Abstract
Purpose
Activation of the mitogen-activated protein kinase (MAPK) pathway and the phosphatidylinositol 3-kinase/AKT pathway seems to be critical for melanoma proliferation. Components of these pathways are client proteins of heat-shock protein 90 (hsp90), suggesting that inhibition of hsp90 could have significant antimelanoma effects. We conducted a phase II trial using the hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin (17-AAG) in melanoma patients. The primary end points were clinical responses and whether treatment inhibited MAPK pathway activity.
Experimental Design
Melanoma patients with measurable disease were stratified on the basis of whether or not their tumor harbored a V600E BRAF mutation. The hsp90 inhibitor 17-AAG was administered i.v. once weekly ×6 weeks at 450 mg/m2. Tumor biopsies were obtained pretreatment and 18 to 50 hours after the first dose of 17-AAG, and were snap-frozen.
Results
Fifteen evaluable patients were treated; nine had BRAF mutations and six were wild-type. No objective responses were observed. Western blot analysis of tumor biopsies showed an increase in hsp70 and a decrease in cyclin D1expression in the posttreatment biopsies but no significant effect on RAF kinases or phospho - extracellular signal-regulated kinase expression. Plasma analyzed by mutant-specific PCR for V600E BRAF showed 86% sensitivity and 67% specificity in predicting tumor DNA sequencing results.
Conclusions
At this dose and schedule of 17-AAG, the effects of 17-AAG on RAF kinase expression were short-lived, and no objective antimelanoma responses were seen. Future trials in melanoma should focus on a more potent hsp90 inhibitor or a formulation that can be administered chronically for a more prolonged suppression of the MAPK pathway.
Most melanomas are driven by activating mutations within the mitogen-activated protein kinase (MAPK) pathway, specifically in NRAS and BRAF (1 -3). Because melanomas harboring BRAF mutations often have concurrent mutations in the phosphatidylinositol 3-kinase/AKT pathway (2, 4), effective antimelanoma strategies will likely require blockade of both the MAPK and phosphatidylinositol 3-kinase/AKT pathways.
17-allylamino-17-demethoxygeldanamycin (17-AAG) is a derivative of the natural product geldanamycin. Both 17-AAG and geldanamycin bind to a conserved pocket in the heat-shock protein 90 (hsp90) family of chaperone proteins and inhibit (5) hsp90 function. This leads to the degradation of hsp90 client proteins, such as Raf-1 (6, 7), mutated BRAF (8), AKT (9), and cdk4 (10). This results in an Rb-dependent G1 growth arrest and, in some systems, differentiation and/or apoptosis (11). Thus, 17-AAG is an attractive agent for the treatment of melanoma because it can block both the MAPK and AKT pathways.
In phase I trials, 17-AAG has been evaluated using dosing schedules of 1, 2, 3 and 5 doses within a week, although these studies included relatively few melanoma patients. Using daily dosing of 5 days every 3 weeks, the maximum tolerated dose was only 40-56 mg/m2 (12, 13) with dose-limiting toxicity being transaminitis. In twice-weekly dosing, the maximum tolerated dose was found to be 175-220 mg/m2 in adult patients (13, 14). If 17-AAG was given three times weekly, dose-limiting toxicities were noted at the 157 mg/m2 dose level (13). At this dose, toxicity was primarily gastrointestinal, with patients developing nausea, diarrhea, and hepatotoxicity. These trials included pharmacokinetic studies and pharmacodynamic analyses of peripheral blood mononuclear cells but limited investigation of pharmacodynamic effects on tumor.
Using a weekly schedule, hepatic toxicity was less prominent and doses up to 450 mg/m2 were tolerated (15, 16), although diarrhea and hepatotoxicity were seen in some patients. In the trial done by Banerji and colleagues, pretreatment and 24-hour posttreatment tumor biopsies were done in 9 patients. CDK4 depletion was reported in 8 of 9 patients and RAF-1 depletion seen in 4 of 6 informative patients. Based on this experience, we decided to explore the 450 mg/m2 weekly dose and schedule.
We carried out a phase II trial in melanoma using a weekly dose of 450 mg/m2 and obtained pretreatment and posttreatment tumor biopsies. The primary goals of the study were to (a) calculate the proportion of clinical responses; (b) determine if 17-AAG can disrupt the MAPK pathway by depleting intratumor stores of RAF kinases and/or downstream proteins such as phospho-extracellular signal-regulated kinase (ERK), CDK4, and cyclin D1; and (c) determine if these clinical or pharmacodynamic effects correlate with the presence of mutated BRAF within the melanoma tumor. A secondary goal was to assess if plasma could be a source of circulating tumor-derived DNA sufficient to accurately determine the BRAF status of the patient's tumor.
Translational Relevance.
The heat-shock protein 90 (hsp90) inhibitor 17-allylamino-17-demethoxygeldanamycin (17-AAG) represents a novel strategy of treating cancer by depleting hsp90 client proteins. This is particularly appropriate in melanoma as BRAF, CDK4, and AKT are all hsp90 client proteins. Currently, most of the effort in developing new therapies for melanoma focuses on identifying inhibitors of either the mitogen-activated protein kinase or AKT pathways;17-AAG inhibits both. This is the first phase II trial in melanoma and the first 17-AAG trial to make pharmacodynamic tumor assessment a major goal. We showed that at the dose and schedule selected (450 mg/m2 i.v. weekly), the Raf kinases were not depleted. This may explain the lack of clinical effects observed. We conclude that future trials will require more prolonged hsp90 inhibition to test the principle that depletion of hsp90 client proteins will result in antimelanoma effects. Although this is a negative phase II trial, it is particularly timely for CCR in light of the recent paper by Goulart (CCR 13:6719, 2007) and the accompanying editorial by Ratain arguing that correlative studies are more appropriate in phase II trials than in phase I trials. Our study follows this paradigm in that we selected the phase II dose based on classic parameters: toxicity, feasibility, and an early hint of clinical activity. This phase II study was built heavily around correlative studies and, in fact, was terminated early on the strength of the correlative studies. Rather than simply rejecting hsp90 inhibition as a strategy, we are able to report that we did not adequately “hit the target”; thus, hsp90 inhibition remains an appealing approach. A better drug or formulation, however, is needed.
Materials and Methods
This was a multicenter phase II trial in which patients were accrued from one of three centers: the Memorial Sloan-Kettering Cancer Center, the H. Lee Moffitt Cancer Center, or the Cancer Institute of New Jersey.
Patient eligibility
Patients with stage III or IV cutaneous melanoma were eligible if they had measurable disease and were not potentially curable by surgery. Patients were stratified based on whether or not their tumors harbored a BRAF mutation as detected by sequencing of tumor-derived DNA. The first 10 patients in each cohort were required to have tumor accessible for pretreatment and posttreatment tumor biopsies. Patients had to be ≥18 y old, have a Karnofsky performance status ≥60, have a life expectancy ≥3 mo, and have adequate bone marrow function (WBC ≥3,000/μL, absolute neutrophils ≥1,500/μL, platelets ≥100,000/μL), liver function (aspartate aminotransferase/alanine aminotransferase <2.5× normal), and normal serum creatinine.
Patients were excluded if they had received >1 prior chemotherapy regimen, had brain metastases (unless they had been brain metastasis-free for ≥6 mo), or were allergic to eggs (17-AAG was formulated with egg phospholipids). Patients were not allowed to be on concomitant anticancer medicines and had to have recovered from the effects of any prior antimelanoma treatment. Patients were not allowed to take medications known to affect CYP3A4 activity or medications known to prolong the Q-Tc interval. Men with Q-Tc intervals ≥450 ms or women with Q-Tc intervals ≥470 ms were excluded.
Treatment regimen
The 17-AAG for this trial was obtained through the Cancer Therapy Evaluation Program of the National Cancer Institute as sterile single-use vials containing 50 mg of 17-AAG in 2 mL DMSO. Patients received 17-AAG by i.v. infusion over approximately 1 to 2 h at a dose of 450 mg/m2 weekly × 6 wk followed by 2 wk with no treatment. Infusion rates were prolonged up to 6 h if needed because of blood pressure changes or nausea. Electrocardiograms were done before each infusion, 6 h after the first infusion, and whenever clinically indicated. Electrolytes were followed carefully and done within 24 h before each infusion. Abnormalities in K+, Mg2+, and Ca2+ were corrected before administering 17-AAG. Patients underwent a physical exam before treatments 1, 5, 8, and on removal from study.
A computed tomography scan of the chest/abdomen/pelvis was done within 4 wk of starting therapy and again on week 7 or upon coming off study. Initially, only patients with at least a partial response were allowed to receive a second cycle of therapy, but halfway through the study this was amended to allow patients with stable disease to receive a second cycle. Tumor responses were measured using the Response Evaluation Criteria in Solid Tumors.
Pharmacodynamic effects of 17-AAG
The plan was to obtain tumor biopsies before the first dose of 17-AAG and 18 to 48 h after the first dose of 17-AAG, although in some cases, the posttreatment biopsy was obtained a few hours later than intended. We selected this time point based on our animal data and on the assumption that a biological effect of interest would have to persist at least 48 h after each dose. Tumor specimens were snap-frozen and stored at -80°C until needed. For analysis of hsp90 client proteins, tumor tissue was homogenized in 2% SDS lysis buffer [50 mmol/L Tris-HCl (pH 7.4), 2% SDS] for 30 s, boiled for 10 min, and sonicated briefly. Cell lysates were cleared by centrifugation at 14,000 × g for 10 min and supernatants were collected. Lysates were added to sample buffer [0.3125 mol/L Tris-HCl (pH 6.8), 10% SDS, 50% Glycerol, 77.5 mg/mL DTT], and equal amounts of protein were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Blots were blocked in 5% nonfat milk in TBS-T [0.1% Tween 20 TBS, 10 mmol/L Tris (pH 7.4), and 150 mmol/L NaCl]. Primary antibodies against the following targets were used: A-RAF, B-RAF, RAF-1, cyclin D1 (Santa Cruz Biotechnology), p85 phosphatidylinositol 3-kinase (Upstate Biology), Phospho-ERK and ERK (Cell Signaling), Hsp70 (StressGene), tyrosinase (Neomarker), and actin (Sigma). After incubation with horseradish peroxidase-conjugated secondary antibodies, proteins were visualized by chemiluminescence (ECL; Amersham Corp.). Band densities were quantitated using FujiFilm Multi Gauge software.
BRAF typing
H&E-stained slides were reviewed to ensure adequate tumor tissue was present. Unstained cut sections mounted on slides were macrodissected to remove contaminating normal cells when possible, and tissue sections were scraped into a 1.5-mL microcentrifuge tube. Genomic DNA was isolated from the paraffin-embedded material using a QIAmp Mini DNA Kit (Qiagen). Conventional DNA sequencing was accomplished as described (17). Sequences were analyzed using SeqScape software (Applied Biosystems, Inc.). Retrospectively, 20 of 21 patients had sufficient DNA remaining to be analyzed by a mutationspecific (MS)-PCR assay as described (18). This MS-PCR assay was also used to test pretreatment blood samples from 13 patients (12 plasma, 1 whole blood), for circulating BRAF-mutant DNA. Each patient contributed a single pretreatment blood sample for analysis. The human melanoma cell line SK-MEL 29, mutant for BRAF, and human placental DNA (Sigma-Aldrich, Inc.), wild-type for BRAF, were included in every run as positive and negative controls, respectively.
DNA was extracted from plasma using the QIAmp DNA Blood Mini Kit (QIAgen) as previously described (18). MS-PCR was done essentially as previously described using a fluorescent tagged 5′ primer (GGCCAAAAATTTAATCAGTGGA) and a mutant-specific 3′primer (GGTGATTTTGGTCTAGCTACATA) specific for the V600E BRAF mutation (18). Amplified PCR product was analyzed using an Applied Biosystems 310 Genetic Analyzer.
Biostatistics
Patients were stratified into one of two cohorts depending on whether their tumor had a mutation in BRAF. For each group, a Simon two-stage design was planned in which a 10% response rate would be considered not promising, a 30% response rate would be considered promising, and the probabilities of a type I error (falsely accepting a nonpromising therapy) and type II error (falsely rejecting a promising therapy) were both set at 0.10. If, at the first stage of the trial, 0 or 1 of the first 16 patients in a group had a clinical response, further accrual into that group would be terminated and declared negative. If 2 or more responses were observed, then additional patients would be accrued into that group until a total of 25 evaluable patients had been accrued. Under this scenario, the maximum trial size would be 50 patients (25 in each group). At the end of the trial, if 5 or more responses were observed in a group, then the therapy would be considered worthy of further testing for that group. This design would yield at least 90% probability of a positive result if the true response rate is at least 30%.
Western blot densitometry measurements were analyzed by comparing the fold-change observed for each protein with the fold-change for actin using a nonpaired t test.
Results
A total of 21 patients signed informed consent for the study from June 2004 to May 2006. Of these, five either progressed or were deemed otherwise ineligible for the trial before being formally registered. Another patient was registered but never received treatment. Of the six patients registered but not treated, two withdrew consent to participate in another clinical trial, one was found to have brain metastases and so was ineligible, and three rapidly progressed within a week of signing consent. Thus, there are 15 evaluable patients. We had originally planned to accrue at least 32 patients (16 in each cohort) but terminated the trial after these 15 evaluable patients had been treated. The reason for early termination was the lack of a sustained effect on tumor BRAF expression or MAPK pathway activity as measured by the expression of phospho-ERK (see below).
Patient characteristics
The age, gender, tumor-node-metastasis stage, sites of metastatic disease, history of prior therapy, and BRAF genotype are shown in Table 1 for the 15 evaluable patients.
Table 1.
Patient characteristics
| Wild-type BRAF group | V600E BRAF group | |
|---|---|---|
| n | 6 | 9 |
| Gender | 5 men/1 woman | 7 men/2 women |
| Median age, y (range) | 66 (42-78) | 55 (44-71) |
| Stage | ||
| III | 1 | 0 |
| IV M1a | 2 | 4 |
| IV M1b | 1 | 1 |
| IV M1c | 2 | 4 |
| Prior therapy* | ||
| None | 1 | 0 |
| Chemotherapy | 5 | 9 |
Adjuvant immunotherapy was not considered prior therapy for metastatic melanoma.
BRAF sequencing was done on all 21 patients. Although stratification was based on results obtained by directly sequencing tumor-derived DNA, the final determination of BRAF mutation status was based also on the MS-PCR results, which are more sensitive (18). In fact, in three patients who were classified as wild-type BRAF by tumor sequencing, we could detect a V600E BRAF mutation in tumor-derived DNA by MS-PCR techniques. Thus, we considered 9 tumors (43%) to be wild-type for BRAF; 12 tumors had mutant BRAF (57%). Of the 15 treated patients, 6 were wild type (40%) and 9 had BRAF mutations (60%). Plasma or whole blood-derived DNA was available for PCR analysis in 13 patients whose tumor DNA had also been analyzed by MS-PCR. In 6 of 7 patients with tumor BRAF mutations, we could detect mutated BRAF in plasma (sensitivity = 86%). Among the six patients with no detectable BRAF mutation in tumor-derived DNA, circulating mutated BRAF was detected in two patients (specificity = 67%).
Clinical responses
Among the 15 treated patients, the median number of 17-AAG doses delivered was 5 (range, 2-6). No patient received a second course of therapy. No objective complete or partial responses were observed. In one patient, stable disease was observed for 6 weeks during the course of therapy. All other patients progressed on therapy.
Toxicity
Table 2 lists all toxicities of at least grade 2 severity that were thought to be possibly attributable to 17-AAG. The most common toxicities were odor and nausea, which were attributable to the DMSO solvent. Grade 3 toxicity was observed only occasionally and only in single patients.
Table 2.
Toxicities
| Toxicity | No. patients |
||
|---|---|---|---|
| Grade 2 | Grade 3 | Grade 4 | |
| Odor | 6 | ||
| Nausea/vomiting | 2 | 1 | |
| Fatigue | 4 | ||
| Anorexia | 3 | ||
| Musculoskelatal pain | 2 | ||
| Cardiac ischemia | 1 | ||
| ↑ Transaminases | 1 | ||
| Edema | 1 | ||
| ↓ K+ | 1 | ||
| Urinary frequency | 1 | ||
| ↑ Alkaline phosphatase | 1 | ||
| ↑ Bilirubin | 1 | ||
| ↑Creatinine1 | 1 | ||
| ↓ Albumin | 1 | ||
| Cough | 1 | ||
| Fever | 1 | ||
| Diaphoresis | 1 | ||
One patient suffered a myocardial infarction while on study. This was a 74-year-old man with known coronary artery disease and hypertension who had previously undergone single vessel coronary artery bypass. Before receiving the 5th dose of 17-AAG, he was found to have acute myocardial ischemia and was admitted to a local hospital. He was taken off protocol and remains alive with melanoma more than 17 months later.
Pharmacodynamic results
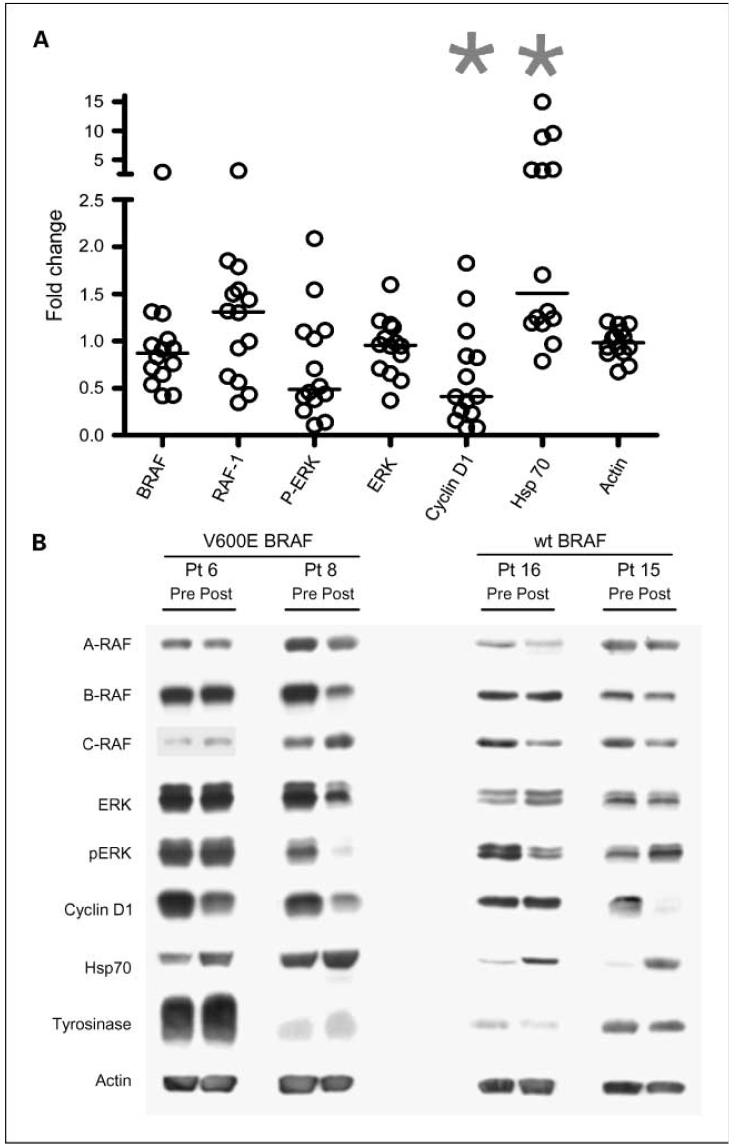
Tumor biopsies were obtained pretreatment and a median of 44.4 hours after the first 17-AAG treatment; in two patients the posttreatment biopsy was outside the planned window (49.4 and 49.7 hours). Tumor samples were analyzed by Western blot for expression of Raf kinases (A, B, C), ERK, phospho-ERK, CDK4, and cyclin D1. Tyrosinase, p85 phosphatidylinositol 3-kinase, and actin were used as loading controls. Expression of hsp70 was also measured as inhibition of hsp90 has previously been shown to induce hsp90 expression (14, 15). Of the 15 pairs of tumor biopsies, 2 pairs were not interpretable because either the pretreatment or posttreatment sample did not contain detectable melanoma as assessed by Western blot expression of tyrosinase and/or the p85 phosphatidylinositol 3-kinase loading controls (data on all 15 patients are presented in Supplementary Fig. S1).
As expected, the level of hsp70 increased in many of the posttreatment tumors, which is indicative of hsp90 inhibition. However, there was no reproducible evidence of a treatment effect on the tumor levels of any of the Raf kinases or phospho-ERK (Fig. 1A). Even in tumors harboring BRAF mutations, we did not detect a significant decrease in BRAF or phospho-ERK expression. We noted a decrease in cyclin D1 expression in many tumors, but this did not correlate with the presence of a BRAF mutation.
Fig. 1.
Western blot analyses of pretreatment and posttreatment tumors. A, densitometric measurements were expressed as fold-change (posttreatment reading/pretreatment reading). Each circle represents a single patient. Results from patients 1and 7 were excluded from the analyses because of lack of tumor material in the pretreatment (patient 7) or posttreatment (patient 1) biopsy. Horizontal lines indicate median values. *, indicate P = 0.02 compared with actin values using a nonpaired t test. B, Western blot results from four representative patients. Two of the patients shown harbored mutated BRAF genes in their tumors; two patients had wild-type BRAF, as indicated.
We considered the possibility that a subset of tumors was resistant to hsp90 blockade and so analyzed separately the tumors showing an increase in hsp70 expression. In this subset we observed no difference in the changes among the other markers (data not shown).
Figure 1B illustrates the Western blot results in four representative patients. Hsp70 increased posttreatment in all four patients. Patient 8 showed a small decrease in BRAF expression. Phospho-ERK declined in patients 8 and 16, but not in the other two patients shown. Cyclin D1 declined in each of the patients except patient 16. The level of tyrosinase was variable between patients although consistent between pretreatment and posttreatment lanes, suggesting that equivalent amounts of tumor-derived protein was loaded for the pre- and posttreatment samples.
Discussion
Recent publications from many investigators have identified the importance of the MAPK pathway in melanoma (1, 2). Sixty to 80% of melanomas have activating BRAF or NRAS mutations (4, 19). In addition, activation of the AKT pathways, through loss of PTEN, is seen in a proportion of melanoma tumors, implying that activation of this pathway may also drive tumor progression in many melanoma tumors (2, 4). These observations suggest that strategies to block both of these pathways in melanoma will be needed to interrupt melanoma growth. The observation that both mutated BRAF and AKT are hsp90 client proteins suggested that using 17-AAG to inhibit hsp90 could be an efficient strategy to block both the MAPK and AKT pathways in melanoma. Several phase I trials have been reported exploring a variety of 17-AAG treatment schedules (12-16, 20, 21). To maximize feasibility, we elected to use a weekly schedule for which the maximum tolerated dose was identified as 450 mg/m2 weekly. Also, at this dose and schedule, 2 of 11 melanoma patients had shown prolonged stable disease in a previous trial (15). In that study, tumors were biopsied after 24 hours in a subset of patients with a variety of tumor types treated at doses of 320 to 450 mg/m2 and compared with pretreatment biopsies. RAF-1 (CRAF) was decreased in 4 of 6 patients measured; CDK4 decreased in 8 of 9 patients biopsied. Here, we have conducted a phase II trial in melanoma at the maximum tolerated dose, stratifying for mutation in the BRAF gene with tumor pharmacodynamic data on 13 patients. Although we observed no objective clinical responses, the pharmacodynamic results may provide an explanation. The effects of 17-AAG on tumors seem to be minimal and short-lived. At the time of the posttreatment biopsy (median 44 hours after dose 1), we could detect an increase in hsp70 levels and a decrease in cyclin D1 levels. These findings, together with the observation of changes in RAF-1 and CDK4 at 24 hours in an earlier study (15), suggest that there may have been transient decreases in raf kinases, phospho-ERK, and CDK4 that had already recovered by the time of the posttreatment biopsy but that in several of the tumors, cyclin D1 levels were still depressed. This suggests that there was a biological effect of 17-AAG, but it was short-lived. Further, these changes in the components of the MAPK pathway were not sufficient to cause tumor shrinkage. Future trials in melanoma will require a more potent hsp90 inhibitor that can be administered chronically, resulting in more prolonged suppression of the MAPK pathway.
We tested whether the tumor BRAF mutation status could be reliably assessed from plasma DNA. Previous investigators have shown that in colorectal and pancreas cancer patients, tumor DNA harboring KRAS mutations is readily shed into the plasma and can be detected by PCR (22-25). With this in mind, we analyzed plasma DNA in our patients for the presence of mutated BRAF to test whether plasma could be used instead of tumor to determine BRAF status. In 6 of the 7 patients tested who harbored a BRAF mutation in their tumors, we were able to detect a BRAF mutation in plasma-derived DNA. However, among the six BRAF wild-type patients tested, we detected mutant BRAF in plasma DNA in two of them. Although the number of patients tested is small, this experience indicates that the specificity of this approach may be too low to yield reliable information about the BRAF status of the tumor. An alternative explanation is that direct Sanger sequencing of tumor DNA is insufficiently sensitive for detecting BRAF mutations. However, when we analyzed tumor DNA by MS-PCR, the plasma PCR results still lacked specificity (data not shown).
Another issue raised by this trial is the feasibility of pretreatment and posttreatment tumor biopsies. Melanoma patients with easily biopsied tumors are not seen as often as many investigators assume. Thus, accrual to these trials is slow and is optimized using a multicenter design. Even in the best of hands, not all biopsies contain viable tumor sufficient for Western blotting, and it is difficult to obtain more than one posttreatment sample. Because the exact pharmacodynamic time course is rarely known beforehand, this makes it easy to miss the critical biological effects, which is what we believe happened in our trial. These issues must be taken into account in future trials studying drugs that target specific pathways.
This is the first 17-AAG phase II trial in melanoma. The lack of objective tumor responses is consistent with what has been reported in hormone-refractory prostate cancer (26) and in renal cell carcinoma (27) using lower doses of 17-AAG. However, tumor biopsies were not obtained in those trials and so we do not know if there were pharmacodynamic effects of 17-AAG.
Preclinical data indicate that among hsp90 client proteins, there is a range of sensitivity to hsp90 inhibition. One of the most sensitive client proteins is HER2 (28) and consistent with this, recent data from a phase I/II trial showed multiple partial responses in HER2+ metastatic breast cancer patients using the same dose and schedule of 17-AAG used in this trial (29). This indicates that inhibitors of hsp90 can be used to deplete client proteins critical for tumor survival and that antitumor effects can be observed in patients. However, the current formulation of 17-AAG seems to be suboptimal for inhibiting BRAF and RAF-1 and therefore for patients with melanoma. As a weekly infusion, the drug is inconvenient, associated with toxicity related to the DMSO diluent, and results in only transient inhibition of the MAPK pathway in melanoma. Depletion of less sensitive hsp90 client proteins, such as BRAF, will require novel hsp90 inhibitors or formulations that can more effectively suppress RAF kinase expression.
Acknowledgments
We thank Percy Ivy of CTEP for help in making 17-AAG available and for advice on trial design, Dr. Philip Friedlander for helping to get the trial started, Qing Ye for technical assistance with the immunoblots, and Adam Litterman for carrying out the mutant-specific PCR analyses. Ron Elamparo and Melanie Heywood served as the data managers for the study.
Grant support: National Cancer Institute grants NO1-CM-17105, NO1 NCI-SAIC-Fredrick 24XS097-01, CA112899-01, and R21 CA109388 and the Sergei S. Zlinkobb Fund for Medical Education (D. Polsky).
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest D. Solit: Honorarium, Kosan Pharmaceuticals.
References
- 1.Fecher LA, Cummings SD, Keefe MJ, Alani RM. Toward a molecular classification of melanoma. J Clin Oncol. 2007;25:1606–20. doi: 10.1200/JCO.2006.06.0442. [DOI] [PubMed] [Google Scholar]
- 2.Kalinsky K, Haluska FG. Novel inhibitors in the treatment of metastatic melanoma. Expert Rev Anticancer Ther. 2007;7:715–24. doi: 10.1586/14737140.7.5.715. [DOI] [PubMed] [Google Scholar]
- 3.Johansson P, Pavey S, Hayward N. Confirmation of a BRAF mutation-associated gene expression signature in melanoma. Pigment Cell Res. 2007;20:216–21. doi: 10.1111/j.1600-0749.2007.00375.x. [DOI] [PubMed] [Google Scholar]
- 4.Goel VK, Lazar AJ, Warneke CL, Redston MS, Haluska FG. Examination of mutations in BRAF, NRAS, PTEN in primary cutaneous melanoma. J Invest Dermatol. 2006;126:154–60. doi: 10.1038/sj.jid.5700026. [DOI] [PubMed] [Google Scholar]
- 5.Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–50. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 6.Schulte TW, Blagosklonny MV, Romanova L, et al. Destabilization of Raf-1 by geldanamycin leads to disruption of the Raf-1-MEK-mitogen-activated protein kinase signalling pathway. Mol Cell Biol. 1996;16:5839–45. doi: 10.1128/mcb.16.10.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stancato LF, Silverstein AM, Owens-Grillo JK, Chow YH, Jove R, Pratt WB. The hsp90-binding antibiotic geldanamycin decreases Raf levels and epidermal growth factor signaling without disrupting formation of signaling complexes or reducing the specific enzymatic activity of Raf kinase. J Biol Chem. 1997;272:4013–20. doi: 10.1074/jbc.272.7.4013. [DOI] [PubMed] [Google Scholar]
- 8.Grbovic OM, Basso AD, Sawai A, et al. V600E B-Raf requires the Hsp90 chaperone for stability and is degraded in response to Hsp90 inhibitors. Proc Natl Acad Sci U S A. 2006;103:57–62. doi: 10.1073/pnas.0609973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci U S A. 2000;97:10832–7. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stepanova L, Leng X, Parker SB, Harper JW. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 1996;10:1491–502. doi: 10.1101/gad.10.12.1491. [DOI] [PubMed] [Google Scholar]
- 11.Munster PN, Srethapakdi M, Moasser MM, Rosen N. Inhibition of heat shock protein 90 function by ansamycins causes the morphological and functional differentiation of breast cancer cells. Cancer Res. 2001;61:2945–52. [PubMed] [Google Scholar]
- 12.Grem JL, Morrison G, Guo XD, et al. Phase I and pharmacologic study of 17-(allylamino)-17-demethoxygeldanamycin in adult patients with solid tumors. J Clin Oncol. 2005;23:1885–93. doi: 10.1200/JCO.2005.12.085. [DOI] [PubMed] [Google Scholar]
- 13.Ramanathan RK, Egorin MJ, Eiseman JL, et al. Phase I and pharmacodynamic study of 17-(allylamino)-17-demethoxygeldanamycin in adult patients with refractory advanced cancers. Clin Cancer Res. 2007;13:1769–74. doi: 10.1158/1078-0432.CCR-06-2233. [DOI] [PubMed] [Google Scholar]
- 14.Solit DB, Ivy SP, Kopil C, et al. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer 10.1158/1078-0432.CCR-06-1863. Clin Cancer Res. 2007;13:1775–82. doi: 10.1158/1078-0432.CCR-06-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerji U, O'Donnell A, Scurr M, et al. Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol. 2005;23:4152–61. doi: 10.1200/JCO.2005.00.612. [DOI] [PubMed] [Google Scholar]
- 16.Goetz MP, Toft D, Reid J, et al. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. J Clin Oncol. 2005;23:1078–87. doi: 10.1200/JCO.2005.09.119. [DOI] [PubMed] [Google Scholar]
- 17.Gorden A, Osman I, Gai W, et al. Analysis of BRAF and N-RAS mutations in metastatic melanoma tissues. Cancer Res. 2003;63:3955–7. [PubMed] [Google Scholar]
- 18.Yancovitz M, Yoon J, Mikhail M, et al. Detection of mutant BRAF alleles in the plasma of patients with metastatic melanoma. J Mol Diagn. 2007;9:178–83. doi: 10.2353/jmoldx.2007.060135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 20.Bagatell R, Gore L, Egorin MJ, et al. Phase I pharmacokinetic and pharmacodynamic study of 17-N-allylamino-17-demethoxygeldanamycin in pediatric patients with recurrent or refractory solid tumors: a Pediatric Oncology Experimental Therapeutics Investigators Consortium study 10.1158/1078-0432.CCR-06-1892. Clin Cancer Res. 2007;13:1783–8. doi: 10.1158/1078-0432.CCR-06-1892. [DOI] [PubMed] [Google Scholar]
- 21.Weigel BJ, Blaney SM, Reid JM, et al. A phase I study of 17-allylaminogeldanamycin in relapsed/refractory pediatric patients with solid tumors: a Children's Oncology Group Study 10.1158/1078-0432.CCR-06-2270. Clin Cancer Res. 2007;13:1789–93. doi: 10.1158/1078-0432.CCR-06-2270. [DOI] [PubMed] [Google Scholar]
- 22.Urban T, Ricci S, Grange J-D, et al. Detection of c-Ki-ras mutation by PCR-RFLP analysis and diagnosis of pancreatic adenocarcinoma. J Natl Cancer Inst. 1993;85:2008–12. doi: 10.1093/jnci/85.24.2008. [DOI] [PubMed] [Google Scholar]
- 23.de Kok JB, van Solinge WW, Ruers TJ, et al. Detection of tumour DNA in serum of colorectal cancer patients. Scand J Clin Lab Invest. 1997;57:601–4. doi: 10.3109/00365519709055283. [DOI] [PubMed] [Google Scholar]
- 24.Kopreski MS, Benko FA, Kwee C, et al. Detection of mutant K-ras DNA in plasma or serum of patients with colorectal cancer. Br J Cancer. 1997;76:1293–9. doi: 10.1038/bjc.1997.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamada T, Nakamori S, Ohzato H, et al. Detection of K-ras gene mutations in plasma DNA of patients with pancreatic adenocarcinoma: correlation with clinicopathological features. Clin Cancer Res. 1998;4:1527–32. [PubMed] [Google Scholar]
- 26.Heath EI, Hillman D, Vaishampayan U, et al. A phase II trial of 17-allylamino-17-demethoxygeldanamycin (17-AAG) in patients with hormone-refractory metastatic prostate cancer. J Clin Oncol Meet Abstracts. 2007;25:15553. [Google Scholar]
- 27.Ronnen EA, Kondagunta GV, Ishill N, et al. A phase II trial of 17-(allylamino)-17-demethoxygeldanamycin in patients with papillary and clear cell renal cell carcinoma. Invest New Drugs. 2006;24:543–6. doi: 10.1007/s10637-006-9208-z. [DOI] [PubMed] [Google Scholar]
- 28.Basso AD, Solit DB, Munster PN, Rosen N. Ansamycin antibiotics inhibit Akt activation and cyclin D expression in breast cancer cells that overexpress HER2. Oncogene. 2002;21:1159–66. doi: 10.1038/sj.onc.1205184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Modi S, Stopeck AT, Gordon MS, et al. The combination of trastuzumab and tanespimycin (KOS 953, 17-AAG) is safe and active in trastuzumab-refractory HER2 overexpressing breast cancer: a phase 1 dose-escalation study. J Clin Oncol. 2007;25:5410–7. doi: 10.1200/JCO.2007.11.7960. [DOI] [PubMed] [Google Scholar]