a), and full length MLC2a (F). Individual densitometry values were normalized to the % maximum response (pre or post) set to 100%. All proteins were corrected for loading differences using the densitometry value of HSP27. Minimum n=6.
Discussion
The purpose of the following study was to determine changes in the myocardial protein profile in patients undergoing cardiac surgery using CP/CPB. Human male right atrial samples, pre- and post-CP/CPB, were used in 2-dimensional electrophoresis, total protein staining, and mass spectrometry analysis to identify specific proteins regulated in response to CP/CPB. Proteomic techniques documenting changes in response to cardiac surgery have been applied previously. Tomic et al documented comparative changes in the serum protein profile of inflammatory markers in patients undergoing on and off pump CPB.13 However, to our knowledge this is the first report to document global CP/CPB-induced changes in the human myocardial tissue proteome after cardiac surgery. Significant and reproducible protein changes were identified that may have novel implications for detrimental consequences of CP/CPB. Specific changes include: decreases in A1AGP which may promote inflammation-associated vascular and myocardial defects, posttranslational modifications of MLC2a which may promote myocardial stunning, and increases in GSTO1 and PEBP which may respectively regulate myocardial redox function and adrenergic sensitivity.
Extrapolating from known function, alterations of the above-identified proteins may have detrimental consequences after ischemic insults associated with CP/CPB. For example, cleavage modifications of MLC-2v in rabbit ventricular tissue are associated with myocardial stunning attributable to reversible ischemic injury.14,15 The cleavage of N-terminal residues is associated with impaired myosin-actin interaction, thereby limiting force generation and Ca2+ sensitivity, both prominent features of CP/CPB-induced myocardial stunning.16,17 In our study, similar alterations were observed in the atrial specific MLC-2a (Spot 2, Table 1). Although MLC-2a is atrial specific, depressed atrial contractile function is a known cause of cardiac low output syndrome. In addition, MLC2a and MLC2v exhibit a high degree of similarity; therefore it is tempting to speculate that similar truncations may occur in the ventricular tissue after CP/CPB. The total levels of MLC2a were also found increased after CP/CPB. Given the short duration of CP/CPB in this patient sample this likely does not reflect de novo protein synthesis. Rather, the detected increase in the MLC2a levels likely reflect an increase in solubility or shedding of MLC2a from the thick filament resulting in depressed contractile function.17,18 Interestingly, MLC2a was not significantly increased in the immunoblot analysis using conventional lysis methods (polytron) (Figure 4F), but was significantly increased in the 2D analysis (Figures 1, 2a, and 3) which used pressure cycling. Pressure cycling has been previously demonstrated to solubilize more proteins than conventional methods. Alternatively, 1D SDS-PAGE separation, which separates primarily on molecular mass, will not distinguish between specific post-translationally modified variants of mlc2a, and may neglect abundance differences in specific variants that are readily detected using 2D separation. Together, these results indicate that CP/CPB may induce instability in myofilament structure.8In addition, MLC-2a was potentially phosphorylated after CP/CPB, (Table 3, spot 1 and 2, and Figure 3C). The phosphorylation of MLC-2a increases the contractile force and Ca2+ sensitivity of human atrial myocytes, and recent evidence indicates that phosphorylation of MLC2v may positively influence ventricular contraction.19,20 Therefore, cardiac MLC phosphorylation during CP/CPB may be a compensatory response to increase Ca2+ sensitivity and subsequent contractile activity. In addition, phosphorylation of MLC is protective against proteolytic cleavage, and may in fact preserve thick filament structure in response to proteolytic activation associated with CP/CPB-induced ischemic insults.21 Therefore, strategies to inhibit MLC truncation modification and increase MLC phosphorylation may be beneficial in the setting of CP/CPB-induced myocardial stunning.
The multifunctional protein PEBP is known to negatively regulate ERK signaling and is a precursor of the highly active negative inotrope, hippocampal neurostimulating peptide (HCNP).22 We have previously documented rapid inactivation of ERK after CP/CPB in pigs, and enhanced PEBP expression may contribute to this effect.23 Also, increases in PEBP during surgery may elevate local and circulating HCNP and thus contribute to myocardial stunning.
Alpha-1-acid glycoprotein (A1AGP) is a serum glycoprotein expressed as an acute response inflammatory protein secreted by the liver.24 Additionally, it can be deposited in the myocardium during severe ischemia.25 It is currently unclear why detected levels of AGP 1 or 2 decrease after CP/CPB, but may reflect diminished signaling after prolonged surgical stress or effects of extracorporeal circulation. AGP has been demonstrated to inhibit complement activation,20 and it has previously been demonstrated that activation of complement can play a role in inflammatory insults associated with CPB26 Therefore, diminished AGP levels may reflect a propensity toward increased complement mediated injury.
Lastly, GSTO1 is highly expressed in the heart and although has intrinsic GST activity, is known to modulate other cellular functions.27 Important in the setting of CP/CPB may be the inhibition of ryanodine receptor activity and subsequent modulation of Ca2+ levels.28
Given the number of statistical tests used in this study, a controlled FDR analysis was used to account for the large number of repeated measures.12,29 FDR methods developed for microarray studies have been previously applied to 2D gel proteomic analyses.30 However, these methods have been proposed to be too conservative in many 2D gel proteomic analyses.29 An FDR of 0.2 was applied in the current study meaning, of those proteins deemed significantly upregulated, it is expected that approximately 20% will yield falsepositives. However, the current analysis using such a threshold may still use an overly conservative approach as errors of misclassifying true positive results may exist. Indeed, protein spots deemed significant using paired t tests (GSTO1, A1AGP, and PEBP; Figure 3), and verified with independent immunoblot experiments (Figure 4) were not deemed significant using the repeated measures analysis. Many previous studies have relied on statistical significance solely on the results of t tests.14,30 The most appropriate method for statistical analysis of 2D-PAGE proteomic studies remains a matter of debate. As such, caution should be exercised in interpreting unverified findings in these experiments.
Recent advances in proteomic based technologies allow the simultaneous identification of thousands of proteins altered by disease or injury. Building on the present findings will include analysis of a greater amount of modified proteins as well as significantly larger patient populations to yield an overall picture of CP/CPB-induced changes in the proteome. It is our hope this analysis will facilitate: (1) improved cardioprotection strategies (type of delivery, conditions, temperature, blood versus crystalloid) and formulations to address detrimental consequences of specific ischemia-induced protein modification and function, (2) tailoring optimal cardioprotection strategies for individual populations (ie, age, sex, hypercholesterolemia, diabetes, hypertension),6,31,32 and (3) provide biomarkers and an understanding of disease mechanism for specific CAD patient population characteristics.
Limitations
Overall, this preliminary report demonstrates distinct and reproducible changes in the patient myocardial protein profile in response to CP/CPB. Changes were documented in human atrial tissue samples, and the unavailability of the more relevant human ventricular tissue represents a limitation of this study. There are documented biochemical differences between the 2 tissues, as well as atrial versus ventricular differences in intraoperative specific stimuli during CP/CPB (ie, less atrial versus ventricular protection). However, previous work in our laboratory demonstrated similar signaling phenomenon in atrial and ventricular tissue in pigs undergoing CP/CPB.33 In addition, the right atrial appendage is an ideal tissue to study potential biomarkers in the myocardial proteomic profile of CABG, valve replacement, and other cardiac surgical patients given that the atria experiences similar ischemic insults as the ventricular muscle, and the samples are routinely discarded.
In conclusion, we have demonstrated multiple significant and reproducible changes to the myocardial protein profile of patients undergoing cardiac surgery with cardioplegic arrest and cardiopulmonary bypass. The identified CP/CPB regulated proteins may have significant implications for cardiac function following cardiac surgery.
Acknowledgments
Sources of Funding
This study was supported by grants R01-HL69024 and R01-HL46716 from the National Institutes of Health (Dr Sellke). Drs Clements and Sodha were supported by a postdoctoral training grant from the National Institutes of Health (5T32-HL076130-02) and the Irving Bard Memorial Fellowship.
Footnotes
Presented at the American Heart Association Scientific Sessions, November 4-7, 2007, Orlando, Fla.
Disclosures
G.B. Smejkal and A. Lazarev are employed by Pressure Biosciences, West Bridgewater, Mass.
References
- 1.Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 2.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 2. Circulation. 2001;104:3158–3167. doi: 10.1161/hc5001.100039. [DOI] [PubMed] [Google Scholar]
- 3.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation. 2001;104:2981–2989. doi: 10.1161/hc4801.100038. [DOI] [PubMed] [Google Scholar]
- 4.Weisel RD. Myocardial stunning after coronary bypass surgery. J Card Surg. 1993;8:242–244. doi: 10.1111/j.1540-8191.1993.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 5.White MY, Van Eyk JE. Cardiovascular proteomics: past, present, and future. Mol Diagn Ther. 2007;11:83–95. doi: 10.1007/BF03256227. [DOI] [PubMed] [Google Scholar]
- 6.Matt P, Carrel T, White M, Lefkovits I, Van Eyk J. Proteomics in cardiovascular surgery. The J Thorac Cardiovasc Surg. 2007;133:210–214. doi: 10.1016/j.jtcvs.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Clements RT, Sodha NR, Feng J, Mieno S, Boodhwani M, Ramlawi B, Bianchi C, Sellke FW. Phosphorylation and translocation of heat shock protein 27 and alphaB-crystallin in human myocardium after cardioplegia and cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2007;134:1461–1470. doi: 10.1016/j.jtcvs.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 8.Smejkal GB, Witzmann FA, Ringham H, Small D, Chase SF, Behnke J, Ting E. Sample preparation for two-dimensional gel electrophoresis using pressure cycling technology. Anal Biochem. 2007;363:309–311. doi: 10.1016/j.ab.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smejkal GB, Robinson MH. Tris interference in IEF and 2-DE. Electrophoresis. 2007;28:1601–1606. doi: 10.1002/elps.200600583. [DOI] [PubMed] [Google Scholar]
- 10.Smejkal GB. Protein Staining in Polyacrylamide Gels. In: Smejkal GB, editor. Separation Methods in Proteomics. CRC Press; 2005. pp. 439–452. [Google Scholar]
- 11.Asara JM, Christofk HR, Freimark LM, Cantley LC. Label-free quantification method by MS/MS TIC compared to SILAC and spectral counting in a proteomics screen. Proteomics. 2008;8:994–999. doi: 10.1002/pmic.200700426. [DOI] [PubMed] [Google Scholar]
- 12.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. (Series B).Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- 13.Tomic V, Russwurm S, Moller E, Claus RA, Blaess M, Brunkhorst F, Bruegel M, Bode K, Bloos F, Wippermann J, Wahlers T, Deigner HP, Thiery J, Reinhart K, Bauer M. Transcriptomic and proteomic patterns of systemic inflammation in on-pump and off-pump coronary artery bypass grafting. Circulation. 2005;112:2912–2920. doi: 10.1161/CIRCULATIONAHA.104.531152. [DOI] [PubMed] [Google Scholar]
- 14.White MY, Cordwell SJ, McCarron HC, Prasan AM, Craft G, Hambly BD, Jeremy RW. Proteomics of ischemia/reperfusion injury in rabbit myocardium reveals alterations to proteins of essential functional systems. Proteomics. 2005;5:1395–1410. doi: 10.1002/pmic.200400995. [DOI] [PubMed] [Google Scholar]
- 15.White MY, Cordwell SJ, McCarron HCK, Tchen AS, Hambly BD, Jeremy RW. Modifications of myosin-regulatory light chain correlate with function of stunned myocardium. J Mol Cell Cardiol. 2003;35:833–840. doi: 10.1016/s0022-2828(03)00141-x. [DOI] [PubMed] [Google Scholar]
- 16.Podlubnaya ZA, Kakol I, Moczarska A, Stepkowski D, Udaltsov S. Truncation of vertebrate striated muscle myosin light chains disturbscalcium-induced structural transitions in synthetic myosin filaments. J Struct Biol. 2000;131:225–233. doi: 10.1006/jsbi.2000.4265. [DOI] [PubMed] [Google Scholar]
- 17.Morano I. Tuning smooth muscle contraction by molecular motors. J Mol Med. 2003;81:481–487. doi: 10.1007/s00109-003-0451-x. [DOI] [PubMed] [Google Scholar]
- 18.Akiyama K, Akopian G, Jinadasa P, Gluckman TL, Terhakopian A, Massey B, Bing RJ. Myocardial infarction and regulatory myosin light chain. J Mol Cell Cardiol. 1997;29:2641–2652. doi: 10.1006/jmcc.1997.0493. [DOI] [PubMed] [Google Scholar]
- 19.Grimm M, Haas P, Willipinski-Stapelfeldt B, Zimmermann WH, Rau T, Pantel K, Weyand M, Eschenhagen T. Key role of myosin light chain (MLC) kinase-mediated MLC2a phosphorylation in the {alpha}1-adrenergic positive inotropic effect in human atrium. Cardiovasc Res. 2005;65:211–220. doi: 10.1016/j.cardiores.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Davis JS, Hassanzadeh S, Winitsky S, Lin H, Satorius C, Vemuri R, Aletras AH, Wen H, Epstein ND. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell. 2001;107:631–641. doi: 10.1016/s0092-8674(01)00586-4. [DOI] [PubMed] [Google Scholar]
- 21.Levine RJ, Yang Z, Epstein ND, Fananapazir L, Stull JT, Sweeney HL. Structural and functional responses of mammalian thick filaments to alterations in myosin regulatory light chains. J Struct Biol. 1998;122:149–161. doi: 10.1006/jsbi.1998.3980. [DOI] [PubMed] [Google Scholar]
- 22.Goumon Y, Angelone T, Schoentgen F, Chasserot-Golaz S, Almas B, Fukami MM, Langley K, Welters ID, Tota B, Aunis D, Metz-Boutigue MH. The hippocampal cholinergic neurostimulating peptide, the N-terminal fragment of the secreted phosphatidylethanolamine-binding protein, possesses a new biological activity on cardiac physiology. J Biol Chem. 2004;279:13054–13064. doi: 10.1074/jbc.M308533200. [DOI] [PubMed] [Google Scholar]
- 23.Araujo EG, Bianchi C, Sato K, Faro R, Li XA, Sellke FW. Inactivation of the MEK/ERK pathway in the myocardium during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2001;121:773–781. doi: 10.1067/mtc.2001.112933. [DOI] [PubMed] [Google Scholar]
- 24.Hochepied T, Berger FG, Baumann H, Libert C. Alpha(1)-acid glycoprotein: an acute phase protein with inflammatory and immunomodulating properties. Cytokine Growth Factor Rev. 2003;14:25–34. doi: 10.1016/s1359-6101(02)00054-0. [DOI] [PubMed] [Google Scholar]
- 25.Poland DCW, Vallejo JJG, Niessen HWM, Nijmeyer R, Calafat J, Hack CE, Van het Hof B, Van Dijk W. Activated human PMN synthesize and release a strongly fucosylated glycoform of {alpha}1-acid glycoprotein, which is transiently deposited in human myocardial infarction. J Leukoc Biol. 2005;78:453–461. doi: 10.1189/jlb.1004566. [DOI] [PubMed] [Google Scholar]
- 26.Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. The Ann Thorac Surg. 2003;75:S715–S720. doi: 10.1016/s0003-4975(02)04701-x. [DOI] [PubMed] [Google Scholar]
- 27.Whitbread AK, Masoumi A, Tetlow N, Schmuck E, Coggan M, Board PG. Characterization of the Omega Class of Glutathione Transferases. In: Helmut Sies aLP., editor. Methods in Enzymology Gluthione Transferases and Gamma-Glutamyl Transpeptidases. Academic Press; 2005. pp. 78–99. [DOI] [PubMed] [Google Scholar]
- 28.Dulhunty A, Gage P, Curtis S, Chelvanayagam G, Board P. The glutathione transferase structural family includes a nuclear chloride channel and a ryanodine receptor calcium release channel modulator. J Biol Chem. 2001;276:3319–3323. doi: 10.1074/jbc.M007874200. [DOI] [PubMed] [Google Scholar]
- 29.Pounds SB. Estimation and control of multiple testing error rates for microarray studies. Brief Bioinform. 2006;7:25–36. doi: 10.1093/bib/bbk002. [DOI] [PubMed] [Google Scholar]
- 30.Karp NA, McCormick PS, Russell MR, Lilley KS. Experimental and statistical considerations to avoid false conclusions in proteomics studies using differential in-gel electrophoresis. Mol Cell Proteomics. 2007;6:1354–1364. doi: 10.1074/mcp.M600274-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Granger CB, Van Eyk JE, Mockrin SC, Anderson NL, on behalf of the Working Group Members National Heart, Lung, and Blood Institute Clinical Proteomics Working Group Report. Circulation. 2004;109:1697–1703. doi: 10.1161/01.CIR.0000121563.47232.2A. [DOI] [PubMed] [Google Scholar]
- 32.Zerkowski HR, Grussenmeyer T, Matt P, Grapow M, Engelhardt S, Lefkovits I. Proteomics strategies in cardiovascular research. J Proteome Res. 2004;3:200–208. doi: 10.1021/pr034079t. [DOI] [PubMed] [Google Scholar]
- 33.Khan TA, Bianchi C, Ruel M, Voisine P, Li J, Liddicoat JR, Sellke FW. Mitogen-activated protein kinase inhibition and cardioplegia-cardiopulmonary bypass reduce coronary myogenic tone. Circulation. 2003;108(Suppl 1):II348–II353. doi: 10.1161/01.cir.0000087652.93751.0e. [DOI] [PubMed] [Google Scholar]


