Abstract
During the synthetic pursuit of guanosine (triG) and xanthosine (triX) tricyclic nucleosides analogues, an interesting side product was discovered. In an effort to uncover the mechanistic factors leading to this result, a series of reaction conditions were investigated. It was found that by varying the conditions, the appearance of the side product could be controlled. In addition, the yield of the desired products could be manipulated to afford either a 50:50 mix of both triG and triX, or a majority of one or the other. To demonstrate the broad utility of the method, it was also adapted to the synthesis of guanosine and xanthosine from 5-amino-1-β-D-ribofuranosyl-4-imidazolecarboxyamide (AICAR). The mechanistic details surrounding the synthetic efforts are reported herein.
Keywords: tricyclic nucleosides, AICAR, xanthosine, guanosine, expanded nucleosides
1. Introduction
A number of laboratories1–9, including ours10–13, have designed and synthesized various structurally unique unnatural nucleosides to study nucleic acid structure and function. Expanding the "letters" of the genetic alphabet beyond the five natural nucleosides would allow further investigation of the requirements for base pairing and stacking, helix stability and interactions, and recognition by enzyme systems such as polymerases or other nucleoside metabolizing enzymes involved in biological processes. A number of design approaches to realize this goal have been explored, including pairings based on size or shape complementarity.14–18
A more traditional strategy relies on pairing up complementary donor-acceptor patterns between unnatural bases.9,19–21 Recently, use of expanded purine nucleosides such as Nelson Leonard's22–24 lin-benzoadenosine has been explored, an approach we began to pursue some time ago beginning with the synthesis of a series of thieno-expanded tricyclic nucleosides.25,26,11,13
In contrast to Leonard's linear system, which has been extensively studied by Kool2,27,4,3,28,29, and Matteucci's extended cytidine analogues30–33 the use of a heteroaromatic spacer ring provides a number of advantages over the benzene spacer, including offering forth a less dramatic expansion of the helix due to the curvature of the base pairing, which contracts the helix width while still retaining the hydrogen bonding elements involved in recognition and base pairing.
In addition, molecular dynamics calculations have shown that inclusion of the heteroaromatic spacer will increase the overall aromaticity and polarizability for the base, which in turn, will result in an increase in stacking effects, an important factor in stabilization of the DNA helix.34–37
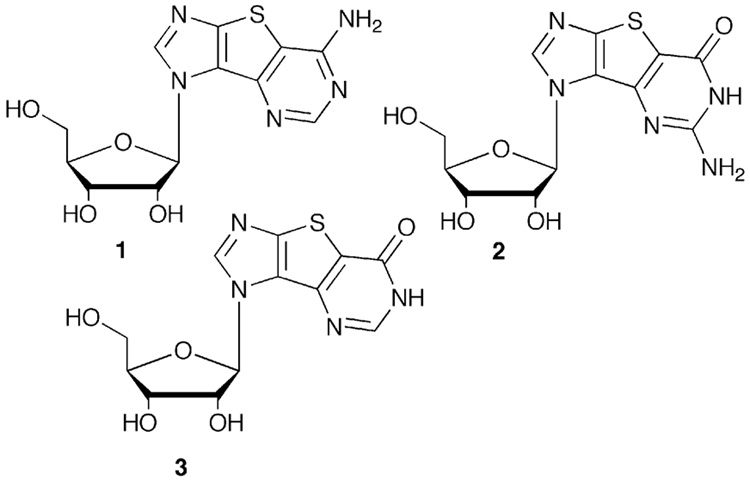
We have previously reported the design and synthesis of the first three tricyclic thieno-expanded purine ribonucleosides11 and their corresponding bases13, tricyclic adenosine (tri-A), guanosine (tri-G) and inosine (tri-I) shown in Figure 1 on the previous page. In parallel to the studies in DNA, the tricyclic nucleosides were investigated in several enzyme systems and not surprisingly, all three were recognized by nucleoside transporters, both in their original nucleoside form as well as the corresponding nucleobases.38 The extended aromatic system resulted in an increase in recognition and uptake over the natural nucleobases. In addition, the tricyclic nucleosides were readily recognized, and in some cases, preferentially, by nucleoside metabolizing enzymes such as guanosine fucose pyrophosphorylase39–41 and RNA dependent RNA polymerases, thus providing additional impetus for expanding our drug design studies with additional analogues.
Figure 1.
Thieno-expanded nucleosides.
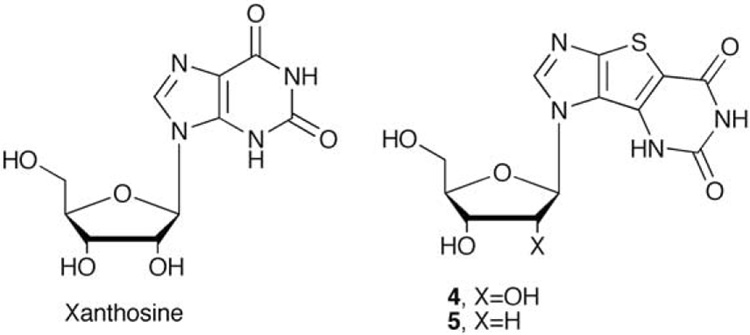
In that regard, xanthosine (Figure 2) is a naturally occurring purine nucleoside that has attracted some interest in recent years due to the ability to act as a universal base.42,43 Xanthosine offers forth a Watson-Crick "acceptor-donor-acceptor" pairing pattern, thus can advantageously pair with a number of unnatural nucleosides. One notable example is Benner's diaminopyrimidine pairing with xanthosine.44–49 This nonstandard pairing was recently shown to be utilized with satisfactory fidelity by variants of HIV reverse transcriptase for synthesis of duplex DNA.44 This finding is highly encouraging to those pursuing expansion of the genetic alphabet, since this is the first example of DNA containing base pairs with alternative hydrogen bonding patterns that was efficiently amplified by PCR.44 Spurred on by those findings, we employed our tricyclic scaffold to produce the corresponding expanded tricyclic xanthosine (tri-X, 4) and the corresponding 2'- deoxyxanthosine 5, shown in Figure 2. In the process of this synthetic effort, an unexpected side product was discovered, thus prompting a mechanistic study of the formation of these analogues.
Figure 2.
Xanthosine and thieno-expanded xanthosine nucleosides.
2. Results and discussion
2.1. Manipulation of the ring closure reaction
In reviewing the literature, only a few routes appeared to be available to obtain xanthosine nucleosides directly, and both were plagued with extremely poor yields, ranging from a low of 8% to a moderate yield of 47%.50–53 Interestingly, a number of literature routes to guanosine have noted xanthosine as a minor side product, however none of these reports evaluated the mechanistic details that would result in successfully obtaining various ratios of the products. Indeed, during the course of scaling up our synthesis of the tri-G analogue 2, we also noted that a small amount of tricyclic xanthosine was formed. Speculating that it might be possible to obtain both 12 and 13 in more satisfactory quantities by altering the reaction conditions, or by manipulating the conditions such that either the xanthosine or the guanosine would be obtained as the major product, we began a mechanistic investigation, since either scenario would prove useful to us and the synthetic community.
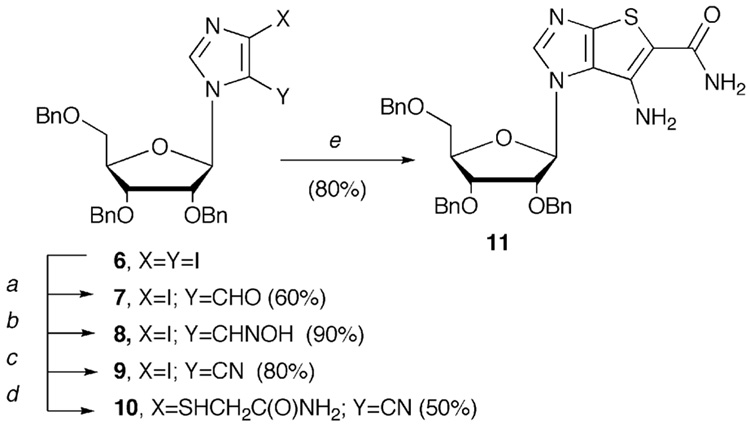
Our original route11,13,26 began with ribosylated 4,5-dibromoimidazole, however more recently12 a switch to the 4,5-diiodo substituted imidazole intermediate 6 shown in above in Scheme 1 was made, since it provided a much more facile workup for several of the steps, no longer requiring tedious column chromatography, since the products could be readily purified via recrystallization. Beginning with 6 (Scheme 1), conversion to the key bicyclic intermediate 11 was accomplished in five steps in a 16% overall yield.
Scheme 1.
Reagents and conditions: (a) (i) EtMgBr, THF, 4 h; (ii) anhydrous DMF; (b) pyrimidine, hydroxylamine hydrochloride, anhydrous EtOH, reflux; (c) Ac2O, reflux; (d) NH2C(O)CH2SH, K2CO3, DMF, 60 °C; (e) NaOEt, EtOH.
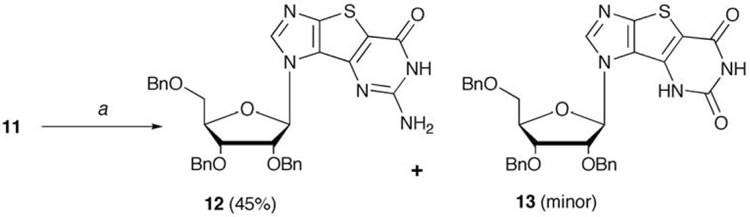
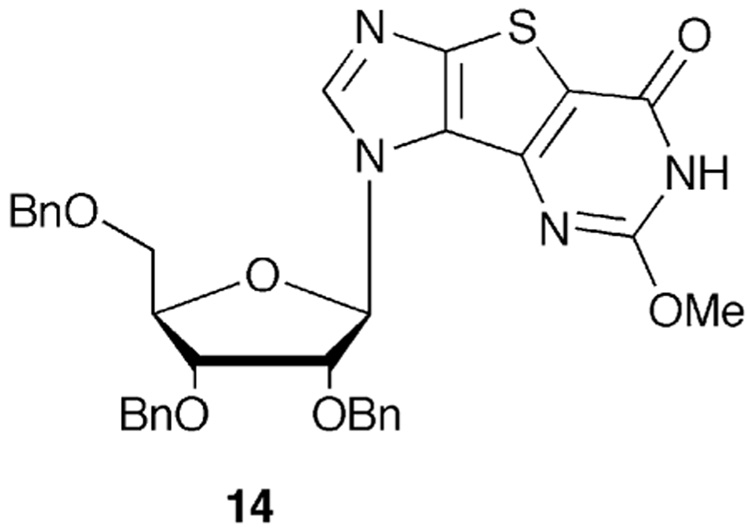
At this point the ring closure was undertaken using a one pot, four step procedure detailed in Scheme 2 to give both benzyl-protected tri-G (12) and a small amount of tri-X (13).11,54 Next, increasing the ratio of sodium hydroxide to substrate in step 1 successfully improved the ratio of tri-G to tri-X (Entry 2) but surprisingly, this also resulted in the formation of a new compound not previously observed. Following characterization by NMR and elemental analysis, this product proved to be a methoxy-substituted nucleoside (14, Figure 3). This was quite unexpected, because to our knowledge, no similar methoxy side product had ever been reported in the literature for these reactions, thus we sought to further investigate the cause of this result.
Scheme 2.
Reagents and conditions: (a) (i) NaOH, MeOH, rt; (ii) CS2, MeOH, 145 °C, 18 h; (iii) H2O2, MeOH, 0 °C, 2h; (iv) NH3, MeOH, 125 °C, 18 h.
Figure 3.
2-Methoxy-substituted thieno-expanded xanthosine.
The most straightforward explanation could be attributed to a methoxy anion acting as a nucleophile due to the increase in base present, however this seemed unlikely given the intermediates proposed in the literature for similar reactions. It should be noted that, in contrast to the literature reports53,52 which utilize NaOH instead of NaOMe in the final step, this was not possible with our compounds due to lack of solubility in aqueous solution as a result of the benzyl protecting groups which were not present in the literature examples. In an effort to more closely follow those procedures, removal of the benzyl groups at the bicyclic stage was carried out, however, the yield for the cyclization reaction for the deprotected bicyclic intermediate (step 1) was unsatisfactory, thus this route was not pursued further.
Speculating that the nucleophilic attack by the OMe could not be occurring in the first step, but most likely in the third or fourth step when the leaving groups would be more favourable, we returned to the benzyl-protected bicyclic intermediate 11. It was rationalized that by significantly increasing the amount of MeOH used in the last step, it would effectively decrease the overall concentration of the NaOH still present in the reaction mixture, thus lowering the concentration of the OMe anion. This indeed proved to be the case; an initial increase of MeOH from 80 mL to 160 mL improved the ratio of 12 to 13, and significantly decreased the formation of the OMe product 14. Further dilution with 250 mL of MeOH produced the desired affect, and resulted in a 1:1 ratio of 12 to 13 with no formation of the tri-OMe side product 14. To test this further, the amount NaOH was increased by one equivalent (Entry 8) and only a trace of tri-OMe was found, while increasing it even further resulting in the reappearance of the tri-OMe product 14, thus supporting our theory.
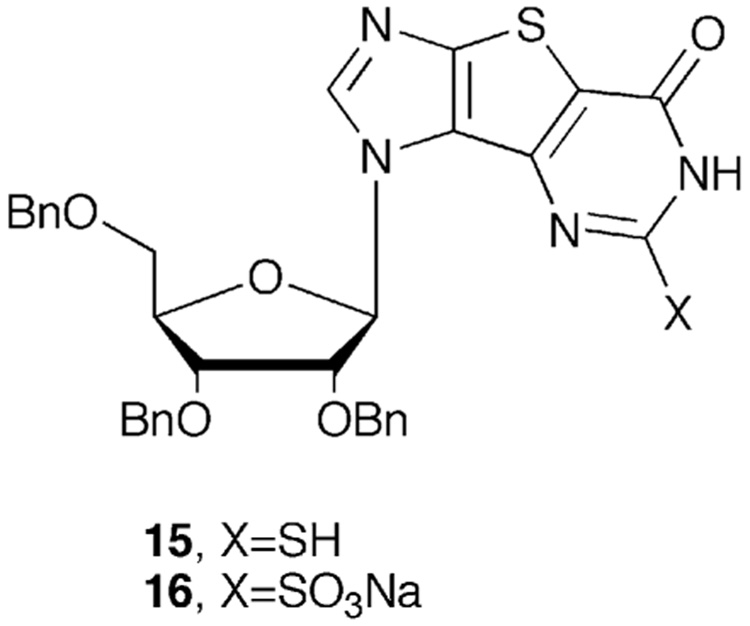
To substantiate this hypothesis, each step of the one pot reaction mixture was monitored closely by mass spectrometry. In the second step, following the addition of CS2, mass spectrometry analysis showed three major components, one with a mass of 649.1 [M+Na] C33H29N4NaO5S2 corresponding to the 2-SH intermediate 15 (Figure 4), the other with a mass of 633.2 [M+Na] C33H29N4NaO6S corresponding to the tri-X 13, and a third corresponding to the mass of the glycosidic bond cleaved sugar moiety, coincidentally, with a methoxy group present, however no mass corresponding to the 2-OMe side product 14.
Figure 4.
Ring-closure reaction intermediates.
After addition of the H2O2 in the next step, TLC showed a new product had formed and mass spectrometry indicated that this was the oxidized sulfonic acid product 16 (Figure 4), however it was not until after addition of the NH3 in the final step that the tri-OMe side product 14 began to appear. This observation supports the premise that the side product was not forming until the last step, despite the fact that the MeOH and NaOH are introduced in the first step of the one pot procedure. The OMe anion present in the reaction mixture is obviously in competition with the ammonia as a nucleophile, however, the other intermediates formed in previous steps are not reactive enough to undergo nucleophilic substitution by the OMe anion. Once the sulfonic acid moiety has formed however, it presents an excellent leaving group that is then attacked by both the OMe and ammonia. Indeed, increasing the amount of NaOH added in the first step leads to the formation of the OMe byproduct 14 as the major product, but only after formation of the -SO3Na adduct (16), thus confirming our mechanistic hypothesis for the formation of the methoxy analogue.
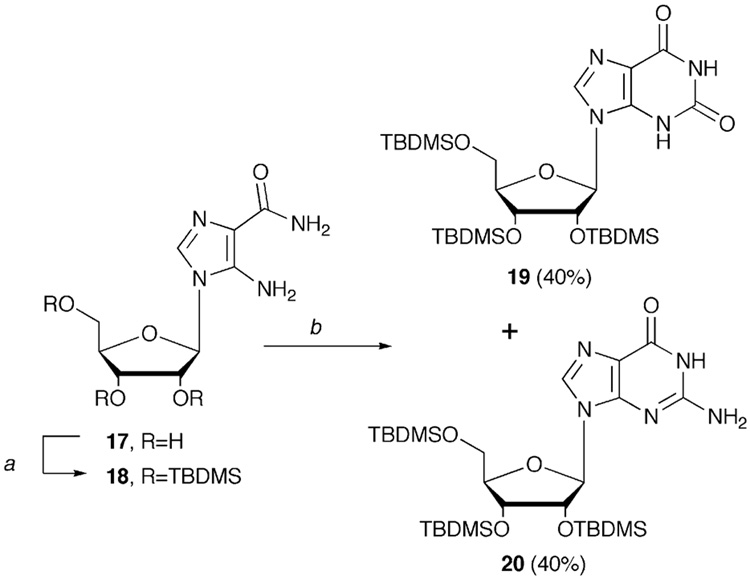
Mechanistic questions answered, we moved on to test the utility of the ring closure conditions for more standard nucleosides. AICAR is a key intermediate in the synthesis of a number of nucleosides such as guanosine and xanthosine, as well as an important player in the field of universal nucleosides. Using the conditions in Table 1, entry 7, AICAR (17, Scheme 3) was converted to a 1:1 ratio of xanthosine (19) and guanosine (20) (40% for each), thus confirming the practicality of this method and the ability to manipulate the reaction conditions to deliver both nucleosides in good yield. It should be noted that the TBDMS group was utilized in place of the benzyl protecting group, since AICAR was obtained commercially, and use of the benzyl group would have required a protracted protection-deprotection strategy, since AICAR contains labile amino groups in addition to the ribose hydroxyls.
Table 1.
Ring closure conditions and product ratio.a
| Entry | Substrate/NaOH equivalents | Temperature (°C) | Reaction Time (h) | Volume of MeOH (mL) | Product Ratio |
|---|---|---|---|---|---|
| (Step 1) | (Step 2) | (Step 2) | (Step 4) | 12:14:13 | |
| 1 | 1:5 | 145 | 18 | 80 | 3:0:1 |
| 2 | 1:6 | 145 | 18 | 80 | 3:1:1 |
| 3 | 1:10 | 145 | 18 | 80 | 2:4:3 |
| 4 | 1:40 | 145 | 18 | 80 | 2:7:3 |
| 5 | 1:5 | 180 | 6 | 80 | 3:2:1 |
| 6 | 1:5 | 145 | 18 | 160 | 2:1:2 |
| 7 | 1:5 | 145 | 18 | 250 | 1:0:1 |
| 8 | 1:6 | 145 | 18 | 250 | 1:trace:1 |
| 9 | 1:8 | 145 | 18 | 250 | 2:1:2 |
based on an average of at least five separate reactions for each set of conditions.
Scheme 3.
Reagents and conditions: (a) TBDMSCl, imidazole, DMF; (b) (i) NaOH, MeOH, rt; (ii) CS2, MeOH, 145 °C, 18 h; (iii) H2O2, MeOH, 0 °C, 2h; (iv) NH3, MeOH, 125 °C, 18 h.
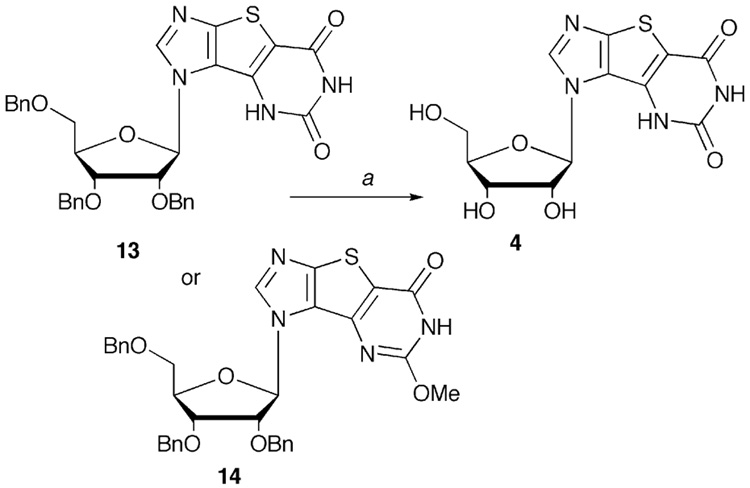
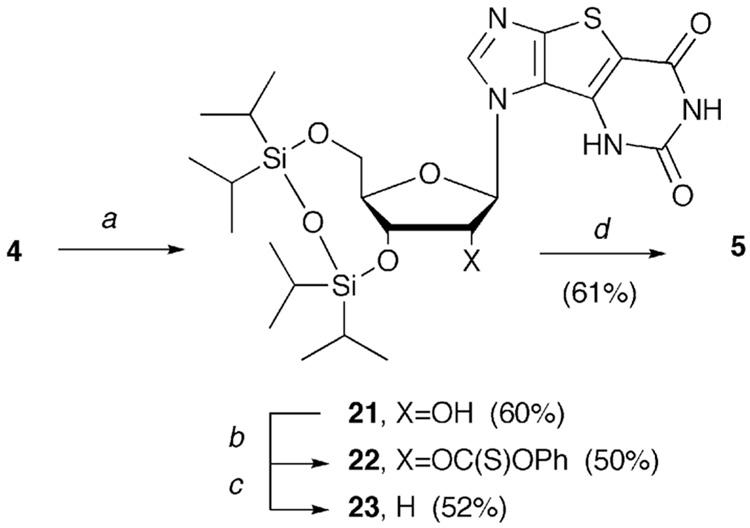
Finally, our efforts turned to conversion of 4 to the requisite 2'-deoxy tri-X (5). Deprotection of the OBn groups also fortuitously deblocked the 2-OMe group resulting in an excellent yield of 4 (Scheme 4).
Scheme 4.
Reagents and conditions: (a) THF•BF3, CH2Cl2, rt, 24h.
Next, using the well-known Barton deoxygenation procedures55,56, bis-silyl protection of the 3'- and 5'- hydroxyl groups (Scheme 5 on the next page), followed by treatment with phenyl chlorothionoformate to give the thioester resulted in 22. Treatment with AIBN and tributyl tin hydride, removed the 2'-hydroxyl group to give 23. Subsequent deblocking of the silyl protecting group with standard conditions then provided 5 in a 9.5% overall yield in four steps from 4.
Scheme 5.
Reagents and conditions: (a) 1,3-dichloro-1,1,3,3-tetraisopropyldisiloxane, pyridine, rt, 24 h; (b) phenyl chlorothionoformate, DMAP, rt, 24 h; (c) AIBN, Bu3SnH, toluene, reflux, 6 h; (d) 1M TBAF, THF, rt, 4 h.
3. Summary
In summary, reaction conditions were developed to provide a facile route to realize both guanosine and xanthosine analogues in a 1:1 ratio in excellent yield. In addition, two new tricyclic xanthosine analogues were synthesized and a mechanistic puzzle for the formation of an unexpected, but potentially interesting, side product was unravelled. Current efforts are focused on the synthesis of the triphosphate and phosphoramidite of 5, for use in studying the unique hydrogen bonding properties of xanthosine analogues, as well as their recognition by biological significant systems. The details of those studies will be reported in due time as they become available.
4. Experimental
4.1. General
All chemicals were obtained from commercial sources and used without further purification unless otherwise noted. Anhydrous DMF, MeOH, DMSO and toluene were purchased from Fisher Scientific. Anhydrous THF, acetone, CH2Cl2, CH3CN and ether were obtained using a solvent purification system (mBraun Labmaster 130). Melting points are uncorrected. NMR solvents were purchased from Cambridge Isotope Laboratories (Andover, MA). All 1H and 13C NMR spectra were obtained on a JEOL ECX 400 MHz NMR, operated at 400 and 100 MHz respectively, and referenced to internal tetramethylsilane (TMS) at 0.0 ppm. The spin multiplicities are indicated by the symbols s (singlet), d (doublet), dd (doublet of doublets), t (triplet), q (quartet), m (multiplet), and b (broad). Reactions were monitored by thin-layer chromatography (TLC) using 0.25 mm Whatman Diamond silica gel 60-F254 precoated plates. Column chromatography was performed using silica gel (63–200 µ) from Dynamic Adsorbtions Inc. (Norcross, GA), and eluted with the indicated solvent system. Yields refer to chromatographically and spectroscopically ('H and 13C NMR) homogeneous materials. Mass spectra were recorded at the Johns Hopkins Mass Spectrometry Facility. Elemental analyses were recorded at Atlantic Microlabs, Inc. (Norcross, GA).
4.2. New procedures in the synthesis of bicyclic intermediate 11
4.2.1. 2,3-Dibenzyloxy-5-benzyloxymethyl-1-[(5-iodo-4-carbaldehyde)imidazole-3-yl]-β-D-ribofuranose (7)
Compound 6 (28.47g, 39.41mmol) was dissolved in anhydrous THF (300 mL), then EtMgBr (12.88 mL, 3M solution) was added dropwise under N2. The reaction mixture was stirred for 5 h at room temperature. Anhydrous DMF (20 mL) was added and the reaction mixture stirred overnight, at which point the solvent was removed under vacuum. Saturated NH4Cl (200 mL) was added, and the reaction mixture extracted with CH2Cl2 (3 × 500 mL). The organic layers were combined, washed with brine, dried over anhydrous MgSO4, the solvent removed under vacuum to give a yellow syrup. The crude syrup was purified via column chromatography eluting with hexanes:EtOAc (5:1) to give 16.1 g of 7 as a white solid (60%), mp 160–161.5°C. 1H NMR (400 MHz, CDCl3): 9.70 (s, 1H), 8.63 (s, 1H), 7.25-7.31 (m, 15H), 6.45 (d, 1H, J=4.0 Hz), 4.36-5.01 (m, 6H), 4.27-4.30 (m, 1H), 4.20-4.22 (m, 1H), 3.90-3.99 (m, 2H), 3.63-3.66 (m, 1H); 13C NMR (100 MHz, CDCl3): 181.0, 143.5, 137.5, 137.4, 137.3, 128.8, 128.5, 128.3, 128.1, 128.0, 127.8, 102.5, 89.8, 80.9, 80.1, 74.3, 73.5, 72.7, 72.3, 67.0; ESI-MS calcd for C30H29IN2NaO5 [M+Na]+ 647.11, found 647.2; HR-FAB calcd for C30H30IN2O5 [M+H]+ 625.11995, found 625.12032.
4.2.2. 2,3-Dibenzyloxy-5-benzyloxymethyl-1-[(5-iodo-4-carbaldehydeoxime)imidazole-3-yl]-β-D-ribofuranose (8)
Compound 7 (22.8 g, 36.5 mmol) was dissolved in anhydrous EtOH (300 mL) and hydroxyamine hydrochloride (3.10 g, 44.15 mmol) was added, followed by additional of pyridine (3 mL). The reaction mixture was refluxed for 2.5 h, the solvent removed under vacuum, H2O (200 mL) added, and the reaction mixture extracted with CH2Cl2 (3 × 500 mL). The organic phases were combined, dried over anhydrous MgSO4, then evaporated to afford 23.0 g of 8 (quantitative) as a white solid, which was used directly without further purification; mp 158 °C. 1H NMR (400 MHz, CDCl3): 8.27 (s, 1H), 8.07 (s, 1H), 7.25-7.31 (m, 15H), 6.43 (d, 1H, J=1.8Hz), 4.47-4.70 (m, 6H), 4.31-4.33 (m, 1H), 4.18-4.23 (m, 1H), 3.99-4.03 (m, 1H), 3.83 (dd, J=2.3 Hz, J=11.0 Hz, 1H), 3.56 (dd, J=2.3 Hz, J=11.0 Hz, 1H); 13C NMR (100 MHz, CDCl3): 142.0, 140.6, 138.1, 137.5, 128.7, 128.6, 128.1, 128.0, 127.9, 127.7, 90.1, 81.2, 81.1, 75.3, 73.5, 73.1, 72.9, 67.6; HR-FAB calcd for C30H31IN3O5 [M+H]+ 640.13085, found 640.13096.
4.2.3. 2,3-Dibenzyloxy-5-benzyloxymethyl-1-[(5-iodo-4-carbonitrile)imidazole-3-yl]-β-D-ribofuranose (9)
Compound 8 (22.0 g, 34.5 mmol) was suspended in anhydrous acetic anhydride (300 mL), refluxed for 3 h, at which point the acetic anhydride was removed under reduced pressure to afford a crude brown syrup. Following purification by silica-gel chromatography eluting with hexanes:EtOAc (6:1), 16.6 g of 9 was obtained as a white solid (85%). 1H NMR (400 MHz, CDCl3): 7.84 (s, 1H), 7.22-7.39 (m, 15H), 5.87 (d, 1H, J=4.6 Hz), 4.47-4.70 (m, 6H), 4.38-4.40 (m, 1H), 4.14-4.20 (m, 2H), 3.78 (dd, 1H, J=3.2 Hz, J=10.5 Hz), 3.60 (dd, 1H, J=3.2 Hz, J=10.5 Hz); 13C NMR (100 MHz, CDCl3): 139.0, 137.3, 137.3, 136.7, 128.8, 128.7, 128.3, 128.1, 110.1, 103.9, 89.7, 82.9, 80.7, 75.5, 73.7, 72.8, 72.6, 68.6, 63.9; HR-FAB calcd for C30H29IN3O4 [M+H]+ 622.12028, found 622.12037.
4.3. Ring closure procedures
4.3.1. 2,3-Dibenzyloxy-5-benzyloxymethyl-1-[(2-aminoimidazo-[4′,5′:4,5]-thieno-[3,2-d]pyrimidin-3-yl-7-one)]-β-D-ribofuranose (12), 2,3-Dibenzyloxy-5-benzyloxymethyl-1-[(5-hydroxylimidazo-[4′,5′:4,5]-thieno-[3,2-d]-pyrimidin-3-yl-7-one)]-β-D-ribofuranose (13), and 2,3-Dibenzyloxy-5-benzyloxymethyl-1-[(5-methyloxyimidazo-[ 4′,5′:4,5]-thieno-[3,2-d]-pyrimidin-3-yl-7- one)]-β-D-ribofuranose (14)
In a steel reaction vessel, compound 11 (993 mg, 1.7 mmol) was dissolved in 40 mL anhydrous MeOH, NaOH (680 mg, 17 mmol) added and the mixture stirred at room temperature for 30 min until a clear solution was obtained. CS2 (615 µL, 10.2 mmol) was added, the vessel sealed and the reaction heated in an oil bath at 145 °C for 18 h. The vessel was cooled, and the solvent removed under reduced pressure to give an orange solid to which MeOH (80 mL) was added. The mixture was cooled to 0 °C and 7.5 mL H2O2 added. This mixture was stirred at 0°C for 2 hours, then transferred to a steel reaction vessel, cooled to 0 °C and NH3 bubbled into the solution for 20 min. The reaction vessel was sealed and heated in oil bath at 120 °C for 18 h. The vessel was cooled, the reaction mixture evaporated to dryness under vacuum to give a yellow solid. The crude mixture was purified by chromatography eluting with hexanes:EtOAc (1:1) to afford 424.4 mg of 14 as a white solid (40%), mp 182 °C. 1H NMR (400 MHz, CDCl3): 8.22 (s, 1H), 7.22-7.34 (m, 10H), 7.05-7.16 (m, 5H), 6.41 (d, 1H, J=4.6 Hz), 4.62-4.70 (m, 3H), 4.50-4.58 (m, 3H), 4.41-4.44 (m, 1H), 4.25-4.28 (m, 1H), 4.08-4.13 (m, 1H), 3.80- 3.82 (m, 1H), 3.78 (s, 3H), 3.65 (dd, 1H J=3.6 Hz, J=10.5 Hz); 13C NMR (100 MHz, CDCl3): 161.5, 156.4, 151.6, 144.4, 143.1, 137.5, 137.2, 128.6, 128.0, 127.9, 115.6, 88.9, 82.1, 80.1, 75.6, 73.6, 72.5, 72.4, 68.9, 60.5, 55.3. ESI-MS calcd for C34H32N4O6S: 624.2, found 625.4 [M+1]+; Anal. calcd for C34H32N4O6S: C 65.37, H 5.16, N 8.97, S 5.13, found C 65.22, H 4.86, N 9.04, S 5.19. Further elution with hexanes:EtOAc (1:2) afforded 207.5 mg of 13 as a white foam (20%). 1H NMR (400 MHz, CDCl3): 9.59 (s, 1H), 9.20 (s, 1H), 7.89 (s, 1H), 6.94-7.34 (m, 15H), 5.80 (d, 1H, J=8.2 Hz), 4.99-5.06 (m, 1H), 4.41-4.64 (m, 4H), 4.24-4.27 (m, 2H), 3.89-3.92 (m, 1H), 3.77-3.83 (m, 2H), 3.36-3.39 (dd, 1H, J=1.8 Hz, J=11.0 Hz); 13C NMR (100 MHz, CDCl3): 159.9, 152.4, 151.0, 144.3, 137.1, 135.9, 128.8, 128.7, 128.4, 128.3, 122.0, 110.3, 100.0, 88.0, 84.4, 80.3, 75.1, 73.6, 73.1, 72.9, 67.8; HR-FAB calcd for C33H31N4O6S [M+H]+ 611.19643, found 611.19878. Further elution with hexanes:EtOAc (1:3) provided 12 as a white foam (310.65 mg, 30%) whose spectral data agreed with literature values.11
4.3.2. 2,3-Dibenzyloxy-5-benzyloxymethyl-1-[(2-aminoimidazo-[ 4′,5′:4,5]-thieno-[3,2-d]-pyrimidin-3-yl-7-one)]-β-D-ribofuranose (12) and 2,3-Dibenzyloxy-5- benzyloxymethyl-1-[(5-hydroxylimidazo-[4′,5′:4,5]-thieno-[3,2-d]-pyrimidin-3-yl-7-one)]-β-Dribofuranose (13)
In a steel reaction vessel, 11 (1.35 g, 2.31 mmol) was dissolved in 40 mL anhydrous MeOH and NaOH (462 mg, 11.55 mmol) added. This mixture was stirred at room temperature for 30 min until a clear solution was obtained, then CS2 (836 µL, 13.87 mmol) was added and the vessel sealed and heated in an oil bath at 145 °C for 18 h. The solvent was removed under reduced pressure to obtain an orange solid, and 80 mL MeOH added. The reaction mixture was cooled to 0 °C, 7.5 mL H2O2 added and the mixture stirred at 0 °C for 2 h. The mixture was transferred to a steel reaction vessel, diluted with MeOH (250 mL), cooled to 0 °C and NH3 bubbled in for 20 min. The vessel was sealed and heated in oil bath at 120 °C for 18 h. The vessel was cooled, the solvents removed under vacuum to give a yellow solid. The crude compound was purified by chromatography eluting with hexanes:EtOAc (1:2) to afford 12 as white foam (634.67 mg, 45%). As before, further elution with hexanes:EtOAc (1:3) gave 13 (633.51 mg, 45%). Spectral data was identical to the previous entries.
4.3.3. Synthesis of TBDMS-protected AICAR (18)
To commercially obtained AICAR (17) (300 mg, 1.12 mmol) dissolved in 5 mL anhydrous DMF was added imidazole (613.4 mg, 8.96 mmol) and TBDMSCl (676.1 mg, 4.48 mmol). The reaction solution was stirred at room temperature for two days. The DMF was removed under reduced pressure to give a crude yellow syrup which was purified via column chromatography eluting with hexane: EtOAc (2:1) to give 18 as a colorless syrup (450 mg, 66.6%). 'H NMR (400 MHz, CDCl3): 7.53 (s, 1H), 5.48 (d, 1H, J=5.96Hz), 4.42 (s, 2H, NH2), 4.26-4.25 (m, 1H), 4.17- 4.16 (m, 1H), 4.06-4.03 (m, 1H), 3.88-3.84 (m, 1H), 3.78- 3.75 (m, 1H), 0.91 (s, 9H), 0.89 (s, 9H), 0.83 (s, 9H), 0.87 (s, 3H), 0.82 (s, 3H), 0.80 (s, 3H), 0.77 (s, 3H), 0.06 (s, 3H), −0.11 (s, 3H); 13C NMR (100 MHz, CDCl3): 159.3, 137.4, 132.0, 112.9, 89.9, 85.8, 82.8, 72.1, 62.6, 26.1, 25.9, 25.8, 18.5, 18.1, 17.9, −4.6, −5.3, −5.4. ESI-MS calcd for C27H56N4O5Si3+H: 601.01, found: 601.03.
4.3.4. Synthesis of TBDMS-protected xanthosine (19) and guanosine (20)
In a steel reaction vessel, 18 (150 mg, 0.25 mmol) was dissolved in 10 mL anhydrous MeOH, NaOH (100 mg, 2.5 mmol) added and the mixture stirred at room temperature for 30 min until a clear solution was obtained. CS2 (90 µl, 1.5 mmol) was then added and the steel vessel sealed and heated at 145°C for 18 h. The vessel was cooled, and the solvent removed under reduced pressure to give an orange solid, to which MeOH (10 mL) was added. The mixture was then cooled to 0°C, 1.1 mL H2O2 added, and the mixture stirred at 0°C for 2 hours. Following transfer to a steel reaction vessel, the mixture was again cooled to 0°C and NH3 bubbled into the solution for 20 min. After saturation with NH3, the vessel was sealed and heated at 120°C for 18 h. The bomb was cooled and the reaction mixture evaporated to dryness to give a crude yellow solid, which was purified by column chromatography eluting with hexanes:EtOAc (2:1) to afford 19 (62.4 mg, 40%) as a colorless syrup. 1H NMR (400 MHz, CDCl3): 12.45 (s, 1H), 10.25 (s, 1H), 7.45 (s, 1H), 5.67 (d, 1H, J=5.6Hz), 4.31-4.33 (m, 1H), 4.20-4.22 (m, 1H), 4.04-4.11 (m, 2H), 4.01-4.03 (m, 1H), 0.96 (s, 9H), 0.94 (s, 9H), 0.92 (s, 9H), 0.90 (s, 3H), 0.87 (s, 3H), 0.85 (s, 3H), 0.79 (s, 3H), 0.09 (s, 3H), −0.47 (s, 3H); 13C NMR (100 MHz, CDCl3): 160.1, 152.2, 142.7, 130.9, 122.5, 110.1, 91.2, 83.0, 75.6, 69.5, 60.9, 29.8, 25.8, 22.8, 17.3, 17.2, 17.0, −4.6, −5.4, −5.6. ESI-MS calcd for C28H54N4O6Si3+H: 627.3, found: 627.3. Further elution with hexane:EtOAc (1:3) gave 20 (62.3 mg, 40%). 1H NMR (400 MHz, CDCl3): 12.5 (s, 1H), 8.5 (s, 1H), 8.08 (s, 1H), 6.06 (d, 1H, J=4.6Hz), 4.79-4.88 (m, 1H), 4.15-4.29 (m, 2H), 3.97-4.01 (m, 1H), 3.71-3.74 (m, 1H), 3.40-3.41 (m,1H), 0.95 (s, 9H), 0.93 (s, 9H), 0.91 (s, 9H), 0.90 (s, 3H), (s, 3H), 0.88 (s, 3H), 0.85 (s, 3H), 0.77 (s, 3H), 0.09 (s, 3H), −0.48 (s, 3H); 13C NMR (100 MHz, CDCl3): 157.4, 137.6, 112.8, 89.89, 85.81, 82.9, 72.1, 62.6, 29.1, 26.2, 25.9, 18.8, 18.5, 17.7, −4.6, −5.3, −5.5 ESI-MS calcd for C28H55N5O5Si3+H: 626.02, found: 626.3.
4.4. Deprotection procedures
4.4.1. 1-[(5-hydroxylimidazo[4′,5′:4,5]thieno[3,2-d]pyrimidin-3-yl-7-one)]-β-D-ribofuranose (4)
Compound 13 (200 mg, 0.328 mmol) was dissolved in anhydrous CH2Cl2 (10 mL), and BF3•OEt (586.4 µL, 2.82 mmol) and EtSH (2.82 mL, 37.53 mmol) were added, and the solution stirred at room temperature for 2 days. After evaporation of the solvent and excess reagents, the residue was dissolved in 40 mL H2O, and washed with CH2Cl2 (3 × 75 mL). The water phase was evaporated to dryness to give a yellow syrup, which was dissolved in H2O:MeOH (1:1), then cooled to 4 °C, and a white solid precipitated. The solid was filtered, washed with small portions of cold H2O and MeOH, and dried under vacuum to give 98.27 mg of a hygroscopic white solid (88%) (NOTE: Compound 14 can be deprotected in an analogous manner to afford 4 in similar yields). 1H NMR (400 MHz, DMSO-d6):11.34 (s, 1H) 11.26 (s, 1H), 8.44 (s, 1H), 5.90 (d, 1H, J=7.3 Hz), 4.80-5.40 (br, 3H), 4.02-4.08 (m, 2H), 3.90-3.97 (m, 1H), 3.60-3.69 (m, 2H); 13C NMR (100 MHz, DMSO-d6): 160.4, 151.7, 150.4, 146.0, 131.6, 122.8, 108.8, 89.5, 86.7, 76.1, 70.2, 61.2; HR-FAB calcd for C12H13N4O6S [M+H]+ 341.05558, found 341.05614. Anal. calcd for C12H12N4O6S (1.2 H2O) C, 39.82; H, 4.01; N, 15.48; S, 8.86 found: C, 39.91; H, 4.17; N, 15.65; S, 8.88.
4.5. Synthesis of the 2'-deoxy thieno-expanded xanthosine nucleoside
4.5.1. 1-[(5-hydroxylimidazo[4',5':4,5]thieno[3,2-d]pyrimidin-3-yl-7-one)]-1'-β-D-ribofuranoside-3',5'-O-(tetraisobutyrylsilicoane)ether (21)
To a solution of 4 (292 mg, 0.86 mmol) dissolved in 12 mL anhydrous pyridine stirring under N2 was added 1,3-dichloro-1,1,3,3-tetraisopropylsiloxane (293 µl, 0.93 mmol). This solution was stirred at room temperature for 24 h. The solvent was then evaporated under reduced pressure, and an off-white solid obtained which was dissolved in CH2Cl2 (20 mL), washed with water (10 mL), then saturated NaHCO3 (10 mL). The organic phases were combined, dried over anhydrous MgSO4 and evaporated to dryness. The residue was purified via column chromatography eluting with hexanes:EtOAc (1:1) to give 300 mg of 21 as a white foam (60%). 1H NMR (400 MHz, DMF-d7): 11.34 (s, 1H), 8.48 (s, 1H), 7.99 (s, 1H), 6.36 (d, 1H, J=6.0 Hz), 4.76-4.78 (m, 1H), 4.41-4.45 (m, 1H), 4.27-4.30 (m, 1H), 4.18-4.22 (m, 1H), 4.04-4.07 (m, 1H), 0.93-1.06 (m, 28H); 13C NMR (100 MHz, DMF-d7): 160.2, 151.5, 150.0, 131.5, 108.8, 91.8, 90.3, 83.3, 75.7, 74.3, 70.7, 60.5, 18.7, 18.0, 17.3, 17.1, 16.3, 16.0, 15.5, 14.2, 14.1, 13.7, 13.6, 13.3, 12.9, 12.7; ESI-MS calcd for C24H38N4O7SSi2 [M+1]+ 583.2, found 583.1; HR-FAB calcd for C24H39N4O7SSi2 [M+H]+ 583.2478, found 583.2478.
4.5.2. [(5-hydroxylimidazo[4',5':4,5]thieno[3,2-d]pyrimidin-3-yl-7-one)]-1'-β-D-ribofuranoside-3',5'-O-(tetraisobutyrylsilicoane)-2'-thiocarbonate phenyl ester (22)
Stirring under N2, 21 (274 mg, 0.47 mmol) suspended in 15 mL anhydrous CH3CN was treated with DMAP (314.9 mg, 2.58 mmol), followed by phenyl chlorothionoformate (118.9 µl, 0.86 mmol). The yellow solution was stirred at room temperature for 24 h, the solvent was evaporated and the crude yellow solid was purified via chromatography eluting with hexanes:EtOAc (1:1) to afford 169 mg of 22 as a colorless glass-like solid (50%). 1H NMR (400 MHz, CDCl3): 10.01 (s, 1H), 9.75 (s, 1H), 8.24 (s, 1H), 7.26-7.38 (m, 3H), 7.07-7.09 (m, 2H), 6.06 (d, 1H, J=4.2 Hz), 4.57- 4.61 (m, 1H), 4.32-4.42 (m, 3H), 4.01-4.09 (m, 1H), 0.93- 1.06 (m, 28H); 13C NMR (100 MHz, CDCl3): 194.4, 160.2, 153.3, 151.7, 151.3, 142.2, 130.4, 129.7, 123.2, 120.9, 112.4, 110.5, 89.5, 88.5, 84.7, 84.2, 83.5, 82.5, 17.5, 17.3, 17.1, 16.9, 16.8, 16.0, 12.9, 12.6, 12.5; HR-FAB calcd for C31H43N4O8S2Si2 [M+H]+ 719.20609, found 719.20562.
4.5.3. [(5-hydroxylimidazo[4',5':4,5]thieno[3,2-d]pyrimidin-3-yl-7-one)]-1'-â-D-ribofuranoside-3',5'-O-(tetraisobutyrylsilicoane)ether (23)
Under N2, 22 (122 mg, 0.17 mmol) was suspended in 10 mL anhydrous toluene and AIBN (11.23 mg, 0.068 mmol) added, followed by n-Bu3SnH (499.24 µl, 1.85 mmol). The reaction mixture was refluxed for 6 h, the solvent was evaporated under vacuum to give a yellow syrup. The crude syrup was purified via chromatography eluting with hexanes:EtOAc (1:1) to give 50 mg of 23 (52%). 1H NMR (400 MHz, DMF-d7): 11.60 (s, 1H), 8.67 (s, 1H), 8.17 (s, 1H), 6.88-6.89 (m, 1H), 4.85-4.87 (m, 1H), 4.11-4.16 (m, 3H), 3.85-3.87 (m, 2H), 1.24-1.28 (m, 4H), 0.97-1.06 (m, 24H); 13C NMR (100 MHz, DMF-d7): 160.2, 157.3, 152.0, 148.3, 138.0, 117.3, 85.6, 69.2, 61.5, 41.2, 36.7, 18.1, 16.7, 16.6, 16.5, 16.3, 16.2, 16.1, 13.3, 13.0, 12.8, 12.5; ESI-MS calcd for C24H39N4O6SSi2 [M+H]+ 567.2, found 567.1; HR-FAB calcd for C24H39N4O6SSi2 [M+H]+ 567.18771, found 567.18462.
4.5.4. 1-[5-hydroxylimidazo[4',5':4,5]thieno[3,2-d]pyrimidin-3-yl-7-one)]-β-D-2-deoxyribofuranose 3,5-diol (5)
Compound 23 (86 mg, 0.15 mmol) was dissolved in 10 mL anhydrous THF, then 1M TBAF in THF (680 µL) was added. The solution was stirred at room temperature for 4 h, the solvent was evaporated to provide a pale-brown syrup. Purification with silica-chromatography eluting with hexanes:EtOAc (1:2) gave 30 mg of 5 as a hygroscopic white solid (61%). 1H NMR (400 MHz, DMF-d7): 11.03 (s, 1H), 10.94 (s, 1H), 8.4 (s, 1H), 6.25-6.29 (m, 1H), 5.54 (bs, 1H), 5.25 (bs, 1H), 4.32 (bs, 1H), 3.82-3.83 (m, 1H), 3.50-3.63 (m, 2H); 13C NMR (100 MHz, DMF-d7): 160.2, 151.6, 131.7, 127.7, 113.9, 111.2, 108.7, 88.5, 86.9, 70.5, 61.3, 42.7; HR-FAB calcd for C12H13N4O5S [M+H]+ 325.06067, found 325.06021. Anal. calcd for C12H12N4O5S (1.65 H2O) C, 40.71; H, 4.36; N, 15.83; S, 9.06, found: C, 40.77; H, 3.99; N, 15.56; S, 8.89.
Acknowledgments
We are grateful to the National Institutes of Health (GM073645) for support. We would also like to thank Joshua Sadler for editorial help, and Dr. Pasupathy Krishnamoorthy for his efforts in the large scale synthesis of starting materials.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benner SA. Acc. Chem. Res. 2004;37:784–797. doi: 10.1021/ar040004z. [DOI] [PubMed] [Google Scholar]
- 2.Krueger AT, Kool ET. Curr Opin Chem Biol. 2007;11:588–594. doi: 10.1016/j.cbpa.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee AH, Kool ET. J Am Chem Soc. 2006;128:9219–9230. doi: 10.1021/ja0619004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kool ET, Lu H, Kim SJ, Tan S, Wilson JN, Gao J, Liu H. Nucleic Acids Symp Ser (Oxf) 2006:15–16. doi: 10.1093/nass/nrl008. [DOI] [PubMed] [Google Scholar]
- 5.Henry AA, Romesberg FE. Curr Opin Chem Biol. 2003;7:727–733. doi: 10.1016/j.cbpa.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Romesberg FE, Yu C, Matsuda S, Henry AA. In: Current Protocols in Nucleic Acid Chemistry. Harkins EW, editor. New York: Wiley; 2002. pp. 1.5–1.5.36. [DOI] [PubMed] [Google Scholar]
- 7.Henry AA, Yu C, Romesberg FE. J Am Chem Soc. 2003;125:9638–9646. doi: 10.1021/ja035398o. [DOI] [PubMed] [Google Scholar]
- 8.Yu C, Henry AA, Schultz PG, Romesberg FE. Angew. Chem., Int. Ed. Engl. 2002;41:3841–3844. doi: 10.1002/1521-3773(20021018)41:20<3841::AID-ANIE3841>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 9.Tae EL, Wu Y, Xia G, Schultz PG, Romesberg FE. J Am Chem Soc. 2001;123:7439–7440. doi: 10.1021/ja010731e. [DOI] [PubMed] [Google Scholar]
- 10.Seley KL, Mosley SL, Zeng F. Org Lett. 2003;5:4401–4403. doi: 10.1021/ol035696q. [DOI] [PubMed] [Google Scholar]
- 11.Seley KL, Zhang L, Hagos A, Quirk S. J Org Chem. 2002;67:3365–3373. doi: 10.1021/jo0255476. [DOI] [PubMed] [Google Scholar]
- 12.Seley KL, Salim S, Zhang L, O'Daniel PI. J Org Chem. 2005;70:1612–1619. doi: 10.1021/jo048218h. [DOI] [PubMed] [Google Scholar]
- 13.Seley KL, Januszczyk P, Hagos A, Zhang L, Dransfield DT. J Med Chem. 2000;43:4877–4883. doi: 10.1021/jm000326i. [DOI] [PubMed] [Google Scholar]
- 14.Morales JC, Kool ET. Nat Struct Biol. 1998;5:950–954. doi: 10.1038/2925. [DOI] [PubMed] [Google Scholar]
- 15.Sintim HO, Kool ET. Angew Chem Int Ed Engl. 2006;45:1974–1979. doi: 10.1002/anie.200504296. [DOI] [PubMed] [Google Scholar]
- 16.Matray TJ, Kool ET. Nature. 1999;399:704–708. doi: 10.1038/21453. [DOI] [PubMed] [Google Scholar]
- 17.Berger M, Ogawa AK, McMinn DL, Wu Y, Schultz PG, Romesberg FE. Angew. Chem., Int. Ed. Engl. 2000;39:2940–2942. doi: 10.1002/1521-3773(20000818)39:16<2940::aid-anie2940>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Brotschi C, Haberli A, Leumann CJ. Angew. Chem., Int. Ed. Engl. 2001;40:3012–3014. doi: 10.1002/1521-3773(20010817)40:16<3012::AID-ANIE3012>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 19.McMinn DL, Ogawa AK, Wu Y, Liu J, Schultz PG, Romesberg FE. J Am Chem Soc. 1999;121:11585–11586. [Google Scholar]
- 20.Kool ET. Cold Spring Harb Symp Quant Biol. 2000;65:93–102. doi: 10.1101/sqb.2000.65.93. [DOI] [PubMed] [Google Scholar]
- 21.Leumann CJ, Parel SP. Helvetica Chimica Acta. 2000;83:2514–2525. [Google Scholar]
- 22.Leonard NJ, Hiremath SP. Tetrahedron. 1986;42:1917–1961. [Google Scholar]
- 23.Leonard NJ, Scopes DIC, VanDerLijn P, Barrio JR. Biochemistry. 1978;17:3677–3685. doi: 10.1021/bi00611a001. [DOI] [PubMed] [Google Scholar]
- 24.Leonard NJ, Sprecker MA, Morrice AG. J Am Chem Soc. 1976;98:3987–3994. doi: 10.1021/ja00429a040. [DOI] [PubMed] [Google Scholar]
- 25.Seley KL. In: Recent Advances in Nucleosides: Chemistry and Chemotherapy. Chu CK, editor. Amsterdam: Elsevier Science; 2002. pp. 299–326. [Google Scholar]
- 26.Seley KL, Zhang L, Hagos A. Org Lett. 2001;3:3209–3210. doi: 10.1021/ol0165443. [DOI] [PubMed] [Google Scholar]
- 27.Krueger AT, Lu H, Lee AH, Kool ET. Acc Chem Res. 2007;40:141–150. doi: 10.1021/ar068200o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynch SR, Liu H, Gao J, Kool ET. J Am Chem Soc. 2006;128:14704–14711. doi: 10.1021/ja065606n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Gao J, Maynard L, Saito YD, Kool ET. J Am Chem Soc. 2004;126:1102–1109. doi: 10.1021/ja038384r. [DOI] [PubMed] [Google Scholar]
- 30.Lin K-Y, Matteucci MD. J Am Chem Soc. 1998;120:8531–8532. [Google Scholar]
- 31.Flanagan WM, Wolf JJ, Olson P, Grant D, Lin K-Y, Wagner RW, Matteucci M. Proc Natl Acad Sci U S A. 1999;96:3513–3518. doi: 10.1073/pnas.96.7.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matteucci MD, von Krosigk U. Tetrahedron Lett. 1996;37:5057–5060. [Google Scholar]
- 33.Lin K-Y, Jones RJ, Matteucci MD. J Am Chem Soc. 1995;117:3873–3874. [Google Scholar]
- 34.O'Daniel PI, Jefferson M, Wiest O, Seley-Radtke KL. J. Biomolecul. Struct. Dyn. 2008;3:283–292. doi: 10.1080/07391102.2008.10507243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kool ET. Annu Rev Biophys Biomol Struct. 2001;30:1–22. doi: 10.1146/annurev.biophys.30.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Guckian KM, Schweitzer BA, Ren RX-F, Sheils CJ, Tahmassebi DC, Kool ET. J Am Chem Soc. 2000;122:2213–2222. doi: 10.1021/ja9934854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guckian KM, Schweitzer BA, Ren RX-F, Sheils CJ, Paris PL, Tahmassebi DC, Kool ET. J Am Chem Soc. 1996;118:8182–8183. doi: 10.1021/ja961733f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallace LJ, Candlish D, Hagos A, Seley KL, de Koning HP. Nucleosides Nucleotides Nucleic Acids. 2004;23:1441–1444. doi: 10.1081/NCN-200027660. [DOI] [PubMed] [Google Scholar]
- 39.Quirk S, Seley KL. Biochemistry. 2005;44:13172–13178. doi: 10.1021/bi051288d. [DOI] [PubMed] [Google Scholar]
- 40.Quirk S, Seley KL. Biochemistry. 2005;44:10854–10863. doi: 10.1021/bi0503605. [DOI] [PubMed] [Google Scholar]
- 41.Quirk S, Seley-Radtke KL. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006;62:392–394. doi: 10.1107/S1744309106008529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wuenschell GE, O'Connor TR, Termini J. Biochemistry. 2003;42:3608–3616. doi: 10.1021/bi0205597. [DOI] [PubMed] [Google Scholar]
- 43.Stoychev G, Kierdaszuk B, Shugar D. Eur J Biochem. 2002;269:4048–4057. doi: 10.1046/j.1432-1033.2002.03097.x. [DOI] [PubMed] [Google Scholar]
- 44.Sismour AM, Lutz S, Park J-H, Lutz MJ, Boyer PL, Hughes SH, Benner SA. Nucleic Acids Res. 2004;32:728–735. doi: 10.1093/nar/gkh241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao P, Benner SA. J Org Chem. 2001;66:5012–5015. doi: 10.1021/jo005743h. [DOI] [PubMed] [Google Scholar]
- 46.Jurczyk SC, Horlacher J, Devined KG, Benner SA, Battersby TR. Helvetica Chimica Acta. 2000;83:1517–1524. [Google Scholar]
- 47.Lutz MJ, Horlacher J, Benner SA. Bioorg Med Chem Lett. 1998;8:1149–1152. doi: 10.1016/s0960-894x(98)00177-2. [DOI] [PubMed] [Google Scholar]
- 48.Lutz MJ, Held HA, Hottiger M, Hübscher U, Benner SA. Nucleic Acids Res. 1996;24:1308–1313. doi: 10.1093/nar/24.7.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piccirilli JA, Krauch T, Moroney SE, Benner SA. Nature. 1990;343:33–37. doi: 10.1038/343033a0. [DOI] [PubMed] [Google Scholar]
- 50.Kojima N, Minakawa N, Matsuda A. Tetrahedron. 2000;56:7909. [Google Scholar]
- 51.Chung F-L, Earl RA, Townsend LB. Tetrahedron Lett. 1980;21:1599–1602. [Google Scholar]
- 52.Yamazaki A, Kumashiro I, Takenishi T. J Org Chem. 1967;32:3032. doi: 10.1021/jo01285a021. [DOI] [PubMed] [Google Scholar]
- 53.Chung FL, Schram KH, Panzica RP, Earl RA, Wotring LL, Townsend LB. J Med Chem. 1980;23:1158–1166. doi: 10.1021/jm00185a002. [DOI] [PubMed] [Google Scholar]
- 54.Bennett SM, Nguyen-Ba N, Ogilvie KK. J Med Chem. 1990;33:2162–2173. doi: 10.1021/jm00170a019. [DOI] [PubMed] [Google Scholar]
- 55.Robins MJ, Wilson JS, Hansske F. J Am Chem Soc. 1983;105:4059–4065. [Google Scholar]
- 56.Barton DHR, Jang DO, Jaszberenyl JC. Tetrahedron Lett. 1990;31:3991–3994. [Google Scholar]