Abstract
Recombinant TCR ligands (RTL) represent the minimal interactive surface with antigen-specific T cell receptors. These novel constructs fold similarly to native four-domain MHC/peptide complexes but deliver suboptimal and qualitatively different signals that cause a “cytokine switch” to anti-inflammatory factors in targeted encephalitogenic T cells. RTL treatment can reverse clinical and histological signs of EAE and most dramatically can promote myelin and axonal recovery in the CNS of mice with chronic disease. These properties of RTL suggest that this novel antigen-specific approach may hold unusual promise as a therapy for multiple sclerosis.
Keywords: RTL, EAE, MS, neuroprotection, immunomodulation
BACKGROUND
Autoimmune diseases are a direct consequence of autoreactive T cells mediating tissue damage after undesired activation by self-antigens. New and improved drugs are needed for treatment of multiple sclerosis (MS), given that the approved therapies have only modest effects and fail to prevent accumulation of tissue damage and clinical disability. The working hypothesis for the pathogenesis of MS involves the activation or dysregulation of T cells, due to as yet unidentified events, that recognize myelin protein antigens in genetically susceptible individuals. Antigen specific CD4 T cells appear to be a central component in the pathogenesis of a variety of human diseases including MS. Antigen driven activation of CD4 T cells is a multistep process initiated by co-ligation of the T cell receptor and CD4 by the MHC class II/peptide complex present on APC, as well as co-stimulation through additional T cell surface molecules such as CD28, CD40 or OX40. Stimulation through the TCR by MHC class II/peptide in the absence of co-stimulation, rather than being a neutral event, induces a state of unresponsiveness to subsequent antigen presentation, a phenomenon termed anergy [29,37]. These early experiments provided evidence that a direct approach toward antigen-driven immunosuppression could be to present the complete ligand, antigen plus MHC, in the absence of co-stimulatory signals that are normally provided by specialized antigen-presenting cells. To develop a simple and effective agent that could bind selectively to the TCR, we further refined this concept, engineering molecules consisting of the α1 and β1 domains of MHC class II molecules genetically linked into a single polypeptide chain [5](See Fig. 1). Molecules with this design have been useful for studying MHC class II allele specificity in vitro, for exploring primary TCR signaling events independent of co-stimulatory input associated with the MHC class II α2 and β2 domains or with other molecules expressed by antigen presenting cells, and for treating CD4 T cell mediated autoimmune disease in an MHC II/epitope-specific manner [3,25,42]. One such DR2 construct, RTL1000, containing the MOG-35–55 peptide, is currently in a phase I safety trial in MS subjects, and analogous constructs are under development for uveitis, arthritis, diabetes and celiac disease.
Fig. 1.
Recombinant T Cell Receptor Ligand (RTL).
Design and engineering of RTLs
The basic design of RTLs involves genetically coupling the amino terminus of the alpha-1-domain to the carboxyl terminus of the beta-1 domain. These molecules have also been made with covalently coupled peptides attached by a linker to the N-terminus of the β-chain. We used molecular modeling, combining structural information from X-ray crystallographic data with recombinant DNA technology to design and produce single chain TCR ligands based on the natural MHC class II peptide binding/T cell recognition domain. The structure-based design of the RTLs is based on work described in our previous publications [4,5,9,17]. Circular dichroism revealed that the β1α1 molecules have highly ordered secondary structures. The empty β1α1 molecule contained approximately 30% α-helix, 15% β-strand, 26% β-turn and 29% random coil structures. Comparison with the secondary structures of four-domain class II molecules by X-ray crystallography provided strong evidence that the β1α1 and β1α1 peptide molecules shared the β sheet platform/anti-parallel a-helix secondary structure common to all class II antigen binding domains. Thermal denaturation revealed a high degree of cooperativity and stability of the molecules. These constructs, with or without associated antigenic peptide, retain structural and conformational integrity consistent with the refolded native MHC class II molecules. The biological integrity of RTL has been demonstrated by their ability to bind antigenic peptides in an allele-specific manner, detect and inhibit rat, mouse and human autoreactive T cells and treat experimental autoimmune encephalomyelitis (EAE), collagen induced arthritis (CIA) and experimental autoimmune uveitis (EAU).
Ag specific RTL treatment on cognate T cells triggers partial activation through the TCR and IL-10 secretion in the absence of costimulatory signals
T lymphocytes identify foreign pathogens through the interaction of the clonotypic heterodimeric αβ T cell receptor (TCR) with peptide antigens bound to class I or class II major histocompatibility complex (MHC) molecules [13]. Recognition of MHC/peptide by the TCR is followed by phosphorylation of conserved “ITAM” motifs on the intracellular CD3 chains, which in turn leads to activation of multiple intracellular signaling pathways in the T cell [27]. Agonist peptides are capable of inducing full activation of the cell-mediated immune response, causing a hierarchical series of events, including cytokine secretion, acid release, calcium flux, and, ultimately, proliferation of T cells [50], while suboptimal ligands, “partial” or “weak agonists,” can lead to a partial activation of the T cell [19,30]. These studies strongly suggest that MHC/peptide binding to the TCR can lead to a variety of outcomes depending on the MHC/peptide and context used. The strength of the interaction between TCR and MHC/peptide is a function of affinity and kinetic stability, both of which have been shown to directly correlate with the biological outcome of TCR:MHC/peptide interaction. Structural studies using a refined model system, the murine 2C TCR and a soluble MHC class II/peptide (H-2Kb/dEV8) suggest that “structural plasticity” at the TCR:MHC/peptide interface via an induced fit in the complementarity-determining region (CDR) loops provided the requisite information for productive TCR signaling [15].
The context within which MHC/peptide and TCR interact is also crucial for biological outcome. The natural context, juxtaposed APC and T cell membranes, provides additional features favorable for T cell activation, including density of the MHC-peptide ligand on the APC cell surface (that promotes TCR cross-linking), stabilization contributed by the CD4 co-receptor (which binds primarily to the MHC class II β2 domain) [8,20] costimulatory interactions such as B7:CD28 and adhesion molecules such as ICAM-1:LFA-1, to name a few. All contribute to mediate downstream cellular differentiation and proliferation. It has been suggested that a longer duration of contact between the TCR and its ligand results in a full signal through the TCR and subsequent Th1 differentiation, whereas shorter duration contact or altered interaction, as in the case of altered peptide ligands, results in a partial signal that favors Th2 differentiation [12,33]. Moreover, stimulation through the TCR in the absence of costimulation can induce a range of cellular responses from full activation to anergy or cell death [29,37].
Partial activation of the TCR has been demonstrated to occur when MHC class II-derived cognate RTL molecules, containing the minimal ligand for peptide-specific TCRs, were incubated with peptide specific Th1 cell clones in the absence of APCs or costimulatory molecules [6,46]. Using RTL to activate a T cell hybridoma specific for MBP-72–89, we observed partial activation that included a CD3ζ p23/p21 ratio shift, ZAP-70 phosphorylation, calcium mobilization, NFAT activation, and transient IL-2 production. In comparison, α-CD3ε treatment produced stronger activation of these cellular events, with additional activation of NFκB and extracellular-regulated kinase (ERK), as well as long-term increased IL-2 production. These results demonstrate that RTLs can bind directly to the TCR and modify T cell behavior through a partial activation mechanism, triggering specific downstream signaling events that deplete intracellular calcium stores without fully activating T cells. The resulting Ag-specific activation of the transcription factor NFAT uncoupled from the activation of NFκB or ERKs constitutes a unique downstream activation pattern that accounts for the inhibitory RTL effects on encephalitogenic CD4+ T cells.
With human T cell clones, partial activation by RTL included included rapid TCRζ-chain phosphorylation, calcium mobilization, and reduced extracellular signal-related kinase activity, as well as IL-10 production, but not proliferation, Th1 cytokine response or IL-2Rα expression. Upon restimulation with APC/peptide, the RTL-pretreated Th1 clones had partially reduced proliferation and less IFN-γ secretion, but retained IL-10 production and had normal expression of IL-2Rα. In contrast, anti-CD3ε mAb induced strong initial proliferation and secretion of IL-2, IFN-γ, and IL-4 in the same system [6]. Anti-CD3ε-pretreated T cells upon restimulation with APC-Ag had markedly reduced levels of proliferation and cytokine secretion, including IL-2, but retained expression of IL-2Rα. Therefore, signals delivered by RTLs appear to have different physiological consequences than those that occur after anti-CD3ε antibody treatment. One other difference lies in the observation that anti-CD3 treatment downmodulates TCR off the surface of the clones, whereas RTL pretreatment produced only partial TCR down-regulation. Antigen specific IL-10 secretion by Th1 clones in response to RTL treatment and continued secretion of IL-10 upon restimulation of the RTL-pretreated clones with APC-Ag indicates that TCR interaction with the RTL results in default IL-10 production that persists even upon re-exposure to specific Ag. The elevated level of IL-10 induced in Th1 cells by RTL might have important regulatory implications for autoimmune diseases such as MS because of the known anti-inflammatory effects of this cytokine on Th1 cell and macrophage activation. Our current concept of the effect of RTL on T cells to induce a “cytokine switch” is shown in Fig. 2.
Fig. 2.
Modified T Cell Activation with RTLs.
RTLs reverse clinical and histological signs of Experimental Autoimmune Encephalomyelitis
EAE is an inflammatory demyelinating disease mediated by CD4 T cells specific for CNS myelin antigens and shares a number of immunological similarities with the human demyelinating disease MS [28]. It has, therefore, been a useful model for preclinical testing of therapies for the human illness [16,18,26,41,43,49,51]. The use of the EAE model has allowed us to explore antigen specific immunosuppression by presenting the cognate TCR ligand (the RTL with bound peptide), in the absence of costimulatory signals.
Most of our early work was carried out in the Lewis (LEW) rat model of EAE. In LEW rats, the dominant encephalitogenic determinant resides in the 72–89 peptide of guinea pig MBP (Gp-MBP-72–89) [2], and active immunization with this peptide in CFA can induce a severe paralytic episode starting on day 10–11 and lasting 5–7 days with associated formation of inflammatory perivascular lesions without significant demyelination within the CNS[28]. We produced an RT1.B-derived RTL covalently linked to Gp-MBP-72–89 peptide (RTL-201) and investigated the regulatory effects of this novel construct on actively or passively induced EAE[3]. RTL-201 possessed potent suppressive and therapeutic activity for actively induced clinical and histological disease, and substantially reduced the proliferative response of T cells cultured from draining lymph nodes (DLN). Even more striking, RTL-201 completely suppressed passive EAE induced after transfer of Gp-MBP-72–89-specific T cells, but had no effect on passive EAE induced with a different I-E (RT1.D)-restricted T cell line specific for a distinct encephalitogenic determinant, MBP-87–99.
Because EAE in the LEW rat is a monophasic disease that does not model the relapsing or progressive phases of MS, we extended our studies of RTLs to a relapsing/remitting model of EAE in SJL/J mice and to two different chronic models of EAE using C57BL/6 mice or DR2*1501 transgenic mice. RTL401 (β1α1 domains of I-As covalently linked to PLP-139–151 peptide) given i.v. or s.c., but not “empty” RTL400 (without a covalently linked peptide) or free PLP-139–151 peptide, significantly reduced clinical severity and prevented relapses of EAE induced by the immunodominant PLP-139–151 peptide [17,25](Fig. 3). However, RTL401 did not inhibit EAE induced by two different encephalitogenic peptides for SJL/J mice, PLP-178–191 or MBP-84–104, thus demonstrating peptide specificity [17]. Moreover, RTL401 was highly effective in treating passive EAE in SJL/J mice, with kinetics of recovery from disease very similar to treatment of actively induced EAE.
Fig. 3.
RTL401 given i.v. or s.c. to SJL mice treats relapsing EAE.
EAE in C57BL/6 mice is a chronic disease that is induced upon injection of MOG-35–55 peptide/CFA/pertussis toxin. For RTL treatment experiments, we produced RTL551 (the β1α1 domains of I-Ab covalently linked to the MOG-35–55 peptide). Similar to our previous experiments, RTL551 was highly effective at treating clinical and histological disease [38]. Of additional interest, we tested RTL401 for treating EAE in (C57BL/6×SJL) F1 mice, in which relapsing disease could be induced with PLP-139–151 (restricted only by I-As) or chronic disease with MOG-35–55 peptide (restricted only I-Ab). We found that RTL401 was effective at treating only PLP-139–151 induced EAE but not MOG-35–55 peptide-induced EAE in these (C57BL/6×SJL/J) F1 mice[17]. Although the converse treatment with RTL551 and dual treatment experiments with both RTL constructs have not yet been carried out, these initial data indicate that RTL treatment is highly specific for the encephalitogenic peptide, even in the presence of an additional MHC allele.
Further support for the efficacy of RTL therapy for chronic EAE comes from the fact that DR2 (DRA:DRB1*1501)-derived RTLs covalently linked to the encephalitogenic MOG-35–55 peptide completely reversed clinical and histological signs of EAE in Tg DR2 mice [42]. This model of EAE has been crucial in the development of a humanized RTL for MS that contains the β1α1 domains of DR2b (a recognized risk factor for MS) covalently linked to a widely encephalitogenic peptide, MOG-35–55[35].
RTL therapy induces a cytokine switch that curbs the encephalitogenic potential of inflammatory cells
The initial immune response in EAE is inflammatory and is mediated by cytokines such as IFN-γ, TNF-α, IL-1, IL-2, IL-6 and IL-8. Recent studies have placed the pro-inflammatory cytokine, IL-17, at center stage for induction of EAE since it has been shown to be responsible for activation of T cells leading to production of a variety of cytokines, chemokines and matrix metalloproteases[1,48]. The recovery phase of EAE is associated with the production of Th-2 like cytokines, including IL-4, IL-5, IL-10, IL-13 and TGF-β. Splenocytes from RTL-treated mice had strongly reduced levels of IFN-γ during remission and the first relapse, with a sustained increase in IL-10 levels during onset, remission and relapse of EAE[25]. When tested in mice developing EAE after transfer of GFP+ encephalitogenic T cells, RTL treatment significantly inhibited clinical and histological disease and downregulated IL-17 and TNF-α secretion by sorted GFP+ cells from the recipient mice [38]. Interestingly, IL-17 and TNF-α were not produced by the GFP negative cells even in placebo-treated mice. The selectivity of the RTL treatment for both Th1 and Th17 cells is a key observation clinically, given the fact that IFN-γ and IL-17 have been detected in the sera and CNS target tissue of MS subjects. In spinal cord, RTL treatment strongly reduced mRNA expression of the chemokines MCP-1, RANTES, MIP-2 and IP-10 and enhanced expression of TGF-β3 [17], consistent with our earlier report suggesting a protective role for this cytokine [24]. These pronounced RTL effects on chemokine and cytokine expression in EAE will be of potential importance when evaluating RTL treatment effects in subjects with MS.
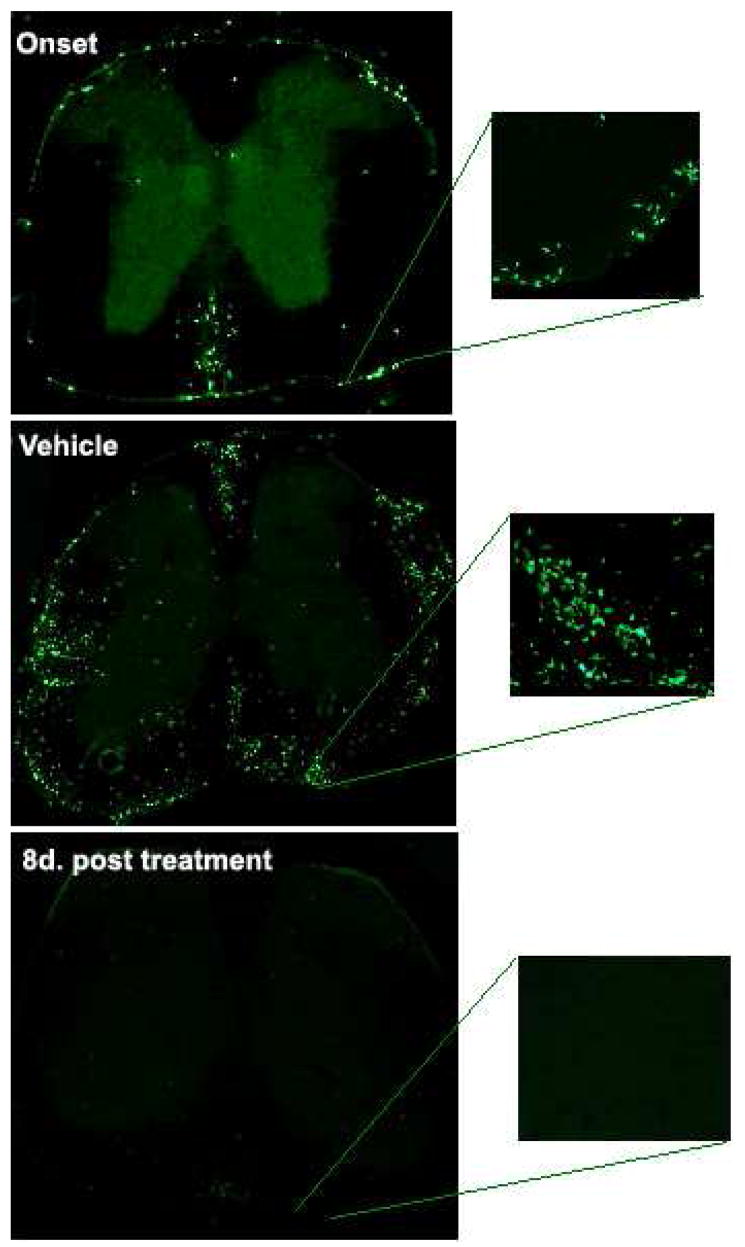
RTL treatment dramatically reduces infiltrating encephalitogenic cells in the CNS of mice with EAE
The transfer of GFP+ MOG-35–55 primed T cells into WT GFP negative C57BL/6 recipient mice enabled us to study the migration pattern of encephalitogenic cells after RTL treatment of mice with ongoing clinical and histological EAE. Treatment with eight daily doses of RTL551 not only prevented infiltration of newly recruited cells, but also eliminated nearly all existant GFP+ encephalitogenic cells from the spinal cords of treated mice [38](Fig. 4). The remaining cells in the RTL-treated group were restricted to the subarachnoid space in the spinal cords, but were not present in the CNS parenchyma or perivascular space, and were therefore positionally restricted in their ability to contribute to CNS damage. This reduction in inflammatory infiltrates after RTL treatment was reflected by a concomitant reduction in clinical disease to a negligible score of 0.5 starting on the fourth day of treatment.
Fig. 4.
RTL551 reverses autoimmune inflammation in spinal cords of C57BL/6 mice with passive EAE induced by GFP+ encephalitogenic T cells.
A common histopathological feature shared by EAE and MS is that both diseases appear to be induced by auto-reactive T cells in the CNS, with an effector phase mediated by cells of the myeloid lineage. Macrophages and CD4+ and CD8+ T cells have been identified in the active MS lesions in the CNS. Natalizumab, a humanized monoclonal antibody to α4 integrins on T cells and other inflammatory cells, has been reported to be more effective than interferon-β or glatiramer acetate for the treatment of MS. This antibody apparently exerts its therapeutic benefits by suppressing leukocyte entry into the CNS [32]. Similarly, reduction of autoimmune inflammation in the CNS by RTL treatment might also have a significant impact on the treatment of MS. Natalizumab treatment unfortunately may have adverse effects. The non-specific blockade of CD4+ T cell entry into the CNS following Natalizumab treatment has resulted in the development of a rare but potentially fatal opportunistic infection of oligodendrocytes by JC polyomavirus. This problem may be avoided by more selective treatments such as RTL. Furthermore, it has been recently shown that Tregs are recruited but are insufficient to control inflammation within the CNS due to prevailing inflammatory conditions [21]. Therefore, by reducing inflammation in the CNS, RTL treatment might indirectly favor regulatory T cells to function under conditions which are otherwise impaired in MS subjects.
RTL treatment creates an unfavorable environment for inflammatory cells in the CNS of mice with EAE
Blood brain barrier disruption and entry of inflammatory cells into the CNS is regulated by chemokines and chemokine receptors along with the expression of adhesion molecules such as VCAM-1 and ICAM-1 on the vascular endothelium. In the experiments described above, we did not observe any evidence of apoptotic cell death of infiltrating GFP+ cells in the CNS in response to RTL treatment. Rather, we observed significant modulation of chemokines and receptors in the spinal cords of treated mice. Most of the chemokines/receptors, including CXCL2/CXCR2, CCL6/CCR1, CCL2/CCR2, CCL5/CCR1,3,5 and CXCL1/CXCR2, were significantly down-regulated in the spinal cords of treated mice, suggesting a loss of chemotactic gradient due to which the inflammatory infiltrates could not be sustained in the spinal cords [38]. Further evidence for loss of an inflammatory milieu was provided by the significant down-regulation of ICAM-1 and VCAM-1 (Fig. 5) on the vascular endothelial cells in response to RTL treatment [38]. Expression of these adhesion molecules by endothelial cells is required for the attachment, rolling, and transmigration of leukocytes from the vasculature into the CNS parenchyma. This interaction between α4β1 integrins on the T cells and counter receptors on the endothelium blocked by Natalizumab has also been shown to be crucial in MS pathogenesis [34,44]. Treatment with IFN-β, an approved therapy for MS, results in a rapid reduction in contrast enhancing MRI lesions [22,39] and this is correlated with an increase in sVCAM-1, suggesting perturbation of cell adhesion molecules on the endothelium with this therapeutic agent as well [7].
Fig. 5.
Treatment of passive EAE in C57BL/6 mice with RTL551 down-regulates VCAM-1 on CNS endothelial cells.
RTL treatment promotes myelin and neuronal-regenerative process
It is now apparent that in addition to extensive primary demyelination that has long been the hallmark feature of CNS damage in MS, axonal injury may be the main factor responsible for the accrual of neurological deficits manifested in progressive MS [14,31,36,47]. To model this important cascade, we treated SJL/J mice with RTL401 beginning 10 days after onset of clinical signs (Fig. 6), when histological evidence confirmed a significant degree of demyelination and axonal damage. We found that RTL treatment when given at this later time point enabled a nearly complete reversal of demyelination (Fig. 7) and axonal damage (Fig. 8) to near normal levels observed prior to onset of disease [45]. Moreover, reduction of inflammatory cells in the CNS after RTL therapy correlated well with the enhanced axonal staining and eventual clinical improvement [45]. Thus, RTL treatment might lead to natural myelin and axonal repair process through antigen-specific inhibition of inflammatory cells within the CNS. Electron microscopy showed that RTL-treated mice had reduced pathology compared with mice treated with vehicle alone and mice at the peak of disease, as demonstrated by a decrease in continued degeneration, an increase in remyelinating axons and the presence of an increased number of small, presumably regenerative axonal sprouts [45]. There is no direct evidence from our study that RTL treatment can stimulate neuroregeneration by acting directly on neurons. However, we cannot exclude the possibility that RTL401 may provide neuroprotection by promoting an anti-inflammatory environment. While excessive production of Th1 proinflammatory cytokines has been shown to cause neuronal cell injury and death, the presence of Th2 anti-inflammatory cytokines, including IL-4 and IL-10, tends to promote neuronal protection and survival in the CNS [11,40]. Interestingly, as described above, RTL therapy can induce a Th1 to Th2 cytokine switch in encephalitogenic T cells [6,17]. These findings suggest how RTL therapy targeting encephalitogenic T cells might promote a CNS neuroregenerative process.
Fig. 6.
Delayed treatment of relapsing EAE with RTL401 results in attenuated clinical reversal.
Fig. 7.
RTL401-treatment ameliorates tissue (myelin) damage.
Fig. 8.
RTL401-treatment reverses progression of CNS axonal loss and inflammation.
RTL therapy for MS
Based on these and other pre-clinical data that were patented and published, we have initiated a human Phase 1 safety study for use of a DR2*1501 RTL with covalently bound MOG-35–55 peptide (RTL1000) in subjects with MS. The study is enrolling 5 cohorts of 6 MS subjects each, four in each cohort receiving drug and two receiving placebo. Each succeeding cohort receives an increasing dose of RTL1000 given i.v., starting with the lowest dose of 2mg given as a single injection (starting dose determined from our preclinical studies[23]), and escalating to 6, 20, 60, and 200mg. At this writing (March, 2008), cohorts 1 and 2 have been completed without safety issues, and cohort 3 is near completion. Further information on this trial can be viewed at clinicaltrials.gov (key in “Multiple Sclerosis”) or on the National Multiple Sclerosis website under current clinical trials in MS.
Future directions
Thus far, we have demonstrated successful treatment of clinical and histological EAE using RTL bearing the cognate encephalitogenic peptide for the EAE model under study. We believe it will be worthwhile to also study whether RTL treatment might induce bystander suppression. This will be especially relevant for clinical MS where the relevant neuroantigen targets are still unknown, and as mentioned earlier, increased IL-10 secretion in response to RTL treatment might cause immunoregulation of activated bystander cells as well. That is, the pathogenesis of MS is likely to involve autoreactive Th1 or Th17 cells directed at one or more immunodominant myelin peptides, including MBP-85–99 and MOG-35–55. As discussed above, RTL induce IL-10 production by T cells, thus neutralizing their pathogenic potential. This local production of IL-10 after Ag-stimulation in the CNS could also result in inhibited activation of bystander T cells that may be of the same or different Ag specificity, as well as macrophages that participate in demyelination[10]. Thus, our findings suggest that RTL therapy and its regulatory potential may extend beyond the RTL-ligated neuroantigen specific T cell. RTL induction of IL-10 in specific T cell populations that recognize CNS antigens could potentially be used to regulate the immune system while preserving the T cell repertoire, and may represent a novel strategy for therapeutic intervention of complex T cell mediated autoimmune diseases such as MS. Studies are underway to determine whether the RTL treatment of encephalitogenic cells makes them lose their disease causing potential mainly due to transformation of their Th1 cytokine profile to that of Th2 cells.
The findings so far indicate that RTL treatment of EAE occurs in an antigen specific manner. RTL treatment also appears to intervene at more than one point of disease pathogenesis and this makes RTL therapy even more versatile for the treatment of autoimmune diseases including MS. RTL therapy not only inhibits systemic production of pro-inflammatory cytokines by encephalitogenic cells but also impedes downstream local recruitment and retention of inflammatory cells in the CNS, factors that may be crucial in minimizing myelin and axonal damage in vivo.
Finally, there are alternative mechanisms currently under study through which RTLs may be “processed” and presented through APC, including dendritic cells, macrophages and B cells, in order to induce specific tolerance. We envision that the RTL “carrier” protein may represent an efficient vehicle for delivering Ag differentially to tissue and/or sub-subsets of APCs, as well as to the sub-cellular compartments within APCs where normal processing and re-presentation occurs. While these alternative mechanisms are currently under intensive investigation our working hypothesis is that the profound clinical utility of RTL therapy lies in the ultimate specific triggering of a “cytokine switch” in pathogenic CD4+ T cells.
Acknowledgments
The authors wish to thank Jianya Huan, Ph.D., Yuan K. Chou, Ph.D., Sandhya Subramanian, M.S., and Jeffrey Mooney, B.S. for their outstanding contributions to the cited work, and Ms. Eva Niehaus for assistance in manuscript preparation.
This work was supported by the National Multiple Sclerosis Society Grants RG3794A and RG3468, NIH Grants NS47661, AI43960, NS41965, and NS46877, The Nancy Davis MS Center Without Walls, and the Biomedical Laboratory R&D Service, Department of Veterans Affairs.
Footnotes
Drs. Offner, Burrows, Vandenbark, and OHSU have a significant financial interest in Artielle ImmunoTherapeutics, Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by the OHSU and VAMC Conflict of Interest in Research Committees.
References
- 1.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the cirle of immunity and autoimmunity. Nature Immunol. 2007;8(4):345–50. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 2.Bourdette DN, Vainiene M, Morrison W, Jones R, Turner MJ, Hashim GA, Vandenbark AA, Offner H. Myelin basic protein specific T cell lines and clones derived from the CNS of rats with EAE only recognize encephalitogenic epitopes. J Neurosci Res. 1991;30(2):308–15. doi: 10.1002/jnr.490300205. [DOI] [PubMed] [Google Scholar]
- 3.Burrows GG, Adlard KL, Bebo BF, Jr, Chang JW, Tenditnyy K, Vandenbark AA, Offner H. Regulation of encephalitogenic T cells with recombinant TCR ligands. J Immunol. 2000;164(12):6366–71. doi: 10.4049/jimmunol.164.12.6366. [DOI] [PubMed] [Google Scholar]
- 4.Burrows GG, Bebo BF, Jr, Adlard KL, Vandenbark AA, Offner H. Two-domain MHC class II molecules form stable complexes with myelin basic protein 69–89 peptide that detect and inhibit rat encephalitogenic T cells and treat experimental autoimmune encephalomyelitis. J Immunol. 1998;161(11):5987–96. [PubMed] [Google Scholar]
- 5.Burrows GG, Chang JW, Bachinger HP, Bourdette DN, Offner H, Vandenbark AA. Design, engineering and production of functional single-chain T cell receptor ligands. Protein Eng. 1999;12(9):771–8. doi: 10.1093/protein/12.9.771. [DOI] [PubMed] [Google Scholar]
- 6.Burrows GG, Chou YK, Wang C, Chang JW, Finn TP, Culbertson NE, Kim J, Bourdette DN, Lewinsohn DA, Lewinsohn DM, Ikeda M, Yoshioka T, Allen CN, Offner H, Vandenbark AA. Rudimentary TCR signaling triggers default IL-10 secretion by human Th1 cells. J Immunol. 2001;167(8):4386–95. doi: 10.4049/jimmunol.167.8.4386. [DOI] [PubMed] [Google Scholar]
- 7.Calabresi PA, Tranquill LR, Dambrosia JM, Stone LA, Maloni H, Bash CN, Frank JA, McFarland HF. Increases in soluble VCAM-1 correlate with a decrease in MRI lesions in multiple sclerosis treated with interferon beta-1b. Ann Neurol. 1997;41(5):669–74. doi: 10.1002/ana.410410517. [DOI] [PubMed] [Google Scholar]
- 8.Cammarota G, Scheirle A, Takacs B, Doran DM, Knorr R, Bannworth W, Guardiola J, Sinigaglia F. Identification of a CD4 binding site on the B2 domain of HLA-DR molecules. Nature. 1992;356:799–802. doi: 10.1038/356799a0. [DOI] [PubMed] [Google Scholar]
- 9.Chang JW, Mechling DE, Bachinger HP, Burrows GG. Design, engineering, and production of human recombinant t cell receptor ligands derived from human leukocyte antigen DR2. J Biol Chem. 2001;276(26):24170–6. doi: 10.1074/jbc.M101808200. [DOI] [PubMed] [Google Scholar]
- 10.Cua DJ, Groux H, Hinton DR, Stohlman SA, Coffman RL. Transgenic interleukin 10 prevents induction of experimental autoimmune encephalomyelitis. J Exp Med. 1999;189(6):1005–10. doi: 10.1084/jem.189.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Souza S, Alinauskas K, McCrea E, Goodyer C, Antel JP. Differential susceptibility of human CNS-derived cell populations to TNF-dependent and independent immune-mediated injury. J Neurosci. 1995;15(11):7293–300. doi: 10.1523/JNEUROSCI.15-11-07293.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–44. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 13.Doherty PC, Zinkernagel RM. T-cell-mediated immunopathology in viral infections. Transplant Rev. 1974;19(0):89–120. doi: 10.1111/j.1600-065x.1974.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 14.Dutta R, Trapp BD. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68(S22–31):S43–54. doi: 10.1212/01.wnl.0000275229.13012.32. [DOI] [PubMed] [Google Scholar]
- 15.Garcia KC, Degano M, Pease LR, Huang M, Peterson PA, Teyton L, Wilson IA. Structural basis of plasticity in T cell receptor recognition of a self peptide-MHC antigen. Science. 1998;279(5354):1166–72. doi: 10.1126/science.279.5354.1166. [DOI] [PubMed] [Google Scholar]
- 16.Howell MD, Winters ST, Olee T, Powell HC, Carlo DJ, Brostoff SW. Vaccination against experimental allergic encephalomyelitis with T cell receptor peptides. Science. 1989;246(4930):668–70. doi: 10.1126/science.2814489. [DOI] [PubMed] [Google Scholar]
- 17.Huan J, Subramanian S, Jones R, Rich C, Link J, Mooney J, Bourdette DN, Vandenbark AA, Burrows GG, Offner H. Monomeric recombinant TCR ligand reduces relapse rate and severity of experimental autoimmune encephalomyelitis in SJL/J mice through cytokine switch. J Immunol. 2004;172(7):4556–66. doi: 10.4049/jimmunol.172.7.4556. [DOI] [PubMed] [Google Scholar]
- 18.Jameson BA, McDonnell JM, Marini JC, Korngold R. A rationally designed CD4 analogue inhibits experimental allergic encephalomyelitis. Nature. 1994;368(6473):744–6. doi: 10.1038/368744a0. [DOI] [PubMed] [Google Scholar]
- 19.Kersh GJ, Allen PM. Structural basis for T cell recognition of altered peptide ligands: a single T cell receptor can productively recognize a large continuum of related ligands. J Exp Med. 1996;184(4):1259–68. doi: 10.1084/jem.184.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konig R, Huang L-Y, Germain RN. MHC class II interaction with CD4 mediated by a region analogous to the MHC class I binding site for CD8. Nature. 1992;356:796–9. doi: 10.1038/356796a0. [DOI] [PubMed] [Google Scholar]
- 21.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13(4):423–31. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li DK, Zhao GJ, Paty DW. Randomized controlled trial of interferon-beta-1a in secondary progressive MS: MRI results. Neurology. 2001;56(11):1505–13. doi: 10.1212/wnl.56.11.1505. [DOI] [PubMed] [Google Scholar]
- 23.Link JM, Rich CM, Korat M, Burrows GG, Offner H, Vandenbark AA. Monomeric DR2/MOG-35–55 recombinant TCR ligand treats relapses of experimental encephalomyelitis in DR2 transgenic mice. Clin Immunol. 2007;123(1):95–104. doi: 10.1016/j.clim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Matejuk A, Dwyer J, Hopke C, Vandenbark AA, Offner H. Opposing roles for TGF-beta1 and TGF-beta3 isoforms in experimental autoimmune encephalomyelitis. Cytokine. 2004;25(2):45–51. doi: 10.1016/j.cyto.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Offner H, Subramanian S, Wang C, Afentoulis M, Vandenbark AA, Huan J, Burrows GG. Treatment of passive experimental autoimmune encephalomyelitis in SJL mice with a recombinant TCR ligand induces IL-13 and prevents axonal injury. J Immunol. 2005;175(6):4103–11. doi: 10.4049/jimmunol.175.6.4103. [DOI] [PubMed] [Google Scholar]
- 26.Oksenberg JR, Panzara MA, Begovich AB, Mitchell D, Erlich HA, Murray RS, Shimonkevitz R, Sherritt M, Rothbard J, Bernard CC, et al. Selection for T-cell receptor V beta-D beta-J beta gene rearrangements with specificity for a myelin basic protein peptide in brain lesions of multiple sclerosis. Nature. 1993;362(6415):68–70. doi: 10.1038/362068a0. [DOI] [PubMed] [Google Scholar]
- 27.Pastor MI, Woodrow M, Cantrell D. Regulation and function of p21ras in T lymphocytes. Cancer Surv. 1995;22:75–83. [PubMed] [Google Scholar]
- 28.Paterson PY. Multiple sclerosis: an immunologic reassessment. J Chronic Dis. 1973;26(3):119–26. doi: 10.1016/0021-9681(73)90085-4. [DOI] [PubMed] [Google Scholar]
- 29.Quill H, Schwartz RH. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol. 1987;138(11):3704–12. [PubMed] [Google Scholar]
- 30.Rabinowitz JD, Beeson C, Wulfing C, Tate K, Allen PM, Davis MM, McConnell HM. Altered T cell receptor ligands trigger a subset of early T cell signals. Immunity. 1996;5(2):125–35. doi: 10.1016/s1074-7613(00)80489-6. [DOI] [PubMed] [Google Scholar]
- 31.Rammohan KW. Axonal injury in multiple sclerosis. Curr Neurol Neurosci Rep. 2003;3(3):231–7. doi: 10.1007/s11910-003-0083-0. [DOI] [PubMed] [Google Scholar]
- 32.Ransohoff RM. Natalizumab for multiple sclerosis. N Engl J Med. 2007;356(25):2622–9. doi: 10.1056/NEJMct071462. [DOI] [PubMed] [Google Scholar]
- 33.Redpath S, Alam SM, Lin CM, O’Rourke AM, Gascoigne NR. Cutting edge: trimolecular interaction of TCR with MHC class II and bacterial superantigen shows a similar affinity to MHC:peptide ligands. J Immunol. 1999;163(1):6–10. [PubMed] [Google Scholar]
- 34.Rice GP, Hartung HP, Calabresi PA. Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology. 2005;64(8):1336–42. doi: 10.1212/01.WNL.0000158329.30470.D0. [DOI] [PubMed] [Google Scholar]
- 35.Rich C, Link JM, Zamora A, Jacobsen H, Meza-Romero R, Offner H, Jones R, Burrows GG, Fugger L, Vandenbark AA. Myelin oligodendrocyte glycoprotein-35–55 peptide induces severe chronic experimental autoimmune encephalomyelitis in HLA-DR2-transgenic mice. Eur J Immunol. 2004;34:1251–61. doi: 10.1002/eji.200324354. [DOI] [PubMed] [Google Scholar]
- 36.Rieckmann P, Smith KJ. Multiple sclerosis: more than inflammation and demyelination. Trends Neurosci. 2001;24(8):435–7. doi: 10.1016/s0166-2236(00)01860-9. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz RH. Models of T cell anergy: is there a common molecular mechanism? J Exp Med. 1996;184(1):1–8. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinha S, Subramanian S, Proctor TM, Kaler LJ, Grafe M, Dahan R, Huan J, Vandenbark AA, Burrows GG, Offner H. A promising therapeutic approach for multiple sclerosis: recombinant T-cell receptor ligands modulate experimental autoimmune encephalomyelitis by reducing interleukin-17 production and inhibiting migration of encephalitogenic cells into the CNS. J Neurosci. 2007;27(46):12531–9. doi: 10.1523/JNEUROSCI.3599-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stone LA, Frank JA, Albert PS, Bash C, Smith ME, Maloni H, McFarland HF. The effect of interferon-beta on blood-brain barrier disruptions demonstrated by contrast-enhanced magnetic resonance imaging in relapsing-remitting multiple sclerosis. Ann Neurol. 1995;37(5):611–9. doi: 10.1002/ana.410370511. [DOI] [PubMed] [Google Scholar]
- 40.Szelenyi J. Cytokines and the central nervous system. Brain Res Bull. 2001;54(4):329–38. doi: 10.1016/s0361-9230(01)00428-2. [DOI] [PubMed] [Google Scholar]
- 41.Vandenbark AA, Hashim G, Offner H. Immunization with a synthetic T-cell receptor V-region peptide protects against experimental autoimmune encephalomyelitis. Nature. 1989;341(6242):541–4. doi: 10.1038/341541a0. [DOI] [PubMed] [Google Scholar]
- 42.Vandenbark AA, Rich C, Mooney J, Zamora A, Wang C, Huan J, Fugger L, Offner H, Jones R, Burrows GG. Recombinant TCR ligand induces tolerance to myelin oligodendrocyte glycoprotein 35–55 peptide and reverses clinical and histological signs of chronic experimental autoimmune encephalomyelitis in HLA-DR2 transgenic mice. J Immunol. 2003;171(1):127–33. doi: 10.4049/jimmunol.171.1.127. [DOI] [PubMed] [Google Scholar]
- 43.Vandenbark AA, Vainiene M, Celnik B, Hashim GA, Buenafe A, Offner H. Definition of encephalitogenic and immunodominant epitopes of guinea pig myelin basic protein (Gp-BP) in Lewis rats tolerized neonatally with Gp-BP or Gp-BP peptides. J Immunol. 1994;153(2):852–61. [PubMed] [Google Scholar]
- 44.von Andrian UH, Engelhardt B. Alpha4 integrins as therapeutic targets in autoimmune disease. N Engl J Med. 2003;348(1):68–72. doi: 10.1056/NEJMe020157. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Gold BG, Kaler LJ, Yu X, Afentoulis ME, Burrows GG, Vandenbark AA, Bourdette DN, Offner H. Antigen-specific therapy promotes repair of myelin and axonal damage in established EAE. J Neurochem. 2006;98(6):1817–27. doi: 10.1111/j.1471-4159.2006.04081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang C, Mooney JL, Meza-Romero R, Chou YK, Huan J, Vandenbark AA, Offner H, Burrows GG. Recombinant TCR ligand induces early TCR signaling and a unique pattern of downstream activation. J Immunol. 2003;171(4):1934–40. doi: 10.4049/jimmunol.171.4.1934. [DOI] [PubMed] [Google Scholar]
- 47.Waxman SG. Demyelinating diseases--new pathological insights, new therapeutic targets. N Engl J Med. 1998;338(5):323–5. doi: 10.1056/NEJM199801293380610. [DOI] [PubMed] [Google Scholar]
- 48.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–52. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 49.Weiner HL, Mackin GA, Matsui M, Orav EJ, Khoury SJ, Dawson DM, Hafler DA. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science. 1993;259(5099):1321–4. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]
- 50.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76(2):263–74. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 51.Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356(6364):63–6. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]