Abstract
129Xe NMR biosensors are promising agents for the early detection of diseases, given that biomolecular interactions can perturb 129Xe chemical shifts well beyond the typical field inhomogeneity of clinical MRI. We introduce human carbonic anhydrase (CA) as a single-binding-site enzyme for studying xenon biosensor-protein interactions. A xenon-binding cryptophane was substituted with linkers of varying lengths to p-benzenesulfonamide to yield non-diastereomeric biosensors with a single 129Xe NMR resonance. X-ray crystallography confirmed binding of the 8-bond-linked biosensor containing a single xenon atom in the CAII active site. Biosensor dissociation constants (Kd = 20–110 nM) were determined by isothermal titration calorimetry (ITC) for isozymes CA I and II. The biosensor-CA complexes yielded “bound” hyperpolarized 129Xe NMR resonances of narrow linewidth that were shifted by 3.0 to 7.5 ppm downfield, signifying much larger shifts than seen previously. Moreover, isozyme-specific chemical shifts clearly differentiated CA I and II, despite their similar active-site architectures. Thus, xenon biosensors may provide a powerful strategy for diagnosing human diseases characterized by the upregulation of specific CA isozymes and other protein biomarkers.
Introduction
Magnetic resonance imaging (MRI) is used widely (~40 million procedures annually) to scan deep tissues in human patients with high spatial resolution. Intrinsic 1H MRI signals from water and fat provide low sensitivity, thus contrast is typically enhanced using gadolinium- or iron-oxide-based agents.1,2 However, there have been challenges in developing these contrast agents for in vivo imaging of medically relevant biomarkers, such as proteins. Moreover, U.S. and European agencies have recently issued advisories based on studies showing that gadolinium agents pose risks in patients with impaired renal function for developing nephrogenic systemic sclerosis/dermatosis (NSF/NSD).3 These findings motivate the investigation of non-proton-based, hyperpolarized MRI agents such as 129Xe, 13C, and 3He, which enable physiological assays, for example of blood flow and lung function, and provide sensitive methods for studying proteins and metabolites.4–6
Functional xenon biosensors have attracted recent attention due to their potential for simultaneously detecting multiple frequency-resolved 129Xe resonances associated with medically relevant biomarkers.7,8 Monatomic xenon-129 is spin-1/2 and exhibits a large chemical shift window (~300 ppm),9 while laser polarization enhances 129Xe NMR signals more than 10,000-fold,10 which helps to compensate for a weaker magnetic moment and much lower concentration of hyperpolarized 129Xe compared to 1H in aqueous solutions. Since the first report of hyperpolarized 129Xe MRI in excised mouse organs,11 numerous advances in biological imaging with this agent have been made.12 In a recent study, the xenon gas exchange kinetics of frequency-resolved tissue barrier and red blood cell resonances were measured and used to detect regions of rat lung injury.13 Frequency-resolved MRI of an immobilized xenon biosensor has also been demonstrated,14 showing the promise of imaging biosensors bound to a target macromolecule. Most recently, we showed that peptide-functionalized cryptophanes can be delivered to human cells and achieve intracellular concentrations (≥ 100 μM) that should allow in vivo hyperpolarized 129Xe MRI studies.15
Herein, we systematically varied the interaction of a xenon biosensor with a biomedically relevant protein, human carbonic anhydrase (CA), in order to investigate whether small changes in binding are detectable by 129Xe NMR. Cryptophane-A-based xenon biosensors offer the possibility of tuning the frequency of the bound 129Xe nucleus, through electronic and mechanical perturbations of the cage environment. Water-soluble cryptophane-A derivatives have been shown to bind xenon with micromolar dissociation constants under nearly physiological conditions, such as in blood plasma.16 Functionalizing the cryptophane with a biological recognition motif creates a biosensor for the detection of specific proteins, such as matrix metalloproteinases known to be secreted by various tumor cells.17 Current strategies for constructing protein-targeted xenon biosensors are (i) the use of a peptide to solubilize the large (~1 kD) nonpolar cryptophane,7,8,18 or (ii) the incorporation of cryptophanes into supramolecular dendrimers.19 While the use of polyamidoamine (PAMAM) dendrimers affords narrow 129Xe linewidths, it also shields the cryptophane from solution, and renders the bound 129Xe relatively insensitive to protein binding. In contrast, studies with peptido-biotin cryptophane biosensors have shown that decreasing the linker length to the biotin ligand increases the change in chemical shift but also broadens the linewidth upon avidin binding.18 Attaching a peptide to the chiral cryptophane creates diastereomers, which increases the number of peaks in the 129Xe spectrum, and correspondingly decreases the signal-to-noise. Computational studies have helped to assign the various NMR resonances to their respective diastereomers.20 However, the tetrameric nature of avidin can further complicate the 129Xe NMR spectrum.18
In the current study, carbonic anhydrase was chosen as the target enzyme due to its biomedical relevance and status as a model system for understanding protein-ligand interactions.21 CA is involved in many physiological processes such as carbon dioxide transport and pH homeostasis in tissue.22 However, some CAs also appear to have detrimental effects on human health: For example, CA I, II and other isozymes, were shown to be present and probably involved in the formation of certain tumors and polycystic kidney disease.23, 24 Thus, the development of isozyme-specific CA biosensors holds considerable promise for cancer imaging.
Numerous crystal structures of CA-inhibitor complexes are available to guide biosensor development, and CAII in particular has served as a successful model system for rational drug design.21,25,26 CA is a monomeric protein, which reduces the probability of biosensor-biosensor interactions, which were problematic with the tetrameric avidin target.18 Water-soluble biosensors for CA were developed that avoid the appendage of additional stereocenters to the cryptophane core. Use of the arylsulfonamide ligand allows comparison of the biosensor-CA interaction with other designed CA inhibitors. Solution-based assays for CA can confirm active-site binding, and identify protein-biosensor complexes suitable for crystallization.27,28 We recently reported the crystal structure of CAII bound to a benzenesulfonamide-linked-cryptophane (Figure 1).29
Figure 1.
Crystal structure showing C8B-Xe-CAII interactions.29 The Xe atom (green) is shown with a van der Waals diameter of 4.3 Å, the Zn2+ ion is gray, the CAII backbone and surface are tan, and the MoMo enantiomer of C8B is shown in black (carbon), red (oxygen), and blue (nitrogen), surrounded by its van der Waals surface (dots). C8B binds in the active site with the sulfonamidate anion coordinated to Zn2+.
Experimental Procedures
Chemical synthesis
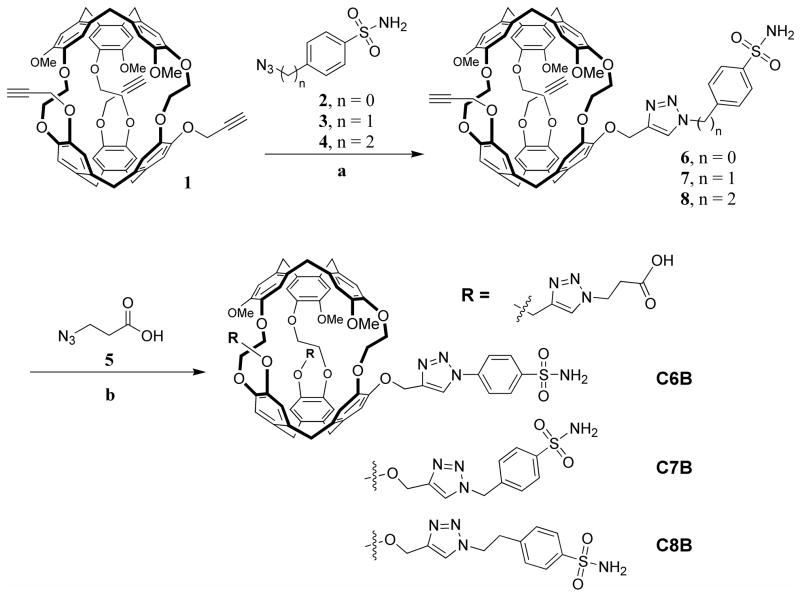
Synthetic protocols and characterization of all new compounds shown in Scheme 1 can be found in Supporting Information (Synthetic Methods and Figure S1).
Scheme 1.
Synthesis of tri-functionalized cryptophane biosensors C6B-C8B, MoMo enantiomers shown. Reagents and conditions for two functionalization steps: (a) Sulfonamide linker (1.1 eq), 100 mM CuSO4 (0.25 eq), 2,6-lutidine (0.25 eq), 300 mM sodium ascorbate (0.75 eq) 10:1 DMSO:H2O, 4 h, 41 – 57% yield; (b) 5 (10 eq), 100 mM CuSO4 (0.25 eq), 2,6-lutidine (0.25 eq), 300 mM sodium ascorbate (0.75 eq), 10:1 DMSO:H2O, 12 h, 41 – 63% yield (17 – 36 % overall yield, as shown, from tripropargyl cryptophane, and 0.8 – 1.8% overall yield from starting materials).16
To conjugate the sulfonamide linker to the tripropargyl cryptophane-A cage, 45 – 90 mg of 1 was dissolved in 2.0 – 4.0 mL dry DMSO at rt with stirring. Sulfonamide linker 2, 3, or 4 (1.1 eq) was added. A 100 mM CuSO4 solution (0.25 eq) was added, followed by 2,6-lutidine (0.25 eq), and 300 mM (+)-sodium-L-ascorbate (0.75 eq). The reaction was allowed to stir overnight and then poured into 50 mL H2O. This solution was extracted with ethyl acetate and the combined organic layer was washed with saturated NaCl solution, dried over Na2SO4, filtered, and evaporated. The yellow oil recovered was purified by silica gel column chromatography to give the pure product as a white solid.
Compound 6, 7, or 8 (27.2 – 54.2 mg) was dissolved in 2.0 mL dry DMSO at rt with stirring. 5 (10 eq) was added. A 100 mM CuSO4 solution (0.25 eq) was added, followed by 2,6-lutidine (0.25 eq), and 300 mM (+)-sodium-L-ascorbate (0.75 eq). The reaction was allowed to stir overnight and then poured into 30 mL H2O. Compound 6, 7, or 8 was dissolved in dry DMSO at rt with stirring. Solubilizing linker 5 was introduced. A 100 mM CuSO4 solution (0.25 eq) was added, followed by 2,6-lutidine (0.75 eq), and 300 mM (+)-sodium-L-ascorbate (0.25 eq). The reaction was allowed to stir overnight and then poured into H2O. This solution was extracted with ethyl acetate and the combined organic layer was washed with saturated NaCl solution, dried over Na2SO4, filtered, and evaporated. The yellow oil recovered was dissolved in minimal 1 M NaOH and a white solid was precipitated with the addition of 1 M HCl. The liquid was decanted after a 5-min centrifugation at 13.2 krpm. The solid was then redissolved in minimal 1 M NaOH, precipitated with 1 M HCl, and isolated. The solid was then sonicated for 10 min in DI H2O and dried under vacuum. If impurities remained, solid was dissolved in minimal CH2Cl2 and precipitated with diethyl ether until desired purity was achieved. Pure product was recovered as a white solid.
Isothermal titration calorimetry (ITC)
All calorimetry experiments were conducted at 298 K on a VP-ITC titration microcalorimeter from MicroCal, Inc. (Northhampton MA), following standard protocols and data analysis.30,31
CA I and II were exhaustively dialyzed against 50 mM Tris-SO4 (pH = 8.0). Compounds (~10 mM stock solution in DMSO) were dissolved at a concentration of 135 to 300 μM in an aliquot of the same buffer, and an equivalent volume of DMSO was added to the enzyme solution. Prior to the titration experiment, samples were degassed under vacuum for 5 min. The sample cell (effective volume 1.4 mL) was overfilled with 1.8 mL of CA at a concentration of 14 to 26 μM and the reference cell was filled with water. The contents of the sample cell were titrated with 30 aliquots (10 μL each) of inhibitor (two initial 2 μL injections were made, but not used in data analysis). After each injection, the heat change was measured and converted to the corresponding enthalpy value. The reaction mixture was continuously stirred at 300 rpm during titration. Control experiments were carried out by titrating the inhibitor into the buffer solution under identical experimental conditions. The calorimetric data are presented with the background titrations subtracted from the experimental data. The amount of heat produced per injection was calculated by integration of the area under each peak. Data were fit to the equation q = VΔH[E]tK[L]/(1 + K[L]), where q is the heat evolved during the course of the reaction, V is the cell volume, ΔH is the binding enthalpy per mole of ligand, [E]t is the total enzyme concentration, K is the binding constant, and [L] is inhibitor concentration. Nonlinear regression fitting to the binding isotherm (ORIGIN 5.0 software, MicroCal) using a one-site model gave the equilibrium dissociation constant of the ligand, Kd, and estimates of the standard error, as provided in Table 1. The error is σi = √(Ciiχ2), where Cii is the diagonal element of the variance-covariance matrix.
Table 1.
Dissociation constants for CA-linker and CA-biosensor complexes at 298K.
| Enzyme | Ligand | Kd (nM) |
|---|---|---|
| CAI | 4 | 30 ± 10 |
| C6B | 20 ± 10 | |
| C7B | 80 ± 10 | |
| C8B | 30 ± 20 | |
| CAII | 4 | 100 ± 10 |
| C6B | 100 ± 20 | |
| C7B | 110 ± 30 | |
| C8B | 60 ± 20 |
Hyperpolarized 129Xe NMR spectroscopy
Isotopically enriched xenon (86% 129Xe and 0.13% 131Xe, Spectra Gases) was polarized via spin-exchange optical pumping with rubidium at 175 °C32 and cryogenically separated33 from the buffer-gas mixture (1% Xe, 10% N2, 89% He by volume) in a Nycomed-Amersham (now GE) IGI.Xe.2000 gas-flow polarizer (output 129Xe polarization 10–20% for 300-mL batches of pure xenon gas). Immediately after thawing, xenon was transferred to a special aluminum container inside a home-built 129Xe probe in the bore of a 9.4 T superconducting magnet (Oxford Instruments). Spin relaxation time T1 of 129Xe gas in the container ranged from 70 to 120 min.
Hyperpolarized 129Xe NMR measurements12 with water-soluble cryptophane biosensors were performed similarly to our previous studies,17 with the exception of frequency-shift referencing described below. For experiments not involving CA, 650 μL solutions of C6B (96 μM), C7B (186 μM), or C8B (121 μM) in 50 mM Tris-SO4 water-based buffer (pH = 8.0) were added to a 5-mm NMR tube fitted vertically onto a removable probe. A 1-mm outer diameter glass capillary was inserted into the NMR tube to allow bubbling of hyperpolarized Xe gas through the sample. NMR samples were interchanged without removing the hyperpolarized Xe container from the vertical magnet bore.
After thermal equilibrium inside the magnet had been achieved (temperature range for all experiments was 20.0–20.5 °C, with thermal fluctuation of only 0.1 °C), hyperpolarized Xe gas was bubbled through the biosensor solution for ~1 min, and the 129Xe@cryptophane signal was monitored via a 1–5 μs pulse every 10 sec. Once the 129Xe@cryptophane signal indicated saturation, Xe bubbling was stopped, and four pulses at 7–11 μs were used to obtain data (n = 4, 129Xe frequency f0 = 110.74668 MHz, 40°–70° tipping pulse) and then averaged. Raw free induction decay (FID) signals were recorded in quadrature. Where useful, multiple processed spectra were averaged to increase signal-to-noise. The biosensor-CA samples were prepared in 50 mM Tris-SO4 buffer (pH = 8.0) using the following concentrations: C6B (188 μM) + CAI (141 μM), C6B (148 μM) + CAII (123 μM), C7B (136 μM) + CAI (100 μM), C7B (132 μM) + CAII (105 μM), C8B (189 μM) + CAI (141 μM), C8B (189 μM) + CAII (153 μM). The biosensor-CA samples were then subjected to the same data collection procedure as the unbound biosensor samples. NMR data were processed using standard fast Fourier transform combined with baseline and phase corrections. Spectra were fitted to linear combinations of Lorentzians using IGOR Pro 5.0 (Wavemetrics, Inc., Oregon). Gaussian broadening of 20 Hz was applied to obtain the shown spectra, however the fits were done without any broadening.
A new procedure, detailed in the Supporting Information (129Xe NMR Methods), was used to reference NMR frequencies to the 129Xe gas line. In the low-frequency region, two narrow 129Xe peaks (from the gas in the vertical capillary and in the spherical bubbles) were observed, superimposed on a broad peak from the gas above the liquid surface. Upon stoppage of bubbling, the down-frequency peak from the bubbles disappeared, and all other peaks shifted by −31 Hz (−0.3 ppm). The narrow gas peaks were split diamagnetically by 343 ± 20 Hz (3.1 ± 0.2 ppm), consistent with −(scyl − ssph)χ w = 3.01 ppm, where scyl = 1 and ssph = 2/3 are magnetization shape factors for a vertical cylinder and a sphere, and χw is diamagnetic susceptibility of the surrounding solution based on water (−9.04 ppm in SI units). Because the chemical shift of 129Xe gas at 20 °C and 1 atm is 0.509 ppm relative to 129Xe gas at 0 atm,34 we used the following expression to convert peak frequencies f to ppm: σ[ppm] = 106(f − fc + 343)/f0 + 0.509, where fc is the measured peak frequency of 129Xe gas in the capillary and f0 is the spectrometer frequency, both in Hz. The uncertainties in 129Xe chemical shifts from peak fits were small (~4 Hz, 0.04 ppm), with additional sources of error (such as the fit to the gas reference peak) being less than the linewidths of approximately 20 Hz or more. These contributions resulted in peak uncertainties of approximately ± 0.07 ppm. 129Xe chemical shifts are reported to a precision of ± 0.1 ppm. The calculated 129Xe NMR chemical shifts for biosensor C7B were decreased by 0.1 ppm to compensate for the slightly higher temperatures (20.3–20.46 °C, see Table S1) at which these data were collected, using the measured temperature dependence of 0.28 ppm/°C.18
Results
Biosensor design and synthesis
Unlike prior biotin-functionalized Xe biosensors, which gained water solubility through the attachment of a chiral peptide,7,8,18 biosensors for CA were designed to avoid the incorporation of tetrahedral stereocenters. Parent cryptophane-A is chiral, with D3 symmetry,35 as the top and bottom cyclotriveratrylene moieties are oriented in an anti configuration. With the original biotin-labeled Xe biosensor, the use of chiral peptide and maleimide functionalized biotin generated four diastereomers,8 which was later reduced to two diastereomers through direct coupling of the biotin to an orthogonally protected lysine residue.18 In each case, avidin binding typically produced numerous overlapping, and correspondingly broadened resonances. In targeting CA, biosensors were modeled into CAII and structures were identified that allowed coordination of the sulfonamidate anion to the active-site Zn2+. We designed relatively low molecular weight biosensors (1372–1423 g/mol, not including Xe), in order to maximize rotational mobility, and achieve narrow hyperpolarized 129Xe NMR resonances. Scheme 1 shows cryptophanes attached via triazole linkers with 6-, 7-, and 8-bonds to benzenesulfonamide (C6B, C7B, C8B). The shortest biosensor, C6B fit inside the protein cleft and positioned the cryptophane edge at the protein surface, approximately 15 Å from the Zn2+ active site. Longer biosensors C7B and C8B were conceived in order to modulate the interaction between the protein and cryptophane, and observe the resulting changes in 129Xe NMR chemical shift. Modeling studies were validated by the X-ray crystal structure determination of C8B-CAII (Protein Data Bank accession code 3CYU, Figure 1), which used anomalous scattering information to confirm the encapsulation of Xe within the cryptophane.29
Benzenesulfonamide was para substituted with azido-functionalized linkers 2–4 that were readily synthesized from the amine analogues (see Supporting Information, Synthetic Methods), and provided a variable 6–8 bond spacer between the cryptophane and benzenesulfonamide recognition unit. As seen in Scheme 1, linkers 2–4 were reacted with tripropargyl cryptophane (1) by a copper (I)-catalyzed [3+2] cycloaddition process previously developed by others,36,37 and applied by our lab.16,17 It was determined that 2 was degraded with light when dissolved in DMSO, thus conjugate 6 was successfully synthesized in the absence of light. Conjugates 7 and 8 did not require this additional precaution.
Compounds 6–8 exhibited poor solubility in aqueous media. To enable subsequent solution NMR and crystallography experiments, two carboxylic acid groups were attached to the cryptophane. 3-Azidopropionic acid 5 was readily prepared following literature protocols,38 and reacted in 5-fold excess with the two remaining alkynes by the same copper (I)-catalyzed [3+2] cycloaddition process. In neutral aqueous solutions, the dianionic biosensors C6B-C8B exhibited favorable solubility for X-ray crystallography studies with CAII.
Biosensor binding studies
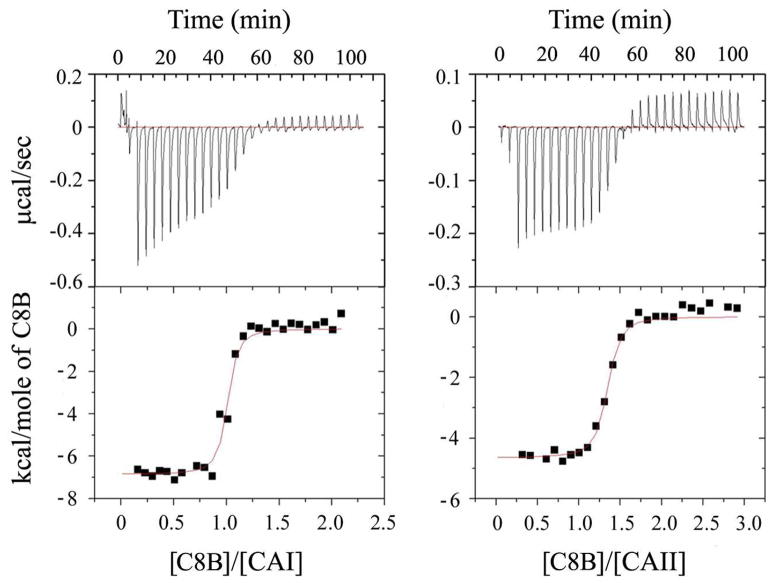
Initial binding studies were conducted using a dansylamide displacement assay,27,28 which confirmed biosensor binding to the active sites of CA I and II in solution (data not shown). Isothermal titration calorimetry was used to determine the affinity of binding of 4, C6B, C7B, and C8B to CA I and II (Figure 2 and Table 1). Biosensor C8B exhibited higher affinity for CAII than the other compounds. However, all four compounds bound slightly better to CAI, with C6B exhibiting the highest affinity for CAI and five-fold discrimination over CAII. Further discrimination may be possible by varying the linker joining benzenesulfonamide and cryptophane, based on small differences between the isozymes in active-site architecture.39,40 Data in Table 1 indicate that the cryptophane and tether length had little effect on the CA I and II dissociation constants (Kd = 20–110 nM) for this range of linkers. Larger refined thermal (B) factors for C8B (<B> = 42 Å2) compared with the overall CAII model (<B>main chain = 31 Å2; <B>side chain = 35 Å2) reflect the mobility and conformational heterogeneity of the bound biosensor.29 Indeed, the cryptophane in C8B sits a few angstroms above the active-site cleft, and there are few hydrogen bonds or other specific interactions with the protein. These structural data are consistent with the relative insensitivity of affinity to tether length. A more detailed investigation into the thermodynamics of the biosensor-protein interaction will be reported in due course.
Figure 2.
Representative ITC experiment with enthalpograms for CA:biosensor complexation at 298 K in 50 mM Tris buffer, pH = 8.0. (left) CAI (14.2 μM) titrated with C8B (135.7 μM); (right) CAII (14.9 μM) titrated with C8B (200.0 μM).
Hyperpolarized 129Xe NMR spectroscopy
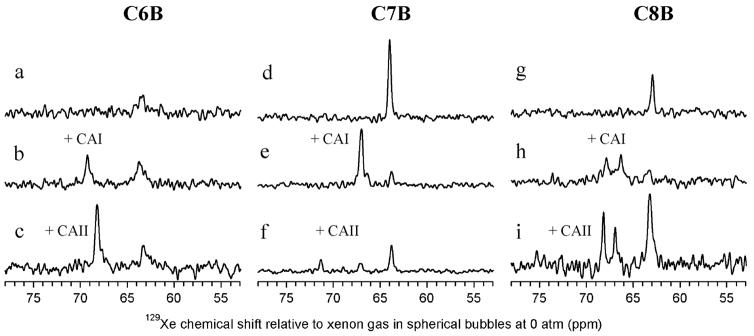
As expected, the racemic mixture of unbound MoMo and PoPo enantiomers produced a single isotropic peak for C6B (63.5 ppm), C7B (63.9 ppm), and C8B (62.9 ppm) in their hyperpolarized 129Xe NMR spectra (Figures 3a, 3d, 3g and Table 2).20 In the presence of substoichiometric CA, the 129Xe NMR peak corresponding to unbound biosensor was typically shifted 0.2–0.4 ppm downfield, which reflects differences in solution properties.
Figure 3.
Laser-polarized 129Xe NMR spectra with biosensors in 50 mM Tris, pH = 8.0, buffer solution. (a,d,g) 129Xe NMR spectra showing individual biosensors free in solution, (b,e,h) 129Xe NMR spectra showing each biosensor bound to CAI. (c,f,i) 129Xe NMR spectra showing each biosensor bound to CAII. (a) C6B alone (96 μM); (b) C6B (188 μM) and CAI (141 μM); (c) C6B (148 μM) and CAII (123 μM); (d) C7B alone (186 μM); (e) C7B (136 μM) and CAI (100 μM); (f) C7B (132 μM) and CAII (105 μM); (g) C8B alone (121 μM); (h) C8B (189 μM) and CAI (141 μM); (i) C8B (189 μM) and CAII (153 μM).
Table 2.
129Xe NMR chemical shifts for C6B, C7B, C8B free in solution, bound to CA I or II.
| Biosensor | Unbound [ppm] | CAI [ppm] Unbound; Bound | Δδ CAI [ppm] | CAII [ppm] Unbound; Bound | Δδ CAII [ppm] |
|---|---|---|---|---|---|
| C6B | 63.5 | 63.7; 69.2 | 5.5 | 68.2; 63.1 | 5.1 |
| C7B | 63.9 | 63.7; 66.9 | 3.2 | --*; 67.0, 71.2 | 3.3, 7.5 |
| C8B | 62.9 | 63.3; 67.9, 66.3 | 4.6, 3.0 | 63.2; 68.2, 66.9 | 5.0, 3.7 |
A slight excess of CAII in solution led to the loss of the unbound signal.
Trials with the three biosensors demonstrated repeatable and significant changes in 129Xe NMR chemical shift upon addition of CA I or II. Addition of CAI to C8B gave an unbound resonance at 63.3 ppm and two peaks at 67.9 ppm and 66.3 ppm for the bound enantiomers (Figure 3h), with Δδ equal to 4.6 and 3.0 ppm. The spectrum of C8B with CAII (Figure 3i) gave an unbound resonance at 63.2 ppm and also showed two resonances for the bound cryptophane enantiomers, with the first at 68.2 ppm (Δδ = 5.0 ppm) and the second at 66.9 ppm (Δδ = 3.7 ppm). The difference between the peaks observed for C8B when bound to CAI (1.5 ppm) and CAII (1.3 ppm) are at the upper bound of the values observed by Lowery et al. for the diastereomeric biotin-labeled cryptophanes bound to avidin.18 The paired resonances observed for C8B are also likely diastereomeric in nature, based on differences in the chiral potential of 129Xe in the two enantiomers bound to the chiral protein. The protein crystal structure identifies one possible site of interaction between C8B and CAII, with the MoMo and PoPo enantiomers occupying very similar positions around a central xenon atom.29
Interestingly, the laser-polarized 129Xe NMR spectrum of C6B with CAI (Figure 3b) showed only a single peak for bound cryptophane biosensor at 69.2 ppm with the unbound peak at 63.7 ppm (Δδ = 5.5 ppm). The spectrum of C6B with CAII (Figure 3c) also showed a single peak for bound cryptophane, at 68.2 ppm, with the unbound peak at 63.1 ppm (Δδ = 5.1 ppm). Based on previous results with biotin-labeled biosensors, it was expected that the short linker of C6B would cause a strong binding-induced shift on the encapsulated 129Xe resonance due to the proximity of the biosensor to the protein.18 It was not expected, however, that C6B bound to CA I or II would exhibit only a single bound 129Xe resonance. Diastereomerically resolved resonances were previously identified in Lowery’s biotin-linked cryptophanes, and were also observed in C8B-CA. It is not possible that only a single enantiomer of C6B is binding CA, based on the stoichiometries obtained from ITC measurements (see Supporting Information, Figure S2). These data and the relatively narrow (40 ± 10 Hz) 129Xe@C6B-CA linewidth, indicate that the two enantiomers of C6B must induce the same change in the potential of the 129Xe nucleus when bound to the chiral protein pocket. In addition to the single “bound” peak, the short linker of C6B provides significantly larger chemical shift changes compared to the previous biosensors that targeted avidin,18 without sacrificing linewidth.
The laser-polarized 129Xe NMR spectrum of C7B with CAI (Figure 3e) also showed only a single peak for bound cryptophane biosensor at 66.9 ppm with the unbound peak at 63.7 ppm (Δδ = 3.2 ppm). Most interestingly, when complexed with CAII (Figure 3f), C7B showed a bound resonance at 67.0 ppm, and a second bound resonance at 71.2 ppm (Δδ = 7.5 ppm, relative to the unbound resonance at 63.7 ppm). This shift is the largest ever reported for a xenon biosensor. Moreover, the peak at 71.2 ppm for C7B-CAII clearly provides isozyme discrimination between CA I and II, which creates opportunities for 129Xe NMR multiplexing experiments.
The observation of a single “bound” peak for C6B-CAI, C6B-CAII, and C7B-CAI (Table 2) suggests that this is a common feature of xenon biosensors when the cryptophane is confined by a rigid linker within the CA environment. The cryptophane in close proximity to CA will be partially desolvated and experience a lower dielectric environment,29 but is not expected to bind to the protein surface. The net environmental changes felt by the xenon in this case appear to dominate the observed 129Xe NMR chemical shift, and outweigh diastereomeric considerations.
To try to explain the spectral differences between C7B-CAI (one “bound” peak) and C7B-CAII (two “bound” peaks), we inspected the two isozymes. CA I and II were calculated using EMBOSS-Align:Needle to have high sequence identity (60%) and sequence similarity (72%).41 Structural homology (r.m.s.d. = 0.92 Å) was calculated using the TOP3D program of the CCP4 package,42 with ligand-free crystal structures for CAI (Protein Data Bank accession code 2CAB),43 and CAII (Protein Data Bank accession code 2CBA).44 CA I and II possess active-site clefts of virtually identical width (15 Å) and depth (15 Å).40 Thus, the 10-Å diameter cryptophane experiences similar steric environments in the two CA isozymes.
Despite these structural similarities, the cavities of CA I and II vary considerably in the distribution of polar and nonpolar residues. For example, two hydrophobic patches that are unique to the CAII active-site channel previously guided the synthesis of high-affinity enzyme inhibitors.45 These patches, and other sites rich in aromatic residues, are accessible to the Zn2+-bound cryptophane biosensor and may serve to discriminate between the enantiomers of C7B. We hypothesize that in C7B-CAI, the bound enantiomers produce equivalent 129Xe NMR resonances by being in close proximity to the protein environment, whereas in C7B-CAII, cryptophane interactions with the protein surface lead to diastereomerism. The variation in 129Xe NMR chemical shift observed for the two enantiomers of C7B-CAII (Δδ = 4.2 ppm) compared to C8B-CAII (Δδ = 1.3 ppm) may be due to conformational differences between the two biosensors. In C7B, the benzenesulfonamide is kinked by 109° relative to the linker, which may enforce interactions of one or both enantiomers with the protein surface.
Importantly for biosensing applications, the linewidths of “bound” xenon biosensors (23–50 Hz) were only somewhat broadened compared to free xenon in solution (12 ± 1 Hz) and were relatively unchanged from the “free” xenon biosensors C7B (27 ± 1 Hz) and C8B (25 ± 3 Hz). The apparently broader linewidth of C6B (79 ± 20 Hz) was likely the result of poor mixing and low cryptophane-bound hyperpolarized 129Xe NMR signal. All three biosensors exhibit excellent solubility in aqueous solutions. In the crystal structure of C8B-CAII,29 the enantiomers appear to interact very little with the CAII surface, which should allow rotational and translational motions of the biosensor, and promote narrow NMR linewidths. Furthermore, because the cryptophane in C8B-CAII appears to be unperturbed by the protein,29 the xenon within the cryptophane can continue to behave isotropically. This should make the noncovalently bound 129Xe nucleus fairly insensitive to the larger rotational correlation time of the biosensor-CA complex. By comparison, the previous use of polypeptide solubilizing moieties and linkers in biotin-functionalized biosensors may have promoted binding interactions with avidin that contributed to line-broadening.18 The CA data support the design of minimally chiral, low molecular weight 129Xe biosensors that target proteins with single binding sites, and also minimize binding interactions between the cryptophane and linker with the protein surface.
Discussion
In cancer patients, tumors can exist for long periods in a dormant, asymptomatic state before diagnosis and development of disease.46 Furthermore, human cancers are characterized by the over-expression of many different proteins.47 Thus, the ability to detect the overexpression of multiple protein biomarkers that colocalize and identify a population of abnormal cells has the potential to improve vastly the accuracy and efficacy of early cancer detection.
Towards this goal, we have shown that water-soluble 129Xe NMR biosensors can be targeted with nanomolar affinity to biomedically relevant proteins, human carbonic anhydrases I and II. A xenon-binding tri-propargyl cryptophane was functionalized in two facile copper (I)-catalyzed [3+2] cycloaddition steps, where the linker to benzenesulfonamide was varied between 6 and 8 bonds. X-ray crystal structure analysis of CAII complexed with C8B confirmed ligation of the sulfonamide moiety to Zn2+ in the CA active site, with the cryptophane at the upper rim of the active-site cleft. CA is the first monomeric, single-binding-site protein that has been studied with a xenon biosensor. ITC measurements revealed that C6B, C7B, and C8B exhibit similar dissociation constants for CA as the bare linker 4, indicating that the cryptophane has little effect on CA binding affinity for this range of linkers.
CA serves as a useful model system for studying xenon biosensor-protein interactions. In order to improve detection sensitivity, it has been proposed to use the HyperCEST technique,48,49 in order to irradiate and quench selectively the hyperpolarized 129Xe NMR signal(s) corresponding to the bound species, and subsequently detect a decrease in the bulk 129Xe NMR signal, based on rapid exchange between xenon populations. 129Xe NMR peaks with narrow linewidths and large changes in chemical shift will facilitate selective irradiation of one or more “bound” xenon species in solution, as required for indirect detection. Additionally, well-resolved resonances will facilitate direct detection. 129Xe NMR studies with C6B, C7B, and C8B indicated significant and reproducible downfield shifts of 5.5, 7.5, and 5.0 ppm upon binding to CA, which are considerably larger than those observed previously for 129Xe biosensors targeting avidin.18 The magnitude of the shifts, relative to typical MRI field inhomogeneity of ~1 ppm, holds considerable promise for in vivo imaging studies.
Finally, isozyme discrimination was observed for all three 129Xe biosensors, with a 129Xe NMR chemical shift difference of ~0.5 ppm between C6B-CAI and C6B-CAII and between C8B-CAI and C8B-CAII. Significantly, a peak was observed exclusively for C7B-CAII that was 4.3 ppm downfield from the single bound resonance for C7B-CAI. Isozyme-specific chemical shifts clearly differentiate between CA I and II, despite their homologous structures.
Conclusion
In summary, we synthesized 129Xe NMR biosensors for CA I and II by modifying a cryptophane-A cage with two water-solubilizing groups and attaching an arylsulfonamide recognition moiety via a triazole linker. The linker was varied between 6 and 8 bonds, in order to tether the biosensors to active-site Zn2+ and modulate the interaction between cryptophane and CA. ITC confirmed nanomolar affinity for CA I and II. When bound to CA, biosensors C6B, C7B, and C8B demonstrated repeatable changes in 129Xe chemical shifts of 5.5, 7.5, and 5.0 ppm, with 7.5 ppm being the largest chemical shift change ever reported for a xenon biosensor. CA I and II were distinguished by C6B and C8B by reproducible 0.5 ppm chemical shift differences. Remarkably, C7B produced a unique resonance when bound to CAII that was shifted 4.3 ppm downfield from the resonance assigned to CAI. Based on the design principles have been advanced through this work, xenon biosensors now provide avenues for detecting diseases associated with CA and other proteins by in vivo hyperpolarized 129Xe NMR spectroscopy.
Supplementary Material
Complete ref 4, detailed synthetic methods and compound characterization, 129Xe NMR methods, 129Xe NMR dataset parameters and full spectrum, ITC data for 4, C6B, and C7B. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This work was supported by an NIH Chemical Biology Interface training grant to J.A.A., NIH grant GM49758 to D.W.C., and DOD (W81XWH-04-1-0657), NIH (1R21CA110104), and Camille and Henry Dreyfus Teacher-Scholar awards to I.J.D. We thank Ronen Marmorstein and Jeffery Saven for access to instrumentation, and Luigi Di Costanzo, George Furst, and M.G. Finn for valuable discussions. Thanks go to Kevin Jude for providing CAII, and Garry Seward and Jan-Oliver Janda for experimental assistance.
References
- 1.Degani H, Gusis V, Weinstein D, Feilds S, Strano S. Nat Medicine. 1997;3:780–782. doi: 10.1038/nm0797-780. [DOI] [PubMed] [Google Scholar]
- 2.Foster-Gareau P, Heyn C, Alejski A, Rutt BK. Mag Res Med. 2003;49:968–971. doi: 10.1002/mrm.10417. [DOI] [PubMed] [Google Scholar]
- 3.Broome DR, Girguis MS, Baron PW, Cottrell AC, Kjellin I, Kirk GA. Am J Roentgenology. 2007;188:586–592. doi: 10.2214/ajr.06.1094. [DOI] [PubMed] [Google Scholar]
- 4.Mugler JP, et al. Magn Reson Med. 1997;37:809–815. doi: 10.1002/mrm.1910370602. [DOI] [PubMed] [Google Scholar]
- 5.Golman K, Zandt RI, Lerche M, Pehrson R, Ardenkjaer-Larsen JH. Cancer Res. 2006;66:10855–10860. doi: 10.1158/0008-5472.CAN-06-2564. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins SR, Levin DL, Emami K, Kadlecek S, Yu J, Ishii M, Rizi RR. J Appl Physiol. 2007;102:1244–1254. doi: 10.1152/japplphysiol.00738.2006. [DOI] [PubMed] [Google Scholar]
- 7.Spence MM, Rubin SM, Dimitrov IE, Ruiz EJ, Wemmer DE, Pines A, Yao SQ, Tian F, Schultz PG. Proc Natl Acad Sci USA. 2001;98:10654–10657. doi: 10.1073/pnas.191368398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spence MM, Ruiz EJ, Rubin SM, Lowery TJ, Winssinger N, Schultz PG, Wemmer DE, Pines A. J Am Chem Soc. 2004;126:15287–15294. doi: 10.1021/ja0483037. [DOI] [PubMed] [Google Scholar]
- 9.Raftery D. Annu Rep NMR Spectros. 2006;57:205–270. [Google Scholar]
- 10.Ruset IC, Ketel S, Hersman FW. Phys Rev Lett. 2006;96:053002. doi: 10.1103/PhysRevLett.96.053002. [DOI] [PubMed] [Google Scholar]
- 11.Albert MS, Cates GD, Driehuys B, Happer W, Saam B, Springer CS, Wishnia A. Nature. 1994;370:199–201. doi: 10.1038/370199a0. [DOI] [PubMed] [Google Scholar]
- 12.Cherubini A, Bifone A. Prog Nuc Magn Res Spec. 2003;42:1–30. [Google Scholar]
- 13.Driehuys B, Cofer GP, Pollaro J, Mackel JB, Hedlund LW, Johnson GA. Proc Natl Acad Sci USA. 2006;103:18278–18283. doi: 10.1073/pnas.0608458103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilty C, Lowery TJ, Wemmer DE, Pines A. Angew Chem-Int Ed. 2006;45:70–73. doi: 10.1002/anie.200502693. [DOI] [PubMed] [Google Scholar]
- 15.Seward GK, Wei Q, Dmochowski IJ. Bioconjug Chem. 2008 doi: 10.1021/bc8002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill PA, Wei Q, Eckenhoff RG, Dmochowski IJ. J Am Chem Soc. 2007;129:9262–9263. doi: 10.1021/ja072965p. [DOI] [PubMed] [Google Scholar]
- 17.Wei Q, Seward GK, Hill PA, Patton B, Dimitrov IE, Kuzma NN, Dmochowski IJ. J Am Chem Soc. 2006;128:13274–13283. doi: 10.1021/ja0640501. [DOI] [PubMed] [Google Scholar]
- 18.Lowery TJ, Garcia S, Chavez L, Ruiz EJ, Wu T, Brotin T, Dutasta JP, King DS, Schultz PG, Pines A, Wemmer DE. Chembiochem. 2006;7:65–73. doi: 10.1002/cbic.200500327. [DOI] [PubMed] [Google Scholar]
- 19.Mynar JL, Lowery TJ, Wemmer DE, Pines A, Frechet JMJ. J Am Chem Soc. 2006;128:6334–6335. doi: 10.1021/ja061735s. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz EJ, Sears DN, Pines A, Jameson CJ. J Am Chem Soc. 2006;128:16980–16988. doi: 10.1021/ja066661z. [DOI] [PubMed] [Google Scholar]
- 21.Krishnamurthy VM, Kaufman GK, Urbach AR, Gitlin I, Gudiksen KL, Weibel DB, Whitesides GM. Chem Rev. 2008;108:946–1051. doi: 10.1021/cr050262p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Supuran CT, Scozzafava A. Bioorg Med Chem. 2007;15:4336–4350. doi: 10.1016/j.bmc.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 23.Pastorekova S, Parkkila S, Zavada J. Adv Clin Chem. 2006;42:167–216. [PubMed] [Google Scholar]
- 24.Kaneta S, Ishizuki S, Kasahara M, Nagao S, Takahashi H. Exper Animals. 1999;48:161–169. doi: 10.1538/expanim.48.161. [DOI] [PubMed] [Google Scholar]
- 25.Greer J, Erickson JW, Baldwin JJ, Varney MD. J Med Chem. 1994;37:1035–1054. doi: 10.1021/jm00034a001. [DOI] [PubMed] [Google Scholar]
- 26.Supuran CT. Nature Rev Drug Discovery. 2008;7:168–181. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- 27.Chen RF, Kernohan JC. J Biol Chem. 1967;242:5813–5823. [PubMed] [Google Scholar]
- 28.Nair SK, Elbaum D, Christianson DW. J Biol Chem. 1996;271:1003–1007. doi: 10.1074/jbc.271.2.1003. [DOI] [PubMed] [Google Scholar]
- 29.Aaron JA, Chambers JM, Jude KM, Di Costanzo L, Dmochowski IJ, Christianson DW. J Am Chem Soc. 2008 doi: 10.1021/ja802214x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiseman T, Williston S, Brandts JF, Lin LN. Anal Biochem. 1989;179:131–137. doi: 10.1016/0003-2697(89)90213-3. [DOI] [PubMed] [Google Scholar]
- 31.Fisher HF, Singh N. Meth Enzymol. 1995;259:194–221. doi: 10.1016/0076-6879(95)59045-5. [DOI] [PubMed] [Google Scholar]
- 32.Walter DK, Griffith WM, Happer W. Phys Rev Lett. 2001;86:3264–3267. doi: 10.1103/PhysRevLett.86.3264. [DOI] [PubMed] [Google Scholar]
- 33.Kuzma NN, Patton B, Raman K, Happer W. Phys Rev Lett. 2002;88:147602. doi: 10.1103/PhysRevLett.88.147602. [DOI] [PubMed] [Google Scholar]
- 34.Jameson CJ, Jameson AK, Cohen SM. J Chem Phys. 1973;59:4540–4546. [Google Scholar]
- 35.Collet A. Comp supramol chem. 1996;2:325–365. [Google Scholar]
- 36.Tornoe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 37.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem-Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Leffler JE, Temple RD. J Am Chem Soc. 1967;89:5235–5246. [Google Scholar]
- 39.Turkmen H, Durgun M, Yilmaztekin S, Emul M, Innocenti A, Vullo D, Scozzafava A, Supuran CT. Bioorg Med Chem Lett. 2005;15:367–372. doi: 10.1016/j.bmcl.2004.10.070. [DOI] [PubMed] [Google Scholar]
- 40.Srivastava DK, Jude KM, Banerjee AL, Haldar M, Manokaran S, Kooren J, Mallik S, Christianson DW. J Am Chem Soc. 2007;129:5528–5537. doi: 10.1021/ja068359w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rice P, Longden I, Bleasby A. Trends Genet. 2000;16:276–277. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- 42.CCP4 Collaborative Project. Acta Crystallogr. 1994;D50:760–763. [Google Scholar]
- 43.Kannan KK, Ramanadham M, Jones TA. Ann NY Acad Sci. 1984;429:49–60. doi: 10.1111/j.1749-6632.1984.tb12314.x. [DOI] [PubMed] [Google Scholar]
- 44.Hakansson K, Carlsson M, Svensson LA, Liljas A. J Mol Biol. 1992;227:1192–1204. doi: 10.1016/0022-2836(92)90531-n. [DOI] [PubMed] [Google Scholar]
- 45.Jain A, Whitesides GM, Alexander RS, Christianson DW. J Med Chem. 1994;37:2100–2105. doi: 10.1021/jm00039a023. [DOI] [PubMed] [Google Scholar]
- 46.Almog N, Henke V, Flores L, Hlatky L, Kung AL, Wright RD, Berger R, Hutchinson L, Naumov GN, Bender E, Akslen LA, Achilles EG, Folkman J. FASEB J. 2006;20:947–949. doi: 10.1096/fj.05-3946fje. [DOI] [PubMed] [Google Scholar]
- 47.Welsh JB, Sapinoso LM, Kern SG, Brown DA, Liu T, Bauskin AR, Ward RL, Hawkins NJ, Quinn DI, Russell PJ, Sutherland RL, Breit SN, Moskaluk CA, Henry F, Frierson J, Hampton GM. Proc Natl Acad Sci USA. 2003;100:3410–3415. doi: 10.1073/pnas.0530278100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schroder L, Lowery TJ, Hilty C, Wemmer DE, Pines A. Science. 2006;314:446–449. doi: 10.1126/science.1131847. [DOI] [PubMed] [Google Scholar]
- 49.Garcia S, Chavez L, Lowery TJ, Han SI, Wemmer DE, Pines A. J Magn Reson. 2007;184:72–77. doi: 10.1016/j.jmr.2006.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complete ref 4, detailed synthetic methods and compound characterization, 129Xe NMR methods, 129Xe NMR dataset parameters and full spectrum, ITC data for 4, C6B, and C7B. This material is available free of charge via the Internet at http://pubs.acs.org.