Abstract
Background
Sleep quality is thought to be an important predictor of immunity and in turn susceptibility to the common cold. This article examines whether sleep duration and efficiency in the weeks preceding viral exposure are associated with cold susceptibility.
Methods
Participants were 153 healthy men and women volunteers, ages 21–55. For 14 consecutive days, they reported their sleep duration and sleep efficiency (percent of time in bed actually asleep) for the previous night, and whether they felt rested. Average scores for each sleep variable were calculated over the 14-day baseline. Subsequently, participants were administered nasal drops containing a rhinovirus, quarantined and monitored on the day before and for five days following exposure for development of a clinical cold (infection in the presence of objective signs of illness).
Results
There was a graded association with average sleep duration, with those with <7 hours sleep 2.94 times (CI[95%]=1.18–7.30) more likely to develop a cold than those with ≥ 8 hours. The association with sleep efficiency was also graded with those with < 92% efficiency 5.50 times (CI[95%]=2.08–14.48) more likely to develop a cold than those with efficiencies ≥98%. These relations could not be explained by differences in pre-challenge virus-specific antibody, demographics, season of the year, body mass, socioeconomic status, psychological variables or health practices. Percent of days feeling rested was not associated with colds.
Conclusions
Poorer sleep efficiency and shorter sleep duration in the weeks preceding an exposure to a rhinovirus were associated with lower resistance to illness.
It is commonly thought that poor sleep increases our susceptibility to the common cold. However, there is little direct evidence for this assertion. Experimental studies have demonstrated that sleep deprivation results in poorer immune function such as reduced natural killer cell activity, suppressed interleukin-2 production and increased levels of circulating pro-inflammatory cytokines (1–3). Sleep deprivation has also been found to attenuate antibody response to both Hepatitis A (4) and influenza immunizations (5). The only direct evidence that sleep habits are associated with cold susceptibility derives from a secondary analysis of data from a rhinovirus-challenge study where a single retrospective questionnaire assessing sleep habits during the last month was used to assess sleep efficiency—percentage of time one actually sleeps between lying down to sleep and waking up the next morning (6). Efficiencies below 80% predicted greater risk of developing verifiable illness.
Here we examine whether sleep habits are associated with resistance to a common cold. Instead of retrospective reports, we obtain estimates of sleep habits by averaging respondent reports of sleep duration, efficiency and “feeling rested” across 14 consecutive days. After sleep assessments were completed, subjects were exposed to a rhinovirus (RV) and followed for development of clinical illness. Infection and signs and symptoms of illness were assessed the day before and for 5 days after viral-challenge. This design extends previous work by providing reliable (averaged over 14 days) on-line (collected daily) measures of baseline sleep, allowing the comparison of sleep duration, efficiency and feeling rested and providing the opportunity to test for graded relations between sleep measures and disease susceptibility.
Methods
Design
During the pre-challenge baseline period we assessed virus-specific neutralizing antibody level, demographics, height and weight in healthy volunteers. We also interviewed them on 14 consecutive days about their sleep quality during the previous night. Other interview and questionnaire data collected during pre-challenge baseline included health practices and psychological factors. Volunteers were then quarantined in separate rooms, exposed to RV39 and followed for five days to assess infection, and signs and symptoms of illness.
Subjects
Data were collected between 2000 and 2004. The subjects were 78 men and 75 women aged 21–55 years (mean=37.06, SD= ±8.95) who responded to advertisements, were judged to be in good health and had no missing data (2 lost) on relevant variables. They were studied in six groups and received $800 for their participation. The study received institutional review board approval and informed consent was obtained from each subject.
Experimental Plan
The temporal sequence of study trials is presented in Table 1. Volunteers underwent medical screenings and were excluded if they had a history of nasal surgery, any chronic disorder (e.g., asthma, cardiovascular disorders, sleep apnea), had an abnormal urinalysis, CBC, or blood enzymes, were pregnant or currently lactating, HIV+, or on regular medication (including sleep medication). They were also excluded if they were hospitalized for psychiatric problems during the last five years or were currently taking medications for psychiatric problems. To maximize the infection rate, we also assessed specific serum antibody to the challenge virus and excluded those with titers >4. Demographics, weight, height and perceived social status were also measured at screening, while sleep, health practices and other psychological measures were assessed within the 23 day period just before viral-challenge.
Table 1.
Temporal Sequence of the Trials
| Baseline Period |
| Eligibility Screening (5–10 Weeks BeforeViral Exposure) |
| Physical Exam |
| Blood for Pre-existing Antibody to Virus |
| Demographics |
| Height and Weight |
| Perceived Social Status |
| 14 Daily Interviews (Beginning 20–23 Days BeforeViral Exposure) |
| Sleep |
| Positive Emotions |
| Health Practices |
| Quarantine Baseline Day 0 (Preceding Viral Exposure) |
| Psychological Questionnaires |
| Nasal Lavage for Baseline Virus Culture |
| Baseline Signs and Symptoms of Respiratory Illness |
| Viral-Exposure |
| Quarantine Baseline Day 0 (End of Day) |
| Viral Inoculation |
| Post-Exposure Follow-up |
| Quarantine Days 1–5 |
| Nasal Lavage for Virus Culture |
| Signs and Symptoms of Respiratory Illness |
| 28 Days After Viral Exposure |
| Blood for Antibody to Virus |
During the first 24 hours of quarantine (pre-challenge), volunteers had a nasal examination and nasal lavage. Baseline symptoms, nasal mucociliary clearance and nasal mucus production were also measured. None of the volunteers reported cold signs or symptoms, and no viral pathogen was isolated from any of the obtained nasal lavage samples.
Subjects were then given nasal drops containing 125 Tissue Culture Infectious Dose50 (TCID50) of RV39. On each day of quarantine, volunteers recorded their respiratory symptoms, were assessed for nasal mucociliary clearance and nasal mucus production, and nasal lavage samples were collected for virus culture. Approximately 28 days post-challenge, blood was collected for serological testing. The investigators were blinded to all measures.
Sleep Measures
Volunteers were interviewed by phone on 14 consecutive evenings with the first interview occurring 20–23 days before viral exposure. They were asked the following questions based on key items from the Pittsburgh Sleep Quality Index and Pittsburgh Sleep Diary (7,8): What time did you lie down to go to sleep last night? What time did you get out of bed this morning? How many minutes of sleep did you lose between the time you lay down to go to sleep [interviewer stated actual time] and the time you got out of bed [interviewer stated actual time] because you had difficulty falling asleep or you woke up and couldn’t get back to sleep? Did you spend any time in bed between lying down to go to sleep [interviewer stated actual time] and getting out of bed [interviewer stated actual time] intentionally awake (for instance, reading or watching TV)? If yes, for how many minutes? Did you feel rested from your sleep when you awoke this morning? (Yes, No).
Sleep scores were calculated for each of the 14 interview days. Sleep duration was scored as the number of hours slept (from time laid down in bed to go to sleep until time got out of bed minus minutes of sleep lost minus minutes intentionally awake) and sleep efficiency as sleep duration divided by time in bed (time from lying down until getting out of bed) (cf. 7). We then averaged across the 14 days (at least 8 days of complete data were required; mean number of days=13.44, SD=1.31) to create average sleep duration, average sleep efficiency, and percent days the subject felt rested.
Control Variables
We controlled for viral immunity as assessed by pre-challenge antibody titer, for age, body mass index (BMI: weight [kilograms]/height [meters]2), race, income, education, sex, and season of exposure (spring, summer, autumn, winter), for psychological variables previously found to be associated with risk for colds including perceived social status, perceived stress, positive emotional style, extraversion and agreeableness (9–11); and for smoking rate, alcohol consumption, and exercise.
Volunteers described their primary racial or ethnic group by choosing from six categories: White, Caucasian; Black, African-American; Native American, Eskimo, Aleut; Asian or Pacific Islander; Hispanic, Latino; or other. For analysis these were dummy coded with all but whites (N=81) and blacks (N=59) collapsed into a single “other” category (N=13). Income was assessed by the question “which category best describes your yearly household income before taxes?” There were 13 categories ranging from “less than $5,000” to “$150,000 or more.” Income was defined as the median income of the identified category. Income scores were log (base-10) transformed to normalize the distribution.
Education was assessed by the question “what is the highest grade or year of school you have completed?” There were 18 categories ranging from “no formal education” to “doctoral degree.” Subjects were assigned a number of years of education based on their response (e.g., high school=12 years, associates degree=14 years, and PhD=20 years).
During each of the 14 daily baseline interviews we assessed health practices during the last 24 hours. For exercise participants were asked: Did you exercise long enough to work up a sweat or get your heart thumping, (if yes) for how many minutes did you exercise? For smoking: Did you smoke any tobacco product, (if yes) how many cigarettes, cigars, bowls of tobacco? For alcohol consumption: Did you consume any alcoholic drinks, (if yes) how many (a glass of wine, 12 ounce beer, or shot of hard liquor each equal one drink)? Scores for each behavior were computed as the arithmetic mean across the 14 days.
Psychological variables assessed by questionnaire included a 10-item measure of the perceptions of stress in one’s life (12); perceived socioeconomic rank as assessed by subjects placing themselves on a 9-rung ladder in terms of where they stand in their country on income, education and occupation (9); and extraversion and agreeableness using the modified 5-item versions of the subscales from Goldberg’s Big 5 (13). Positive Emotional Style was assessed as the extent to which respondents reported feeling happy, calm, full of pep, lively, and cheerful during each of the 14 interview days and averaging across days (11).
Viral Cultures and Antibody Response
Virus-specific neutralizing antibody titer was measured in serum collected before and approximately 28 days after virus exposure (14); results were expressed as reciprocals of the final dilution of serum. Daily nasal lavage samples were frozen at −80°C and later cultured for RV using standard techniques (14).
Signs and Symptoms
On each quarantine day, subjects rated the severity (during the previous 24 hours) of each of eight illness symptoms (nasal congestion, sneezing, runny nose, earache, sinus pain, sore throat, cough, chest congestion) on a scale of 0 (none) to 4 (very severe) (15).
Daily mucus production was assessed by collecting used tissues in sealed plastic bags (16). The bags were weighed and the weight of the tissues and bags subtracted. Nasal mucociliary clearance function was assessed as the time required for dye administered into the anterior nose to reach the nasopharnyx (16).
Baseline-adjusted daily scores for each measure were calculated by subtracting the appropriate baseline score from each post-exposure daily score. Negative adjusted scores were re-assigned a value of 0. Total adjusted scores for symptoms, mucus weight and nasal clearance were calculated by summing the respective adjusted daily scores over the post-challenge quarantine days.
Volunteers were considered to have a clinical cold if they were both infected and met illness criteria. Infection was defined as recovery of challenge virus on any of the post challenge days or a ≥ four-fold rise in virus-specific serum neutralizing antibody titer (pre-exposure to 28 days post-exposure) (16). We used an objective illness criterion in the primary analyses that required a total adjusted mucus weight of ≥10 grams or a total adjusted nasal clearance time of ≥ 35 minutes (6). For those with clinical colds by this criterion, the mean total adjusted subjective symptom score was 36.07 (SD=±22.36) versus 11.52 (SD=±13.47); for those without colds (t[151]=−8.48, P<.001). We also report analyses using an illness criterion based on subject self-report. This modified Jackson Criterion requires a total adjusted symptom score of ≥ 6, in addition to either reporting having a cold or reporting rhinorrhea on ≥ 3 of the 5-day period (14).
Statistical Analyses
BMI, total symptom, mucus weight, and nasal clearance scores were logged (base-10) to normalize each distribution. Logistic regression was used to predict colds (1=yes, 0=no) and multiple linear regression to predict continuous markers of objective illness and total subjective symptom scores. Sleep measures were treated as continuous variables and we report regression coefficients, their standard errors and probability levels. To help clarify the nature of the relationships and provide a clearer estimate of effect sizes, we also fit regression equations using categorical measures of sleep (tertiles) and report odds ratios (OR) and 95% confidence intervals (CI). In the primary analyses, we report the association of sleep habits and both objective and subjective cold criteria, but the remaining analyses focus on the objectively determined outcome.
Results
Rates of Infection and Colds
Of the 153 subjects, 135 (88.24%) were infected; 54 (35.29%) developed a cold defined as infection and the objective cold criterion and 66 (43.14%) developed a cold defined as infection and the subjective (Jackson) criterion.
Sleep Scores
Means and standard deviations for the average sleep scores were 7.45 hours (SD=1.33) for duration, 94% (SD=.06) for efficiency and 77% (SD=.22) for percent nights rested. The average sleep scores were only moderately intercorrelated: r=.37 for efficiency and duration; r=.22 for efficiency and percent nights rested; and r=.29 for duration and percent nights rested (all Ps<.01). Approximate tertiles for duration were: low <7 hours (N=58), middle ≥ 7 to <8 (N=52) and high ≥ 8 (N=43); and for efficiency were: low <92% (N=48), middle ≥ 92–98% (N=53), and high >98–100% (N=52).
Baseline Sleep and Cold Susceptibility
Because of their traditional associations with cold susceptibility we included age and viral specific antibody as controls in all the primary analyses. We then conducted a series of analyses, each entering 1 of the16 separate control variables. By trimming the number of covariates we reduced risk of “over fitting” the model (17); however, including all covariates in a single model yielded the same conclusions.
When sleep habits were treated as continuous variables, and age and antibody titer were entered as controls, both shorter sleep duration and lower sleep efficiency were associated with increased risk of developing a cold by both objective (B=−.39, SE=.15, P<.02 for duration and B=−8.93, SE=2.97, P<.003 for sleep efficiency} and subjective criteria (B=−.36, SE=.15, P<.02 for duration and B=−12.33, SE=3.35, P<.001 for efficiency). Percent nights rested was unrelated to either cold criterion (P>.17).
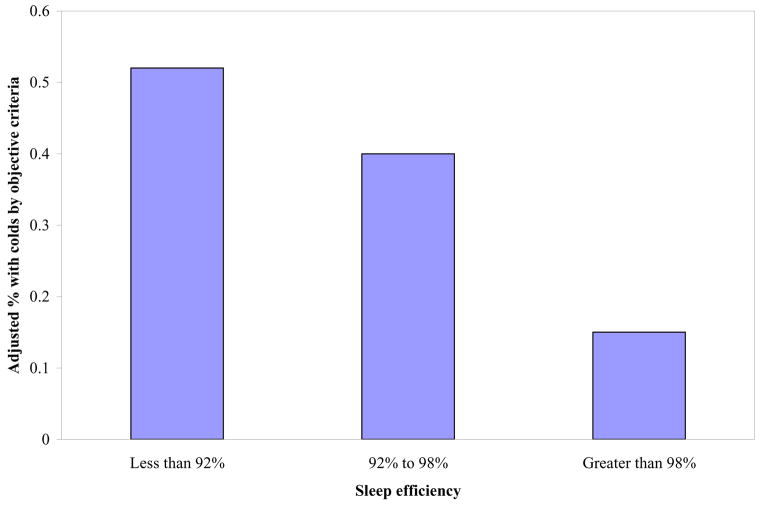
Using tertiles of the sleep variables to predict objectively defined colds illustrates that the relationships between sleep variables and colds were graded for both duration (OR=2.94, CI =1.18–7.30 for low, OR = 1.63, CI =.63–4.19 for middle, and 1 for high) and efficiency (OR= 5.50, CI=2.08–14.48 for low, OR=3.94, CI=1.50–10.37 for middle, 1 for high; Figure 1).
Figure 1.
Sleep efficiency (% time in bed asleep) averaged over 14-days before virus exposure is associated with the percent of persons who subsequently develop a cold. Percent colds are based on predicted values (adjusted for pre-challenge antibody and age) from equation.
To determine if sleep duration and efficiency were independent predictors, we entered both into the same equation predicting the objective cold criterion. Sleep efficiency (B=−7.34, SE=3.14, P<.02) but not duration (B=−.27, SE=.16, P>.10) remained a significant predictor, suggesting overlap between the two predictors and that sleep efficiency accounted for a greater part of the effect.
It is common to consider sleep efficiencies ≤85% as abnormal. Only 8.5% of our sample fell below this norm. To provide a risk estimate based on this common cutoff, we compared those with sleep efficiencies ≤ 85% (N=13) to the remaining subjects (dummy coded). Those with efficiencies ≤ 85% were at a substantially increased risk of getting a cold relative to the rest of the sample (OR=5.37, CI=1.51–19.1).
In a second set of regressions, each analysis contained a single control variable together with either sleep duration or efficiency (32 analyses). In all cases, the sleep variables’ associations with colds remained significant (lowest p<.03) indicating that the sleep effects were independent of all of the controls. Finally, in models including all 16 control variables, both duration (b=−.39, SE=.18, P<.03) and efficiency (b=−6.93, SE=3.37, P<.04) remained independent predictors.
Baseline Sleep and Individual Signs and Symptoms of Illness
In additional analyses, we used individual signs and symptoms as the outcomes instead of clinical illness. Controlling for age and antibody titer, poor sleep efficiency (B=−1.11, SE=.42, P<.01), but not reduced sleep duration (P>.11) was associated with increased mucus weight. Neither efficiency nor duration predicted average increase in nasal clearance time (Ps>.54). Sleep efficiency (B=−1.51, SE=.40, P<.001) but not duration (P>.11) was associated with total symptom score.
Discussion
Poorer sleep efficiency and shorter sleep duration as assessed by self-report over a two-week period in the 23 days preceding exposure to a rhinovirus were associated with increased probability of developing a cold. Both associations were graded. They were also robust in the face of 16 control variables: pre-challenge viral-specific antibody titer, age, BMI, race, income, education, sex, and season of exposure, psychological factors (perceived stress, perceived social status, positive emotional style, extraversion and agreeableness) and health practices (smoking, alcohol consumption, and physical activity). Associations of sleep duration and sleep efficiency overlapped, with efficiency being the primary and independent predictor. Interestingly, while measures estimating when one was sleeping (duration and efficiency) were predictive, the more evaluative sleep measure, “feeling rested,” was not.
Much of what we know about sleep and health derives from prospective cohort studies that use one or two retrospective questions to assess habitual sleep duration. These studies suggest that the lowest morbidity (coronary heart disease) and mortality generally occur among those who report sleeping approximately 7–8 hours a night (18,19). The evidence reported here on risk for the common cold suggests a substantial risk associated with getting < 7 hours of sleep per night. However, unlike some of the mortality studies (18,19), we find that increases in sleep duration above 8 hours are associated with better (rather than poorer) health. One explanation for elevated mortality among long sleepers is that longer-duration sleep is merely a symptom of depression, itself a risk factor for mortality. Our participants were screened for psychiatric disorders and hence depression is not likely to be at play here. Another issue is that our sample is relatively young (mean age 37 and maximum 55) and healthy, while evidence for longer sleep being detrimental derives from samples including a large segment of older adults, many who suffer from chronic illnesses.
The data were quite consistent across outcome measures with the exception of nasal clearance function. The reason for this one anomaly is not clear, although we found surprisingly little variability (as compared to mucus weights or symptoms) in the day-today measures of nasal clearance suggesting a potential insensitivity of this measure.
What mechanisms might link sleep to cold susceptibility? When the components of clinical illness (infection and signs or symptoms) were examined separately, sleep efficiency but not duration was associated with signs and symptoms of illness. However, neither was associated with infection (analysis not reported). This study was designed to maximize the infection rate (participants selected for low antibody levels) and was not powered to fairly test whether sleep is associated with infection (over 88% were infected). Consequently no conclusions about the role of sleep in infection can be made from this null effect. However, the evidence is consistent with increased clinical illness among those with poorer sleep being attributable to symptom expression. A possible explanation for this is that sleep disturbance influences the regulation of pro-inflammatory cytokines, histamines and other symptom mediators released in response to infection (1).
The relative ease of assessing self-reported sleep makes these findings particularly useful to physicians and patients, giving them an approximate indicator they can act on. Studies of both 17–30 and 32–59 year-olds report that about 2/3 report sleeping 7–8 hours a night and approximately 3/4 report 7 hours or more of sleep (20,21). Similar to the mortality studies (eg, 18,19,22), the data reported here support the argument that 7–8 hours of sleep is a reasonable target. However, they also suggest that even a minimal habitual sleep disturbance (sleep losses of 2–8% --10 to 38 minutes for an 8-hour sleeper) is associated with 3.9 times the risk of developing a cold.
Evidence from actigraphy studies suggests that self-reported sleep slightly underestimates both duration and total number of nocturnal wakenings (23–25). Consequently, we may want to be cautious about the exactness of self-reported estimates of sleep duration and efficiency and treat them as broad relative indicators. However, the actigraphy studies suggesting self-report bias were conducted in people with sleep disorders and other psychiatric and physical problems (e.g., 23–25). Self-report biases are likely less marked in healthy samples with less disturbed sleep. Moreover, if there are actually biases in self-reports of duration and efficiency, these same biases would enter in to patients’ attempts to meet specific sleep targets and hence have little significance for clinical intervention. Finally, since existing suggestions about what constitutes health-promoting sleep are based on studies of self-reported sleep habits, our data can be more readily compared with earlier evidence on other morbidities and mortality.
That viral-challenge trials results are applicable to natural colds is supported by concordant evidence of these trials and epidemiologic data and by the close fit between symptom timing and severity patterns with those found in epidemiologic studies (10,26). Generalization of results using RV39 to other cold viruses is provided by challenge studies showing associations hold across multiple upper respiratory viruses (e.g.,10,11), including the earlier study of sleep efficiency and colds (6).
In sum, measures of sleep predicted susceptibility to developing a cold. Although both shorter duration and lower efficiency were associated with risk for illness, duration did not predict independently of efficiency, which was a stronger overall correlate of illness. Although the prospective design does not allow causal inference, it does eliminate reverse causation as an explanation. Because of the prospective design and the controls for multiple confounding factors, these results strongly suggest the possibility of sleep playing a causal role in cold susceptibility. Moreover, the use of a maximally reliable multiple day assessment of sleep habits increases our confidence in the assessments in this study.
Acknowledgments
Research reported here was funded by grants to the Pittsburgh Mind-Body Center from the National Heart, Lung and Blood Institute (HL65111; HL65112), by the National Institute of Allergies and Infectious Disease (AI066367) and by supplementary funds provided by the John D. and Catharine T. MacArthur Foundation Network on Socioeconomic Status and Health. The funders played no role in design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review or approval of the manuscript. None of the authors have any conflicts of interest including specific financial interests and relationships and affiliations relevant to the subject of this manuscript. Dr. Cohen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Cohen was involved in all phases of the study; Dr. Doyle in conception and design, acquisition of data, interpretation of data and drafting of the article; Dr. Turner in the design and conduct of the study, interpretation of the data and in the drafting of the article; Dr. Janicki-Deverts in data analysis and interpretation and drafting of the article, and Dr. Alper in the conduct of the study and drafting of the article. The authors are indebted to Dan Buysse, MD and Martica Hall, PhD of the University of Pittsburgh School of Medicine for their comments on an earlier draft, to Wesley Barnhart, B.S., and Ellen Conser, M.A. of Carnegie Mellon University for their assistance in preparing this article and to the study staff and volunteers.
References
- 1.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166(16):1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 2.Opp MR, Born J, Irwin MR. Sleep and the immune system. In: Ader R, editor. Psychoneuroimmunology. 4. New York, NY: Academic Press; 2007. pp. 579–618. [Google Scholar]
- 3.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89(5):2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 4.Lange T, Perras B, Fehm HL, Born J. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med. 2003;65(5):831–835. doi: 10.1097/01.psy.0000091382.61178.f1. [DOI] [PubMed] [Google Scholar]
- 5.Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA. 2002;288(12):1471–1472. doi: 10.1001/jama.288.12.1471-a. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Social ties and susceptibility to the common cold. JAMA. 1997;277(24):1940–1944. [PubMed] [Google Scholar]
- 7.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 8.Monk TH, Reynolds CF, Kupfer DJ, Buysse DJ, Coble PA, Hayes AJ, et al. J Sleep Res. 1994;3(2):111–120. [PubMed] [Google Scholar]
- 9.Cohen S, Alper CM, Doyle WJ, Adler N, Treanor JJ, Turner RB. Objective and subjective socioeconomic status and susceptibility to the common cold. Health Psychol. 2008;27(2):268–274. doi: 10.1037/0278-6133.27.2.268. [DOI] [PubMed] [Google Scholar]
- 10.Cohen S, Tyrrell DAJ, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325(9):606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 11.Cohen S, Alper CM, Doyle WJ, Treanor JJ, Turner RB. Positive emotional style predicts resistance to illness after experimental exposure to rhinovirus or influenza A virus. Psychosom Med. 2006;68(6):809–815. doi: 10.1097/01.psy.0000245867.92364.3c. [DOI] [PubMed] [Google Scholar]
- 12.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 13.Goldberg LR. The development of markers for the Big-Five factor structure. Psychol Assess. 1992;4(1):26–42. [Google Scholar]
- 14.Gwaltney JM, Jr, Colonno RI, Hamparian VV, Turner RB. Rhinovirus. In: Schmidt NI, Ernmons RW, editors. Diagnostic Procedures for Viral, Rickettsial, and Chlamydial Infections. 6. Washington, DC: American Public Health Association; 1989. pp. 579–614. [Google Scholar]
- 15.Farr BM, Gwaltney JM, Jr, Hendley JO, et al. A randomized controlled trial of glucocorticoid prophylaxis against experimental rhinovirus infection. J Infect Dis. 1990;162(5):1173–1177. doi: 10.1093/infdis/162.5.1173. 1990. [DOI] [PubMed] [Google Scholar]
- 16.Doyle WJ, McBride TP, Swarts JD, Hayden FG, Gwaltney JM., Jr The response of the nasal airway, middle ear, and Eustachian tube to experimental rhinovirus infection. Am J Rhinol. 1988;2(4):149–154. [Google Scholar]
- 17.Bagley SC, White H, Golomb BA. Logistic regression in the medical literature: standards for use and reporting, with particular attention to one medical domain. J Clin Epidemiol. 2001;54(10):979–985. doi: 10.1016/s0895-4356(01)00372-9. [DOI] [PubMed] [Google Scholar]
- 18.Kojima M, Wakai K, Kawamura T, Tamakoshi A, Aoki R, Lin Y, et al. Sleep patterns and total mortality: a 12-year follow-up study in Japan. J Epidemiol. 2000;10(2):87–93. doi: 10.2188/jea.10.87. [DOI] [PubMed] [Google Scholar]
- 19.Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163(2):205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 20.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47(5):833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 21.Steptoe A, Peacey V, Wardle J. Sleep duration and health in young adults. Arch Intern Med. 2006;166(16):1689–1692. doi: 10.1001/archinte.166.16.1689. [DOI] [PubMed] [Google Scholar]
- 22.Ferrie JE, Shipley MJ, Cappuccio FP, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30(12):1659–1666. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kushida C, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement W. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 24.Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999;8(3):175–183. doi: 10.1046/j.1365-2869.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 25.Wilson KG, Watson ST, Currie SR. Daily diary and ambulatory activity monitoring of sleep in patients with insomnia associated with chronic musculoskeletal pain. Pain. 1998;75(1):75–84. doi: 10.1016/S0304-3959(97)00207-8. [DOI] [PubMed] [Google Scholar]
- 26.Gwaltney JM, Jr, Hendley JO, Patrie JT. Symptom severity patterns in experimental common colds and their usefulness in timing onset of illness in natural colds. Clin Infect Dis. 2003;36(6):714–723. doi: 10.1086/367844. [DOI] [PMC free article] [PubMed] [Google Scholar]