Abstract
Background
Although clinicians accept that relapse is probable when successful acute phase pharmacotherapy is discontinued, less is known about when to stop versus continue successful cognitive therapy. This report describes the development of “translational tools” to bridge the gap between research and practice on this and similar decisions that practitioners make daily. We aim to provide patients, clinicians, and public health administrators’ practical tools to facilitate informed decisions about when to stop versus continue cognitive therapy with responders who presented with recurrent major depressive disorder (MDD).
Method
Data are drawn from a randomized clinical trial (Jarrett et al., 2001) showing that continuation-phase cognitive therapy (C-CT; Jarrett, 1989) reduced relapse more over 8 months than an assessment-only control, for responders to acute phase cognitive therapy (A-CT; Beck et al., 1979). We provide tools to translate the additional finding that, over 2 years, responders to A-CT for recurrent depression with higher residual symptoms were more likely to require C-CT to avoid relapse/recurrence than responders with lower or no residual symptoms.
Results
To measure residual symptoms we provide the specific scores from six readily available measures of depressive symptom severity taken at the last acute phase session and their associated probabilities of relapse or recurrence over 8, 12, and 24 months.
Conclusions
These tools can aid individual patient and provider make informed decisions when they decide to continue versus discontinue cognitive therapy.
Limitations
The results are limited to a 20-session trial of A-CT for recurrent depression conducted by highly experienced therapists and require replication.
Keywords: depression, cognitive therapy, continuation-phase treatment, translational research, relapse, recurrence
How Much Cognitive Therapy, for Which Patients, Will Prevent Depressive Relapse?
The aim of this translational research report is to help cognitive therapists decide how many sessions are necessary to prevent relapse in adult responders to acute-phase cognitive therapy (A-CT; Beck et al, 1979) for recurrent depression, who differ in terms of their relapse risk. It is known that A-CT can reduce depressive relapse, even when discontinued, whereas responders to acute phase pharmacotherapy more often need continuation pharmacotherapy to avoid relapse (Vittengl et al., 2007). However, little is known about how much cognitive therapy is necessary to prevent relapse and recurrence (i.e., a new episode): Reports of relapse and recurrence 1 year after A-CT range from 6.9% (Shapiro et al., 1995) to 51% (Hautzinger et al., 1996). This range may result from sampling error or heterogeneous samples with different risks for relapse, and leave practitioners wondering whether—and how much—cognitive therapy can reduce relapse and recurrence for their particular patient.
This report focuses on this question. We build on the robust finding that when acute phase responders to both cognitive therapy or pharmacotherapy have incomplete remission or residual symptoms, depressive illness is more likely to relapse or recur than if they have no or very minimal residual symptoms (Evans et al., 1992; Fava et al., 1994; Judd et al., 1998; Kanai et al., 2003; Lin et al., 1998; Paykel et al., 1995; Prien & Kupfer, 1986; Thase et al., 1992). To this prediction, we add the finding that continuation-phase cognitive therapy (C-CT) can “neutralize” these residual symptoms to lower the risk of relapse and recurrence to a level similar to that of patients who show fewer residual symptoms and a more stable pattern of response during A-CT (Jarrett et al., 2001). In the current report, we show how practitioners can use scores on any one of six readily available measures of depressive symptom severity assessed at the 20th session of A-CT to select those responders who need C-CT to help avoid relapse and recurrence over the next 8 to 24 months.
Specific Findings Requiring Translation for Use in Clinical Practice
Data to develop these clinical tools are drawn from Jarrett et al. (2001)’s randomized clinical trial of A-CT responders’ (N = 84, see definition below), comparing relapse/recurrence in C-CT to assessment-only control in an 8-month experimental phase followed by 16 months of naturalistic follow-up. Over the experimental phase, C-CT significantly reduced relapse (10%) compared to assessment-only (31%). During both the experimental phase and the full 24-months post-A-CT, treatment (C-CT vs. control) interacted with residual symptom severity, which was dichotomized as any HRSD17 score of 7 and above (high residual symptoms or “unstable remission”) versus 6 and below (low residual symptoms or “stable remission”) during the last 7 assessments (A-CT sessions 13–20 plus an independent evaluation approximately 1 week later). Residual symptoms predicted relapse/recurrence in the control group, but not in the C-CT group: Control-group patients with unstable remission relapsed/recurred sooner.
These findings can be used to identify which patients need C-CT, but require simplification to be practicable in routine clinical practice which we do by building on Vittengl et al.’s (2005) findings. Four standard measures of depressive symptoms (two self-report and two clinician-rated; described below) changed similarly over A-CT and marked the same depressive symptom construct, so they may be considered functionally interchangeable in the context of cognitive therapy for depression. Vittengl et al. (2005) offered formulas for converting among the measures based on their common factor. In this report, we (a) extend these empirical conversions to include two additional brief measures, and (b) simplify their application by showing that the prediction holds using a single assessment of residual symptoms as a continuous variable at the last A-CT session.
Method
Participants
Participants were adult outpatients presenting with DSM-IV nonpsychotic, recurrent, major depressive disorder (MDD; American Psychiatric Association, 1994), diagnosed using a supplemented Structured Clinical Interview for DSM-III-R (Spitzer et al., 1989). Inclusion criteria included clear inter-depressive episode recovery (≥ 2 months of at least nearly normal functioning) and a score ≥ 16 on the HRSD17. Exclusion criteria included concurrent medical disorders potentially accounting for depressive symptoms, organic mental disorders, psychotic disorders, active substance abuse or dependence, primary obsessive-compulsive or eating disorders, borderline personality disorder, and inability or unwillingness to complete questionnaires or to comply with the treatment protocol. Participants were recruited though media, printed announcements, and self- and practitioner referrals. They completed telephone screening (N > 3500) and diagnostic interviews (N = 608), and provided informed consent to enter the protocol (N = 156). More detail is available in Jarrett et al. (2001).
Procedure
Acute-phase cognitive therapy
A-CT (Beck et al., 1979) was conducted by five experienced therapists within a 12–14 week protocol, including 20 individual sessions (50–60 minutes) held approximately twice weekly for 8 weeks, then weekly for 4 weeks. No pharmacotherapy was provided. A-CT is designed to reduce depressive symptoms by eliciting thoughts associated with negative affect, and teaching patients to evaluate the validity of such thoughts through logical and empirical methods, to generate more realistic alternatives when negative thoughts are not supported, and to use problem-solving skills when negative conclusions are warranted. C-CT is described in Jarrett (1989) and Jarrett, et al. (in press).
Experimental Phase
The purpose of the experimental phase was to evaluate the efficacy of C-CT. Of the 87 A-CT responders who also completed the immediate post-A-CT assessment, 84 consented to randomization and were assigned to either C-CT (n = 41) or an assessment-only control condition (n = 43). No pharmacotherapy was provided. The C-CT protocol consisted of 10, 60–90 minute sessions of C-CT over 8 months (the first 4 sessions every other week, and the next 6 sessions monthly) from the same therapist who had provided A-CT. Continuation-phase CT is designed to prevent relapse and recurrence of depression through maintenance and generalization of skills learned in A-CT, to reduce residual depressive symptoms, to prepare for stressors, and to reduce modifiable vulnerabilities. In C-CT, patients are taught to use emotional distress and symptoms as cues to implement skills learned in A-CT.
Patients randomized to C-CT completed a mean of 9.5 sessions (mode = 10). Assessment-only control patients were scheduled for evaluations at the same frequency as in C-CT and completed a mean of 9.1 sessions (mode = 10). Evaluators had not provided A-CT and used no psychosocial interventions. Patients in either condition who relapsed were asked to complete all sessions and were referred for extra-protocol treatment if not receiving C-CT.
Longitudinal Follow-Up Phase
This assessment-only period lasted 16 months beyond the experimental phase, and consisted of 10 sessions scheduled monthly at months 9–12 post-A-CT and bimonthly at months 14–24 post-A-CT. Evaluators avoided psychosocial interventions and no pharmacotherapy was provided. Patients completed a mean of 8.9 assessment sessions (mode = 10). Patients with relapse/recurrence were referred for extra-protocol treatment.
Measures
Hamilton Rating Scale for Depression (HRSD17)
The HRSD17 (Hamilton, 1960) is a widely used, 17-item, clinician rating scale of depressive symptom severity. It has shown high interrater reliability (r = .85; Clark & Watson, 1991) and convergence with self-report measures (rs = .70–.83; Clark & Watson, 1991). Alpha internal consistency was .85 in the current dataset.
Beck Depression Inventory (BDI21)
The BDI21 (Beck et al., 1961) is a very widely used, 21-item, self-report measure of depressive symptom severity. Beck et al. (1988) reported an average internal consistency of .87, a mean short-term (< 1 mo.) retest reliability of .60, and strong convergence with other self-report depressive-symptoms measures and the HRSD17. Alpha internal consistency was .93 in the current dataset.
Inventory for Depressive Symptomatology
This 30-item depressive symptom severity scale (Rush et al., 1986, 1996, 2000) has both self-report (IDS-SR30) and clinician (IDS-C30) versions. Rush et al. (1986) reported internal consistencies of .85 (IDS-SR30) and .88 (IDS-C30), and good convergence with the BDI21 (IDS-SR30 r = .78; IDS-C30 r = .61) and HRSD17 (IDS-SR30 r = .67; IDS-C30 r = .92). Alpha internal consistency was .93 (IDS-SR30) and .90 (IDS-C30) in the current dataset.
Quick Inventory for Depressive Symptomatology (QIDS)
This 16-item scale is a subset of IDS items that assess the 9 DSM-IV MDD symptoms (Rush et al., 2003), with both self-report (QIDS-SR16) and clinician (QIDS-C16) versions. Rush et al. (2003) reported an internal consistency reliability of .86 (QIDS-SR16) and a correlation of .81 with the HRSD17. Trivedi et al. (2004) reported an internal consistency of .85 (QIDS-C16). Alpha internal consistency was .88 (QIDS-SR16) and .86 (QIDS-C16) in the current dataset.
Longitudinal Interval Follow-Up Evaluation (LIFE)
The LIFE (Keller et al., 1987) is semi-structured interview to rate DSM-IV Axis I psychopathology, treatment history, and psychosocial functioning retrospectively. Inter-rater reliabilities for various LIFE indices typically have been > .70 (Keller et al., 1987). Experienced, independent evaluators completed LIFE ratings at 4, 8, 12, and 24 months post-A-CT, at exit, and any time patients, therapists, or follow-up evaluators suspected relapse/recurrence of MDD. Weekly psychiatric status ratings of MDD (on a 1–6 scale; ratings ≥ 5 indicate meeting diagnostic criteria) were used to assess relapse/recurrence post-A-CT. Relapse was defined as meeting criteria for DSM-IV MDD before—and recurrence as meeting criteria after—8 consecutive months free of MDD (i.e., recovery). Relapse/recurrence events were collapsed for the current analyses.
Analyses
Previous Common Factor Conversions
Vittengl et al. (2005) analyzed 127 patients’ HRSD17, IDS-SR30, IDS-C30, and BDI21 scores from 15 assessments (two pre-treatment, weekly during A-CT, and a 1-week post-A-CT independent evaluation. These patients were selected from the 156 consenting to A-CT (Jarrett et al., 2001) because they were missing ≤ 2 of these scores. Remaining missing values were imputed using a Markov Chain Monte Carlo procedure (SAS Institute Inc., 1999; Schafer, 1997). Mean symptom severity decreased rapidly on all measures until mid-A-CT, after which gradual decreases continued through the end of A-CT. Cross-time principal axis factor analyses revealed that the major source of variation was time, with distinct “early” and “late” factors, regardless of measure or rater (patient vs. clinician). Within-time principal axis and confirmatory factor analyses using a pooled dataset (14 assessments × 127 participants = 1778 observations; second pre-treatment assessment excluded because self-report measures were not collected) identified a single depressive symptom factor with decreasing stability over longer retest intervals. Factor scores from the within-time principal axis analysis were used to convert among the four depressive symptom measures. These common factor conversions were highly consistent with results obtained with traditional bivariate regression and rank-order (percentile) methods for scale equating.
Current analyses
We report on three extensions of prior findings: (1) Extending common factor conversions to two additional scales, (2) extending the finding (using survival analysis) that C-CT interacts with “unstable remission” to predict relapse/recurrence by using only the last A-CT session (versus the last 7; Jarrett et al., 2001); and (3) computing expected probabilities of relapse/recurrence at different time points for different symptom severity levels.
Results
Extension to Two Brief Measures
In the pooled dataset (N = 1778), the QIDS-SR16 and QIDS-C16 correlated highly with their parent measures, the IDS-SR30 and IDS-C30 (r = .96 and .95, respectively) and with the common factor scores derived from the IDS-SR30, IDS-C30, HRSD17, and BDI21 (r = .94 for both). Consequently, we used the common factor to predict all six measures in regression equations and used the resulting regression constants and coefficients to estimate scores among measures through the common factor (see Table 1). For each pair of measures (e.g., HRSD17 and BDI21), we solved one symptom measure’s regression equation (e.g., HRSD17 = 6.41F + 9.95) for the common factor term (e.g., F = HRSD17/6.41 − 9.95/6.41), substituted this expression for the common factor term in the second symptom measure’s regression equation (e.g., BDI21 = 9.60F + 13.93), and simplified (e.g., BDI21 = 1.50HRSD17 −0.97). A strength of converting scales through a common factor is that “back conversions” are equivalent within rounding error. Thus, clinicians and researchers can use these equations to calculate depressive symptom severity scores on any of the six measures from any of the others.
Table 1.
Formulas for Converting Depressive Symptom Scores Among Six Scales
| Known Score | ||||||
|---|---|---|---|---|---|---|
| Estimated Score (± 1 SE) | BDI21 | HRSD17 | IDS-C30 | IDS-SR30 | QIDS-C16 | QIDS-SR16 |
| BDI21 (± 3.4) | --- | 1.50(HRSD17) − 0.97 | 0.79(IDS-C30) − 0.80 | 0.70(IDS-SR30) − 1.32 | 1.96(QIDS-C16) − 1.11 | 1.79(QIDS-SR16) − 1.56 |
| HRSD17 (± 2.0) | 0.67(BDI21)+ 0.65 | --- | 0.53(IDS-C30) + 0.11 | 0.47(IDS-SR30) − 0.23 | 1.31(QIDS-C16) − 0.09 | 1.20(QIDS-SR16) − 0.39 |
| IDS-C30 (± 2.7) | 1.26(BDI21) + 1.01 | 1.89(HRSD17) − 0.21 | --- | 0.89(IDS-SR30) − 0.65 | 2.47(QIDS-C16) − 0.39 | 2.26(QIDS-SR16) − 0.95 |
| IDS-SR30 (± 3.4) | 1.42(BDI21) + 1.87 | 2.13(HRSD17) + 0.49 | 1.13(IDS-C30) + 0.73 | --- | 2.79(QIDS-C16) + 0.29 | 2.55(QIDS-SR16) − 0.34 |
| QIDS-C16 (± 1.8) | 0.51(BDI21) + 0.57 | 0.76(HRSD17) + 0.07 | 0.40(IDS-C30) + 0.16 | 0.36(IDS-SR30) − 0.10 | --- | 0.91(QIDS-SR16) − 0.23 |
| QIDS-SR16 (± 1.9) | 0.56(BDI21) + 0.87 | 0.84(HRSD17) + 0.33 | 0.44(IDS-C30) + 0.42 | 0.39(IDS-SR30) + 0.13 | 1.09(QIDS-C16) + 0.25 | --- |
Note. Observed ranges: BDI21 = 0–51; HRSD17 = 0–30; IDS-C30 = 0–52; IDS-SR30 = 0–63; QIDS-SR16 = 0–25; QIDS-C16 = 0–21. To convert an HRSD17 score of 20 to the QIDS-SR16, for example, use the formula QIDS-SR16 = 0.84(HRSD17) + 0.33; substitute the known HRSD17 score in the equation, 0.84(20) + 0.33, and solve to yield QIDS-SR16 ≈ 17.
Survival analyses
Using Cox regression, we predicted time-to-relapse/recurrence of depression during 8 and 24 months post-A-CT from treatment group (C-CT or assessment-only), depressive symptoms at the last (20th) A-CT session, and their interaction. Using this model over the first 8 post-A-CT months (the administration of C-CT vs. assessment-only) included 16 relapse/recurrence events and was significant, Wald χ2(3) = 16.53, p < .01. The same model over 24 months (adding the 16 months of assessment-only follow-up) included 33 relapse/recurrence events and also was significant, Wald χ2(3) = 15.39, p < .01. Parameter estimates and hazard ratios for the models are shown in Table 2. Results were consistent with Jarrett et al. (2001): (1) C-CT increased time-to-relapse over 8, but not 24, months compared to assessment-only control, (2) residual symptom severity decreased time-to-relapse/recurrence over 24 months, and (3) residual symptom severity was a stronger risk factor for relapse/recurrence in the assessment-only group compared to C-CT over 24 months.
Table 2.
Cox Regression Predicting Time to Relapse/Recurrence Post-Acute Phase Cognitive Therapy
| Predictor | Δ Model χ2 | Beta | SE | Hazard Ratio |
|---|---|---|---|---|
| During 8 Months (16 Relapse/Recurrence Events) | ||||
| Treatment | 4.61* | − 2.52 | 1.21 | 0.08 |
| Depressive Symptom Severity | 7.18* | 1.03 | 0.60 | 2.81 |
| Treatment × Severity Interaction | 4.74* | − 1.41 | 1.20 | 0.24 |
| During 24 Months (33 Relapse/Recurrence Events) | ||||
| Treatment | 1.82 | − 1.56 | 0.70 | 0.21 |
| Depressive Symptom Severity | 8.36* | 1.04 | 0.36 | 2.82 |
| Treatment × Severity Interaction | 5.21* | − 1.11 | 0.71 | 0.33 |
Note. N = 84. Treatment coded continuation-phase cognitive therapy = 0.5, assessment-only = − 0.5. Depressive symptom severity is a factor z-score from the last (20th) acute phase cognitive therapy session. Δ Model χ2 statistics are increments to omnibus Wald statistics with df = 1 and are derived from forward entry of treatment, symptom severity, and their interaction. Beta, SE, and Hazard Ratio statistics are from the final models including all three predictors.
p < .05.
Prediction of relapse/recurrence
We computed expected probabilities of relapse/recurrence (1 – survival probabilities) at 3 time points (8, 12, and 24 mos. post-A-CT) for different levels of depressive symptom severity at the final A-CT session. As shown in Table 3 and Figure 1, higher residual depressive symptoms predict greater chance of relapse/recurrence for C-CT and assessment-only conditions at each time point, although expected probabilities of relapse/recurrence vary widely (range .09–.97), and the incremental risk of relapse/recurrence is higher for the control group. Based on the standard errors of estimate, we compared relapse/recurrence estimates for C-CT and assessment-only through a range of residual symptom levels. At 8 months post-A-CT (end of the experimental phase), C-CT and assessment-only were equivalent for participants with low residual symptoms (e.g., HRSD17 ≤ 3), whereas patients with moderate residual symptoms (e.g., HRSD17≥ 4) were predicted to benefit from C-CT. A similar pattern was evident at 12 and 24 months post-A-CT, although differentiation of C-CT versus assessment-only occurred at slightly higher levels of residual symptoms (e.g., HRSD17 ≥ 5).
Table 3.
Expected Probability of Relapse/Recurrence (SE) Post A-CT Based on Depressive Symptom Level at Final A-CT Session
| Factor z Score and Scale Approximations | During Treatment Time | After Treatment Time | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IDS- | QIDS- | Within 8 Months | Within 12 Months | Within 24 Months | ||||||||
| z | HRSD17 | BDI21 | C30 | SR30 | C16 | SR16 | Control | C-CT | Control | C-CT | Control | C-CT |
| −1.5 | 0 | 0 | 0 | 1 | 0 | 1 | .13 (.06) | .09 (.06) | .18 (.07) | .19 (.07) | .27 (.09) | .30 (.10) |
| −1.4 | 1 | 0 | 2 | 3 | 1 | 1 | .15 (.07) | .09 (.06) | .20 (.07) | .20 (.07) | .31 (.09) | .31 (.10) |
| −1.3 | 2 | 1 | 3 | 4 | 1 | 2 | .18 (.07) | .09 (.06) | .23 (.07) | .21 (.07) | .36 (.09) | .32 (.09) |
| −1.2 | 2 | 2 | 4 | 5 | 2 | 2 | .20 (.07) | .10 (.05) | .27 (.07) | .22 (.06) | .40 (.09) | .34 (.08) |
| −1.1 | 3 | 3 | 5 | 7 | 2 | 3 | .24 (.07)
|
.10 (.05)
|
.30 (.07) | .23 (.06) | .45 (.09) | .35 (.08) |
| −1.0 | 4 | 4 | 6 | 8 | 3 | 3 | .28 (.07) | .10 (.05) | .35 (.08) | .24 (.06) | .51 (.09) | .36 (.08) |
| −0.9 | 4 | 5 | 8 | 9 | 3 | 4 | .32 (.08) | .11 (.05) | .39 (.08)
|
.25 (.06)
|
.57 (.08)
|
.38 (.08)
|
| −0.8 | 5 | 6 | 9 | 11 | 4 | 4 | .37 (.08) | .11 (.05) | .44 (.08) | .26 (.06) | .62 (.09) | .39 (.08) |
| −0.7 | 5 | 7 | 10 | 12 | 4 | 5 | .42 (.09) | .11 (.06) | .50 (.09) | .27 (.07) | .68 (.09) | .41 (.09) |
| −0.6 | 6 | 8 | 11 | 13 | 5 | 5 | .48 (.10) | .12 (.06) | .55 (.10) | .28 (.08) | .74 (.09) | .42 (.10) |
| −0.5 | 7 | 9 | 13 | 15 | 5 | 6 | .54 (.11) | .12 (.07) | .61 (.11) | .29 (.09) | .79 (.09) | .44 (.11) |
| −0.4 | 7 | 10 | 14 | 16 | 6 | 7 | .60 (.12) | .12 (.08) | .67 (.12) | .30 (.10) | .84 (.09) | .45 (.12) |
| −0.3 | 8 | 11 | 15 | 18 | 6 | 7 | .67 (.14) | .13 (.09) | .73 (.12) | .32 (.11) | .88 (.08) | .47 (.14) |
| −0.2 | 9 | 12 | 16 | 19 | 7 | 8 | .73 (.14) | .13 (.11) | .78 (.13) | .33 (.12) | .92 (.08) | .49 (.15) |
| −0.1 | 9 | 13 | 17 | 20 | 7 | 8 | .79 (.15) | .14 (.12) | .83 (.13) | .34 (.14) | .95 (.06) | .50 (.17) |
| 0.0 | 10 | 14 | 19 | 22 | 8 | 9 | .84 (.14) | .14 (.14) | .88 (.12) | .36 (.15) | .97 (.05) | .52 (.19) |
Note. N = 84. Factor z M = − 1.0, SD = 0.4, range − 1.5–0.6; the displayed range −1.5–0.0 encompasses 96% (81/84) of cases included in the models. A- and C-CT = acute and continuation-phase cognitive therapy, respectively. Estimated probabilities based on Cox regression analyses predicting time-to-relapse/recurrence (from C-CT, depressive symptom severity at last A-CT session, and their interaction) over 8 months and over 24 months (for 12 and 24 month estimates). C-CT vs. control estimates in plain font p > .10, italics p ≤ .10, bold p ≤ .05, 2-tailed.
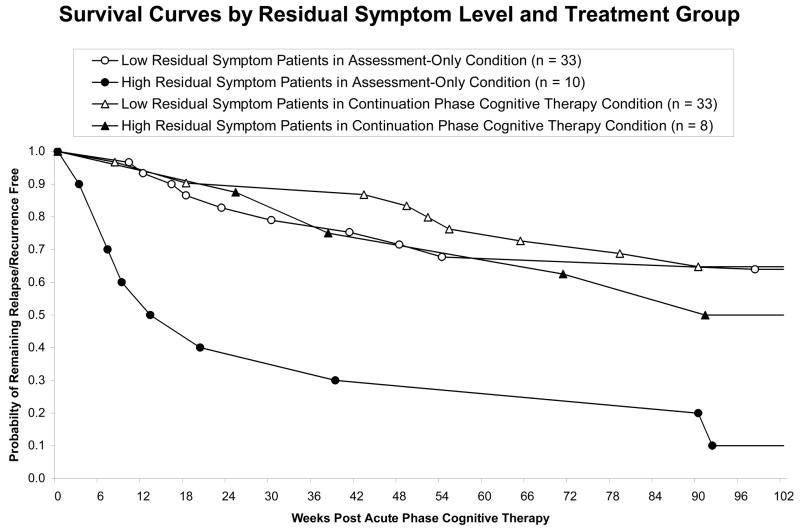
Figure 1.
Patients with higher (factor z > −0.7; see Table 3) compared to lower (factor z ≤ −0.7) depressive symptoms scores at the last acute phase cognitive therapy session relapse/recur less quickly with continuation-phase cognitive therapy vs. assessment-only.
Discussion
This report translates findings from a randomized clinical trial into tools and initial guidelines to inform empirical clinical practice on how to best prevent relapse and recurrence after adults with recurrent MDD respond to A-CT. We focus on residual symptom level as the predictor of relapse/recurrence because it has been shown to be a robust predictor of relapse and recurrence, is easily measured by clinicians, and behaves distinctly given the presence or absence of C-CT. However, there are other predictors of relapse and recurrence (e.g., age of onset [Jarrett et al., 2001]) and cognitive reactivity [Segal, 2006]) for which similar guidelines could be developed. Such development will have the positive effect of increasing our ability to predict relapse/recurrence, but can also complicate prediction.
Using Cox regression models, we provide, in Table 3, probabilities of relapse/recurrence at 8, 12, and 24 months post-A-CT with and without adjunctive C-CT over a range of residual symptom levels measured at the final acute phase session. Using this table, clinicians and researchers can estimate relapse/recurrence probabilities for individual patients based on scores: (a) reflecting residual symptoms from any of six measures at the final A-CT therapy session and (b) associated with the decision to stop or continue treatment.
Based on our estimates, patients with low residual symptom levels (e.g., BDI21 0–3) at the last A-CT session can expect relatively lower, non-trivial probabilities of relapse and recurrence over 8–24 months (e.g., 13–45% and are less likely to benefit from continued cognitive therapy. Conversely, patients with moderate residual symptoms, although still within the “normal” range (e.g., BDI21 scores 4–9), have relatively higher chances of relapse/recurrence (e.g., 28–97%) and are more likely to benefit from C-CT. Although C-CT does not prevent all relapses or recurrences in these high-risk patients, the clinical conclusion is clear—patients with higher residual symptoms after A-CT relapse and recur less and later with C-CT, whereas patients with lower residual symptoms, absent other risk factors, may not require C-CT. However, our conversion formulae are estimates based on one sample of patients with recurrent depression. The need to cross-validate our formulae, and especially cut-points for clinical decisions, reinforces the value of multi-measure symptom assessment in clinical trials. Future studies may show how distinct risk factors interact with each other as well as with treatment.
This guideline is based on a factor-analytic estimate of depressive symptom severity that allows translation among several functionally interchangeable, popular measures. With this conversion method, clinicians and researchers can consider information from classic (i.e., BDI21, HRSD17) as well as newer long- (IDS) and short-form (QIDS) self- and clinician-report measures. Thus, these conversion formulae are tools that clinicians and researchers can use to “translate” and compare findings across studies and clinical practice settings. We note that the measures differ in length and cost; three (i.e., BDI21, IDS-SR30, QIDS-SR16) are patient rather than clinician administrated, five (i.e., HRSD17, all versions of the IDS) are in the public domain.
The extent to which these guidelines generalize beyond adult outpatients with recurrent major depression who respond to a 20-session course of acute phase cognitive therapy delivered by highly competent cognitive therapists is unknown. Extending these guidelines to first-episode depression, therapists with variable levels of competence, or shorter courses of A-CT deserves evaluation. Moreover, the current results focus on relapse prevention and thus apply only to patients who respond to A-CT; as many as 40–50% of similar patients may not respond to or complete A-CT (Jarrett et al., 2001; DeRubeis et al., 2005). Non-responders might be switched to another treatment and, following a response; the impact of C-CT on them is unknown. Similarly, whether and how these guidelines apply also to discontinuation of C-CT is unknown.
Because some A-CT responders (e.g., about half with depression factor z scores ≤ −1.1; see Table 3) do not require C-CT, we emphasize the importance of this initial guideline to identify them for potential savings of patient and clinician time and public health costs. The results are preliminary, limited, and in need of replication on larger samples, and other factors must be weighed in deciding whether to continue treatment, including patient motivation and costs of continued treatment. Nevertheless these results provide empirical estimates to inform this treatment decision and illustrate the utility of developing practical, translational tools.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Robin B. Jarrett, Department of Psychiatry, The University of Texas Southwestern Medical Center at Dallas
Jeffrey R. Vittengl, Department of Psychology, Truman State University
Lee Anna Clark, Department of Psychology, The University of Iowa.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford Press; 1979. [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- Beck A, Ward C, Mendelsohn M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. J Abnorm Psychol. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, et al. Cognitive therapy vs. medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62:409–416. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- Evans MD, Hollon SD, DeRubeis RJ, Piasecki JM, Grove WM, Garvey MJ, Tuason VB. Differential relapse following cognitive therapy and pharmacotherapy for depression. Arch Gen Psychiatry. 1992;49:802–808. doi: 10.1001/archpsyc.1992.01820100046009. [DOI] [PubMed] [Google Scholar]
- Fava GA, Grandi S, Zielezny M, Canestrari R, Morphy MA. Cognitive behavioral treatment of residual symptoms in primary major depressive disorder. Am J Psychiatry. 1994;151:1295–1299. doi: 10.1176/ajp.151.9.1295. [DOI] [PubMed] [Google Scholar]
- Hautzinger M, de Jong-Meyer R, Treiber R, Rudolf GA, Thien U. The efficacy of cognitive behavior therapy and pharmacotherapy, alone or in combination, in nonendogenous unipolar depression. Zeitschrift für Klinische Psychologie. Forschung und Praxis. 1996;25:130–145. [Google Scholar]
- Jarrett RB. Cognitive therapy for recurrent unipolar depressive disorder: The continuation/maintenance phase. 1989 Unpublished manuscript. [Google Scholar]
- Jarrett RB, Basco MR, Risser R, Ramanan J, Marwill M, Kraft D, et al. Is there a role for continuation phase cognitive therapy for depressed outpatients? J Consult Clin Psychol. 1998;66:1036–1040. doi: 10.1037//0022-006x.66.6.1036. [DOI] [PubMed] [Google Scholar]
- Jarrett RB, Kraft D, Doyle J, Foster BM, Eaves GG, Silver PC. Preventing recurrent depression using cognitive therapy with and without a continuation phase: A randomized clinical trial. Arch Gen Psychiatry. 2001;58:381–388. doi: 10.1001/archpsyc.58.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Vittengl J, Clark LA. Preventing Recurrent depression. In: Whisman MA, editor. Cognitive Therapy for Complex and Comorbid Depression: Assessment and Treatment. New York: Guilford Publications; in press. [Google Scholar]
- Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, Mueller TI, Rice JA, Keller MB. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50:97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- Kanai T, Takeuchi H, Furukawa TA, Yoshimura R, Imaizumi T, Kitamura R, et al. Time to recurrence after recovery from major depressive episodes and its predictors. Psychol Med. 2003;33:839–845. doi: 10.1017/s0033291703007827. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, et al. The longitudinal interval follow-up evaluation: A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Lin EH, Katon WJ, VonKorff M, Russo JE, Simon GE, Bush TM, et al. Relapse of depression in primary care. Rate and clinical predictors. Archives of Family Medicine. 1998;7:443–449. doi: 10.1001/archfami.7.5.443. [DOI] [PubMed] [Google Scholar]
- Paykel ES, Ramana R, Cooper Z, Hayhurst J, Kerr J, Barocka A. Residual symptoms after partial remission: An important outcome in depression. Psychol Med. 1995;25:1171–1180. doi: 10.1017/s0033291700033146. [DOI] [PubMed] [Google Scholar]
- Prien RF, Kupfer DJ. Continuation drug therapy for major depressive episodes: How long should it be maintained? Am J Psychiatry. 1986;143:18–23. doi: 10.1176/ajp.143.1.18. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Carmody T, Reimitz PE. The Inventory of Depressive Symptomatology (IDS): Clinician (IDS-C30) and self-report (IDS-SR30) ratings of depressive symptoms. Int J Methods Psychiatr Res. 2000;9:45–59. [Google Scholar]
- Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger JE, Burns CT. The Inventory for Depressive Symptomatology (IDS): Preliminary findings. Psychiatry Res. 1986;18:65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): Psychometric Properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT user’s guide (version 8) Cary, NC: SAS Institute Inc; 1999. [Google Scholar]
- Schafer JL. Analysis of incomplete multivariate data. New York: Chapman & Hall; 1997. [Google Scholar]
- Segal ZV, Kennedy S, Gemar M, Hood K, Pedersen R, Buis T. Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Arch Gen Psychiatry. 2006;63:749–755. doi: 10.1001/archpsyc.63.7.749. [DOI] [PubMed] [Google Scholar]
- Shapiro DA, Rees A, Barkham M, Hardy G, Reynolds S, Startup M. Effects of treatment duration and severity of depression on the maintenance of gains after cognitive-behavioral and psychodynamic-interpersonal psychotherapy. J Consult Clin Psychol. 1995;63:378–387. doi: 10.1037//0022-006x.63.3.378. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R-Outpatient Version (with Psychotic Screen) New York: New York State Psychiatric Institute; 1989. [Google Scholar]
- Thase ME, Simons AD, McGeary J, Cahalane JF, Hughes C, Harden T, Friedman E. Relapse after cognitive behavior therapy of depression: Potential implications for longer courses of treatment. Am J Psychiatry. 1992;149:1046–1052. doi: 10.1176/ajp.149.8.1046. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, Crismon ML, Shores-Wilson K, Toprac MG, Dennehy EB, Witte B, Kashner TM. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C30) and Self-Report (IDS-SR30), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C16) and Self-Report (QIDS-SR16) in public sector patients with mood disorders: A psychometric evaluation. Psychol Med. 2004;34:73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Kraft D, Jarrett RB. Multiple measures, methods, and moments: A factor-analytic investigation of change in depressive symptoms during acute phase cognitive therapy. Psychol Med. 2005;35:693–704. doi: 10.1017/s0033291704004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Dunn TW, Jarrett RB. Reducing relapse and recurrence in unipolar depression: a comparative meta-analysis of cognitive therapy’s effects. J Consult Clin Psychol. 2007;75:475–488. doi: 10.1037/0022-006X.75.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]