Abstract
Three families of ligand-activated ion channels mediate synaptic communication between excitable cells in mammals. For pentameric channels related to nicotinic acetylcholine receptors and tetrameric channels like glutamate receptors, the pore-forming and gate regions have been studied extensively. In contrast, little is known about the structure of trimeric P2X receptor channels, a family of channels that are activated by ATP and serve crucial roles in neuronal signaling, pain transmission and inflammation. To identify the pore-forming and gate regions within P2X receptor channels, we introduced cysteine residues throughout the two transmembrane (TM) segments and studied their accessibility to thiol reactive compounds and ions. Our results show that the TM2 helix lines the central ion conduction pore, that the TM1 helix is positioned peripheral to TM2, and that the flow of ions is minimized in the closed state by a gate formed by the external region of TM2.
In mammals there are seven subtypes of P2X receptor channels (termed P2X1-7) that are widely expressed throughout the nervous system and many other tissues, including muscle and epithelia1, 2. From a structural perspective P2X receptor channels are intriguing because they are formed by three identical or related subunits 3-7, each having a large extracellular segment of ∼ 280 amino acids that forms the ATP binding domain 8, with two flanking transmembrane helices (TM1 and TM2) 9, 10 spanning the membrane and leaving the N and C termini on the intracellular side (Fig. 1). Our objective was to determine the relative contribution of the TMs to forming the ion conduction pore in P2X receptor channels and to localize the gate region, two fundamentally important questions that remain unresolved 1. In the present study we set out to answer these questions by introducing cysteine (Cys) residues throughout both TMs of the P2X2 receptor channel, an isoform that desensitizes slowly after activation by ATP 2, and measuring the apparent rate of chemical modification with thiol reactive compounds and ions applied to the external side of the membrane. For this approach to be informative for our purposes, the thiol reactive compounds and ions should 1) not modify the channel unless a Cys is introduced, 2) modify with rates approaching those observed in aqueous solution when the channel is open, and 3) modify an introduced Cys below the gate with dramatically slower rates when the channel is closed 11-13. These requirements were not achieved in previous Cys accessibility studies on P2X receptors 14-17, leaving the pore-forming and gate regions unclear 1. Although modification of residues in both TM1 and TM2 was observed, no rapid modification rates were obtained, leaving open the possibility that reagents access introduced Cys residues through non-aqueous pathways (e.g. protein or lipid) and/or react with rare conformations of the channel. Furthermore, contradictory conclusions were put forward as to the secondary structure of TM2 and the location of the gate. Egan and colleagues proposed that TM2 forms an outward facing loop with I328 and L334 positioned external and internal to the gate, respectively, with G342 at the apex of the loop and contributing to the gate 15. In contrast, Rassendren and coworkers assumed TM2 is helical and suggested that the gate is positioned deep within the TM, between L338 and D349 16.
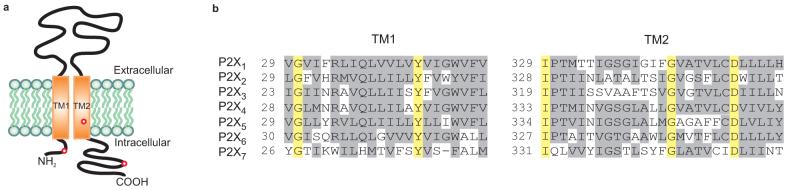
Figure 1.
a, Schematic representation of the general topology of a P2X receptor channel subunit. Three endogenous Cys residues in TM2 and two termini were mutated to Thr as indicated by red circles. b, Sequence alignment of the two putative TMs of rat P2X1–P2X7. Identical residues are highlighted in yellow and similar residues in gray.
RESULTS
To generate an appropriate background for our studies we mutated three native Cys residues to threonine (one in TM2 and one in each of the two termini; Fig. 1; red circles), leaving the ten conserved Cys in the extracellular domain because they are thought to be involved in disulphide bonds that are important for channel function 18, 19. This P2X2-3T channel is robustly activated by ATP when expressed in HEK293 cells (Fig 2a; Supplementary Fig. 1a), desensitizes slowly in the presence of ATP (Fig 2a), and is insensitive to high concentrations of 2-trimethylaminoethyl methanethiosulfonate (MTSET; 1 mM), Ag+ (67 nM) and Cd2+ (20 μM) in either the absence or presence of ATP (Fig. 2a; 3a; 4a). Collectively, these properties of the P2X2-3T construct make it an ideal background for studying the reactivity of subsequently introduced Cys residues with thiol reactive compounds and ions.
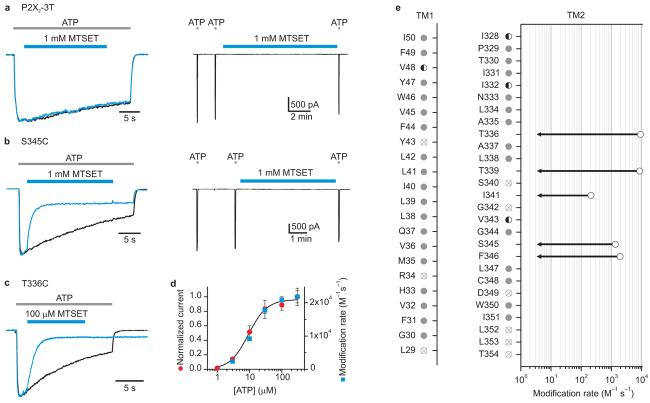
Figure 2. State-dependent accessibility of extracellular MTSET in P2X2 receptor channels.
a, P2X2-3T is insensitive to 1 mM MTSET in both the absence and presence of ATP. Left panel shows superimposed scaled current traces in response to ATP without (black) and with (blue) the application of MTSET (indicated by bar above the current trace). The two current traces were recorded at a 2 min interval from the same cell. Right panel shows a continuous current trace where application of MTSET for 10 min in the absence of ATP has no detectable effect on the current activated by ATP after correcting for rundown. Rundown was assessed using two applications of ATP (2 s duration; 90 s interval) prior to MTSET addition. b, Superimposed scaled current traces for S345C demonstrating modification by MTSET in the presence of ATP (left panel). No modification is observed after applying 1 mM MTSET for 300 s in the absence of ATP (right panel). We conservatively place an upper limit for the modification rate in the absence of ATP at < 3 M−1s−1., the value that would be obtained if the time constant for modification was 300 s at 1 mM MTSET. c, Superimposed scaled current traces for T336C demonstrating that MTSET inhibits the current in the presence of ATP and slows channel closing. d, Plot of MTSET modification rate at T336C (blue squares) or macroscopic current activation (red circles) as a function of ATP concentration. The solid line is a fit of a Hill equation to the concentration-dependence for activation by ATP. Each symbol is the mean of four experiments and error bars are S.E.M.. e, Summary of the effects of MTSET on Cys substitutions in TM1 and TM2 and the corresponding modification rates. Most mutants were not modified by MTSET (gray circles). Modification rates for T336C, T339C, I341C, S345C, and F346C obtained in the presence of ATP are shown as open black circles. Each point is the mean value of four to seven experiments with error bars (S.E.M.) that are smaller than the symbols. In each of these cases the modification rates in the absence of ATP are below that which we can detect (∼3 M−1s−1). Modification rates were not calculated for V48C, I328C, I332C, and V343C (half filled circles) due to the complexity of the response to MTSET (see Supplementary Fig 3). Reactivity with MTSET could not be examined for some mutants (crossed out circles) because they show minimal responses to ATP (L29C, R34C, Y43C, S340C and D349C) or desensitize too fast to study (G342C, L352C, L353C and T354C). Disulfide bond formation in these Cys mutants could not be detected, as the application of 5 mM DTT for 10 min has no detectable effect. An EC50 concentration of ATP was used for each mutant (see Supplementary Fig 1; Table 1), except for T336C where a range of ATP concentrations were examined in d.
Figure 3. State-dependent accessibility of extracellular Ag+ in P2X2 receptor channels.
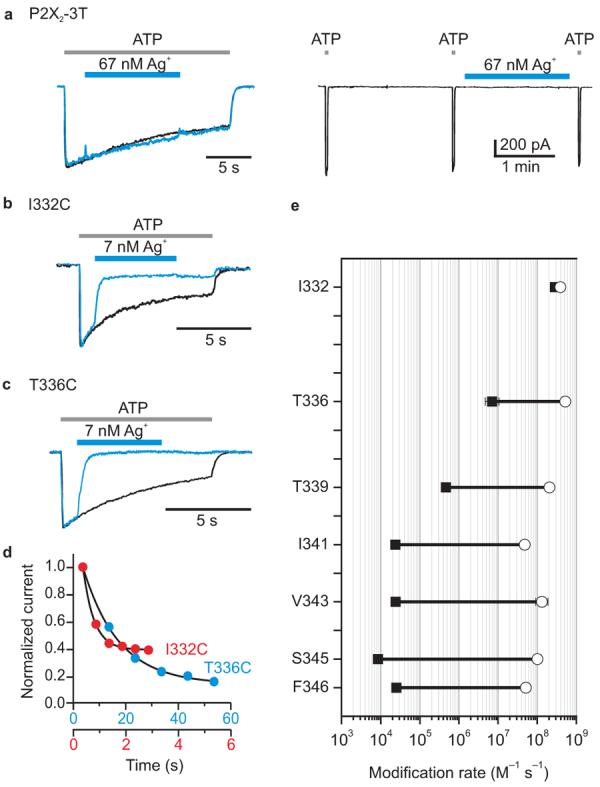
a, P2X2-3T is insensitive to Ag+ in both the absence and presence of ATP. Same experimental paradigm as in Fig. 1a. b, c, Superimposed scaled current traces for I332C and T336C showing that these channels are rapidly modified by 7 nM Ag+ in the presence of ATP. d, Time course for modification of I332C and T336C in the absence of ATP. Red and blue circles indicate the normalized currents in response to 2 s application of ATP. Ag+ was applied for 2 s (I332C) or 20 s (T336C) in the absence of ATP and washed out before each ATP application to assay channel activity. Note the different time scales for the two mutants. e, Summary of Ag+ modification rates for seven residues in TM2 in the absence (black filled squares) or presence (open circles) of an EC50 concentration of ATP. Each point is the mean value from four to seven experiments and error bars are S.E.M. (in most cases these are smaller than the symbols). Fractional inhibition values for Ag+ in the presence of ATP are as follows: 78±7% (I332C), 91±4% (T336C), 93±3% (T339C), 68±5% (I341C), 94±3% (V343C), 82±3% (S345C) and 83±7% (F346C).
Figure 4. Stable coordination of Cd2+ at T336C.
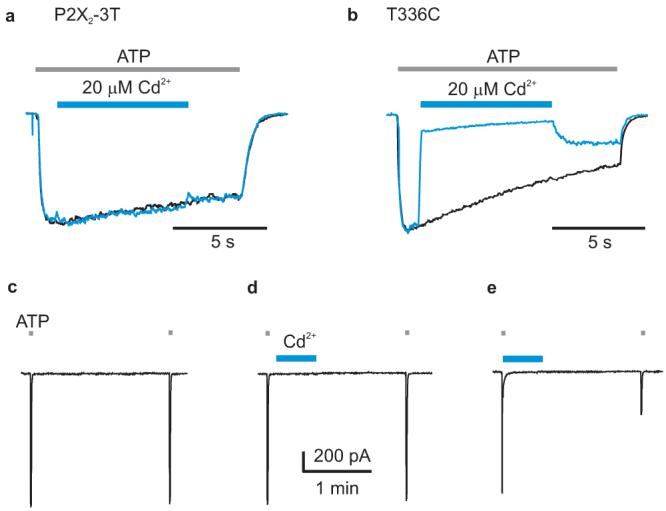
a, P2X2-3T is insensitive to 20 μM Cd2+. Same experimental paradigm as in Fig. 1a. b, Superimposed scaled current traces for T336C showing that the current is rapidly inhibited by 20 μM Cd2+ in the presence of ATP. Only a fraction of the inhibitory effect reverses rapidly. c, Consecutive applications of ATP to T336C without Cd2+ application. d, Consecutive applications of ATP to T336C where 20 μM Cd2+ was applied for 30 s in the absence of ATP. e, Consecutive applications of ATP to T336C where Cd2+ was applied in the presence of ATP. Current records for c-e are from the same cell.
Reactivity of introduced Cys residues with MTSET
We introduced Cys residues throughout the two TMs on the P2X2-3T background and studied their activation by ATP (Supplementary Fig. 1b). Of the 49 mutants, 44 respond robustly to ATP, but four of these desensitize too rapidly to be studied further (Fig 2e; Supplementary Fig 1b). For the remaining 40 Cys mutants we initially tested their accessibility to positively charged MTSET from the external side of the membrane, either in the absence or presence of an EC50 concentration of ATP. For these experiments we infer that the engineered Cys reacts with MTSET if the reagent irreversibly alters the ATP-induced current and that no reaction occurs if the reagent is without effect, a reasonable assumption because mutations throughout TM1 and TM2 have pronounced effects on activation of the channel by ATP. 9, 10, 20 In TM1, only one mutant (V48C) displays accessibility to MTSET, which modifies the residue in either the absence or presence of ATP, resulting in a reduction in current and a dramatic slowing of channel closing (Supplementary Fig 2a)17. The apparent modification rate at V48C is rather modest (∼700 M−1 s−1 in the presence of ATP), implying that the open probability of the mutant is low or that this residue is not readily accessible to MTSET in both open and closed states. The most significant aspect of the results with TM1 is that the dearth of reactive positions strongly suggests that TM1 is not the central pore lining helix.
In contrast to the results with TM1, there are 8 positions within TM2 where Cys substitutions display pronounced responses to MTSET (Fig 2b,c,e; Supplementary Fig 2b,c,d), including I328C, I332C, T336C, T339C, I341C, V343C, S345C and F346C. At positions I328C and I332C towards the external end of TM2, MTSET activates the channel in the absence of ATP, but either inhibits (I328C) or activates (I332C) the channel when applied in the presence of ATP (Supplementary Fig 2b,c). Although the phenomena observed at these two positions are interesting, they do not directly implicate a specific region in forming the pore or the gate. In contrast, the results for deeper positions in TM2 are quite informative because the apparent rates of MTSET modification in the presence of ATP can be quite rapid, and the modification rates drop dramatically in the absence of ATP (Fig 2b,c,e). At position T336C for example, the MTSET modification rate is 9200 M−1s−1 in the presence of an EC50 concentration of ATP, but < 3 M−1s−1 in the absence of ATP (Fig 2c,e). The rate of MTSET modification of T336C closely follows the dose-response curve for ATP activation of the channel (Fig 2d), suggesting that the large change in modification rate is related to channel gating, reaching a maximal value of 22,000 M−1s−1 at saturating ATP concentrations. The modification rate seen for MTSET with T336C in the presence of high ATP concentrations is close to the rate for MTSET reaction with free mercaptoethanol in solution (∼90,000 M−1s−1)21 or that observed within the internal gate region of the Shaker Kv channel 12, 13, in particular when one considers that the maximal open probability of T336C mutant channels could be considerably less than unity. In this case, our estimate of modification rate for the open state (in the presence of ATP) would be an underestimate, which may also explain the somewhat slower modification rates observed for positions I341C, S345C and F346C. Nevertheless, the rates observed for both T336C and T339C are rapid enough to conclude that these residues must be exposed to aqueous solution in the open state and thus that this region of TM2 forms a water-filled pore for MTSET to reach the introduced Cys. The strong gated access of MTSET to T336C and below suggests that a gate for this reagent is located within the external region of TM2.
Another noteworthy feature of MTSET modification is that the reagent can cause a dramatic slowing of channel closing after removal of ATP, in particular for V48C in TM1 (Supplementary Fig 2a) and T336C in TM2 (Fig 2c). These ‘foot in the door’ effects are similar to those reported for quaternary ammonium blockers of the internal pore of Kv channels 22-24, providing direct evidence that the outer regions of TM1 and TM2 move during opening and closing 9, 10, 15, 16, 20, 25.
Reactivity of introduced Cys residues with Ag+
The results with MTSET provide strong evidence that the external region of TM2 forms the external pore of P2X receptor channels and define pore-lining positions where the access of MTSET varies dramatically between closed and open states. Although these results suggest that there is a gate for MTSET in the external region of TM2, they do not directly implicate this region in forming a gate for the smaller ions that permeate P2X receptors under physiological conditions. In cyclic nucleotide-gated (CNG) channels, for example, the gate for moderately large MTS reagents and small ions appear to be in different regions of the pore 26, 27. To explore whether the gate that governs access of MTSET also forms a barrier to small ions, we investigated the gated access of Ag+, a small thiol reactive ion that has dimensions and diffusion properties that are similar to the alkali metal cations that permeate P2X receptor channels 13, 28. We examined Cys substitutions at seven positions in TM2, and in each case find that extracellular application of Ag+ causes irreversible inhibition in either the absence or presence of an EC50 concentration of ATP (Fig. 3b-e). The modification rates obtained in the presence of ATP are very rapid, ranging from 5×107 to 5×108 M−1 s−1, comparable to that seen for the intracellular gate region of the Shaker Kv channel 13. The ten-fold range of modification rates observed in the presence of ATP for these seven TM2 positions could result from differences in maximal open probability between the mutants, but in each case the observed modification rate is sufficiently rapid to conclude that all seven residues likely line an aqueous ion conduction pathway in the open state. As observed in experiments with MTSET, at position T336C and below we observe gated access of Ag+ to introduced Cys residues, with rates that differ by 75 (T336C) to 104 (S345C) fold in the absence and presence of ATP (Fig. 3e), suggesting that the access of small ions to the pore of P2X receptors is also controlled by a gate located in the external region of TM2. Interestingly, the reaction between Ag+ and I332C was very rapid in either the absence or presence of ATP (Fig. 3b,d,e), suggesting that the gate is positioned below this position.
Coordination of Cd2+ by T336C
The results thus far demonstrate that charged thiol reactive reagents like MTSET and Ag+ can access Cys residues in TM2 through an aqueous pathway at very high rates. If the reactive Cys is located at the central axis of the pore, it might be close enough to the equivalent Cys in the other subunits to stably coordinate Cd2+, a metal that seems to require at least three Cys residues for tight binding 12. We therefore investigated whether Cd2+ could be stably coordinated by Cys substituted at position T336, the most external position exhibiting rapid state-dependent modification rates in the presence of ATP. Although extracellular application of Cd2+ to T336C in the absence of ATP is without significant effect (Fig 4c,d), application of Cd2+ in the presence of ATP produces pronounced inhibition of the channel and a substantial fraction of the inhibitory effect does not recover 2 min after removal of the divalent ion (Fig 4b,e). These results indicate that Cd2+ can be stably coordinated in T336C channels and suggest that this region of TM2 lines a centrally located ion conduction pore.
DISCUSSION
Collectively, our results showing rapid and strongly state-dependent modification rates for Cys residues introduced into TM regions of P2X receptor channels have three important implications for the structure of the pore and the location of the gate. First, they demonstrate that a central ion conduction pathway in these trimeric channels is predominantly formed by the TM2 helix (Fig 5a). TM1 may also make significant contributions, in particular if TM1 and TM2 span the membrane at different angles, but the paucity of MTSET reactivity with this helix suggests that it is positioned more peripherally than TM2 (Fig 2e). It is interesting that this structural feature of P2X receptor channels is also found in the trimeric acid-sensing ion channels (ASIC) 29, suggesting that the structures of these channels may be similar even though their amino acid sequences are unrelated. As such, our results may have implications for gating of the ASIC family of channels.
Figure 5. Architecture of the pore of P2X receptor channels.
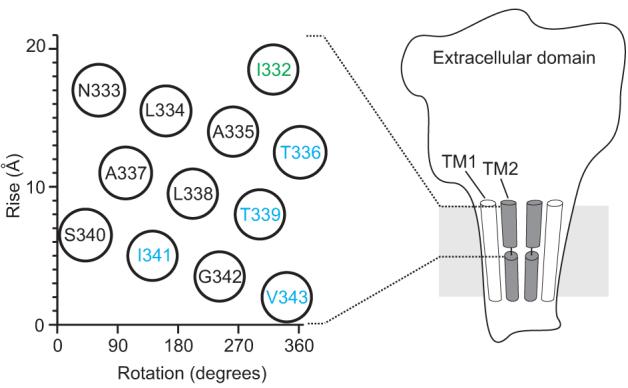
Illustration of the architecture of P2X receptor channels showing TM2 lining the pore. Only two of the three subunits are shown for clarity. The region housing the gate within the extracellular half of TM2 (I332 to V343) is shown on a helical net diagram to the left. I332 (green) reacts with Ag+ at similar rates in the absence and presence of ATP, whereas blue residues show marked gated accessibility to Ag+ and MTSET.
Second, our results suggest that access of ions to the pore is controlled by a gate located within the external half of the TM2 helix. No state-dependent changes in Ag+ modification rates can be seen towards the external end of TM2 at position I332C, whereas the state-dependent changes in Ag+ modification rates are consistently large for all positions at or below I341C, implicating residues between these two extremes in forming the barrier to ion permeation in the closed state. Although TM2 has been proposed to adopt a non-helical structure 15, both mutagenesis 9 and the present modification results (Figs 2e, 3e) suggest that the outer half of TM2 is helical (Fig 5b). The 3 helical turns of TM2 that likely house the gate is sprinkled with aliphatic residues in all P2X receptors, which could form a hydrophobic plug when the channel is closed (Fig 1b, 5b). It is interesting that the rate of Ag+ modification of residues in TM2 in the closed state become progressively slower between I332C and I341C (Fig 3e), raising the possibility that ions experience a growing barrier to movement through the external region of the pore. One interpretation of this intriguing pattern is that the diameter of the pore narrows over an extended region in the closed state, which differs somewhat from the more localized bundle crossing seen for the internal gate region of potassium channels 12, 30. An extended barrier could also explain the very large state-dependent changes in Ag+ reaction rates that we see for positions near the middle of TM2 (∼104), which are considerably larger than have been observed for the internal gate in potassium channels 13.
Finally, it is very interesting that mutations in the region of TM2 that we define as lining the pore in the open state and forming the gate also alter the permeability of P2X receptor channels for divalent ions 31, 32, raising the intriguing possibility that the gate region itself also functions as a selectivity filter of sorts. The selectivity filters of cyclic nucleotide-gated channels and several types of potassium channels may form a gate for either channel activation or inactivation 26, 27, 33, although in those instances the selectivity filter is formed by a reentrant pore helix and turn 34 that is structural very distinct from the present picture for P2X receptor channels where the end of a TM helix influences ion selectivity in the open state and prevents ion conduction in the closed state.
METHODS
Channel constructs
Rat P2X235 cDNA in pcDNA1 was generously provided by Dr. David Julius (University of California, San Francisco). To remove three native Cys residues, Cys9, Cys348 and Cys430 were individually mutated to Ala, Ser, Thr and Val. In all three cases, substitution with Thr resulted in channels that resemble the Wt channel with respect to expression level, sensitivity to ATP and slow desensitization. We therefore designed a final construct with all three mutated to Thr (P2X2-3T), which also closely resembles the Wt channel (Figs 1a; Supplementary Fig 2a and Table 1). All mutations were generated using overlapping PCR and confirmed by DNA sequencing.
Cell Culture and transfection
Human embryonic kidney (HEK293) cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum and 10 mg/L gentamicin. All cell culture reagents were obtained from GIBCO. Trypsin treated HEK293 cells were seeded onto glass coverslips in 12-well plates 1 d before transfection and placed in a 37°C incubator with 95% air and 5% CO2. Transfections were performed using FuGENE6 Transfection Reagent (Roche Applied Science). P2X2 constructs were cotransfected with a green fluorescent protein (GFP) cDNA construct in pGreen-Lantern (Invitrogen) at a ratio of 2:1. Cells were used for whole-cell recording 24-48 hours after transfection.
Electrophysiology
Membrane currents were recorded from HEK cells using the standard whole-cell configuration of the patch-clamp technique 36. Extracellular solutions were rapidly (∼10-50 ms) exchanged using a computer-controlled perfusion system (RSC-200; Biologic). Membrane currents were recorded under voltage-clamp at −60 mV using an Axopatch 200B patch clamp amplifier (Axon Instruments, Inc.) and digitized on-line using a Digidata 1322A interface board and pCLAMP 9 software (Axon Instruments, Inc.). Currents were filtered at 2-5 kHz using either 4- or 8-pole Bessel filters. The standard extracellular solution contained (mM): 140 NaCl, 5.4 KCl, 2 CaCl2, 0.5 MgCl2, 10 HEPES, and 10 D-glucose, adjusted to pH 7.3 with NaOH. The standard pipette solution contained (mM): 140 NaCl, 10 EGTA and 10 HEPES, adjusted to pH 7.0 with NaOH. The external solution for all experiments with Ag+ contained (mM): 140 NaNO3, 5.4 KNO3, 10 EDTA, 10 HEPES at pH 7.3, and 10 EDTA was substitute for 10 EGTA in the pipette solution. Bath and ground chambers were connected by an agar bridge containing 150 mM KCl. Stock aqueous solutions of 1 mM AgNO3 were prepared daily and protected from light until use. For each experiment, freshly prepared extracellular solutions containing a total of 1 μM, 10 μM or 50 μM AgNO3 were used. The free Ag+ concentration in these solutions with 10 mM EDTA was calculated using maxchelator.stanford.edu with appropriate stability constants for our conditions and determined to be 7 nM, 67 nM and 300 nM, respectively. Moderate free concentrations (>70 nM) of Ag+ evoke significant currents in untransfected HEK293 cells, so in most of the experiments low concentrations were used. Solutions containing ATP were freshly prepared every 2 h. Stock solutions of 100 mM MTSET (bromide salt; Toronto Research Chemicals Inc.) were made daily and stored on ice. MTSET was diluted to the desired concentration within 2 min of being applied to cells and the pH of the solution was carefully adjusted with NaOH when 1 mM MTSET was required. ATP, MTSET, Ag+ and Cd2+ were delivered by the rapid perfusion.
Data analysis
Concentration-response relationships for ATP were constructed for each mutant channel and a Hill equation was fit to the data according to:
where I is the normalized current at a given concentration of ATP, Imax is the maximum normalized current, EC50 is the concentration of ATP ([ATP]) producing half-maximal currents and n is the Hill coefficient. Time constants for modification (τ) were obtained by fitting relaxations with a single exponential function and apparent modification rates (R) were calculated according to:
where [M] is the concentration of thiol reactive reagent (MTSET or Ag+).
Supplementary Material
Acknowledgments
We thank Jorge Contreras and Miguel Holmgren for extensive advice in using the thiol reactive compounds described in this study. We also thank Miguel Holmgren, Joe Mindell and members of the Swartz lab for helpful discussions, and the NINDS DNA sequencing facility for DNA sequencing. This work was supported by the Intramural Research Program of the NINDS, NIH.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/neuro
Competing interest statement
The authors declare that they have no competing interest
References
- 1.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–32. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 2.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–67. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 3.Nicke A, et al. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. Embo J. 1998;17:3016–28. doi: 10.1093/emboj/17.11.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoop R, et al. Contribution of individual subunits to the multimeric P2X(2) receptor: estimates based on methanethiosulfonate block at T336C. Mol Pharmacol. 1999;56:973–81. doi: 10.1124/mol.56.5.973. [DOI] [PubMed] [Google Scholar]
- 5.Jiang LH, et al. Subunit arrangement in P2X receptors. J Neurosci. 2003;23:8903–10. doi: 10.1523/JNEUROSCI.23-26-08903.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aschrafi A, Sadtler S, Niculescu C, Rettinger J, Schmalzing G. Trimeric architecture of homomeric P2X2 and heteromeric P2X1+2 receptor subtypes. J Mol Biol. 2004;342:333–43. doi: 10.1016/j.jmb.2004.06.092. [DOI] [PubMed] [Google Scholar]
- 7.Barrera NP, Ormond SJ, Henderson RM, Murrell-Lagnado RD, Edwardson JM. Atomic force microscopy imaging demonstrates that P2X2 receptors are trimers but that P2X6 receptor subunits do not oligomerize. J Biol Chem. 2005;280:10759–65. doi: 10.1074/jbc.M412265200. [DOI] [PubMed] [Google Scholar]
- 8.Evans RJ. Orthosteric and allosteric binding sites of P2X receptors. Eur Biophys J. 2008 doi: 10.1007/s00249-008-0275-2. [DOI] [PubMed] [Google Scholar]
- 9.Silberberg SD, Chang TH, Swartz KJ. Secondary structure and gating rearrangements of transmembrane segments in rat P2X4 receptor channels. J Gen Physiol. 2005;125:347–59. doi: 10.1085/jgp.200409221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Migita K, Samways DS, Voigt MM, Egan TM. Gain and loss of channel function by alanine substitutions in the transmembrane segments of the rat ATP-gated P2X2 receptor. J Neurosci. 2004;24:7378–86. doi: 10.1523/JNEUROSCI.1423-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmgren M, Liu Y, Xu Y, Yellen G. On the use of thiol-modifying agents to determine channel topology. Neuropharmacology. 1996;35:797–804. doi: 10.1016/0028-3908(96)00129-3. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Holmgren M, Jurman ME, Yellen G. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 1997;19:175–84. doi: 10.1016/s0896-6273(00)80357-8. [DOI] [PubMed] [Google Scholar]
- 13.del Camino D, Yellen G. Tight steric closure at the intracellular activation gate of a voltage- gated k(+) channel. Neuron. 2001;32:649–56. doi: 10.1016/s0896-6273(01)00487-1. [DOI] [PubMed] [Google Scholar]
- 14.Haines WR, Voigt MM, Migita K, Torres GE, Egan TM. On the contribution of the first transmembrane domain to whole-cell current through an ATP-gated ionotropic P2X receptor. J Neurosci. 2001;21:5885–92. doi: 10.1523/JNEUROSCI.21-16-05885.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egan TM, Haines WR, Voigt MM. A domain contributing to the ion channel of ATP-gated P2X2 receptors identified by the substituted cysteine accessibility method. J Neurosci. 1998;18:2350–9. doi: 10.1523/JNEUROSCI.18-07-02350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rassendren F, Buell G, Newbolt A, North RA, Surprenant A. Identification of amino acid residues contributing to the pore of a P2X receptor. Embo J. 1997;16:3446–54. doi: 10.1093/emboj/16.12.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang LH, Rassendren F, Spelta V, Surprenant A, North RA. Amino acid residues involved in gating identified in the first membrane-spanning domain of the rat P2X(2) receptor. J Biol Chem. 2001;276:14902–8. doi: 10.1074/jbc.M011327200. [DOI] [PubMed] [Google Scholar]
- 18.Ennion SJ, Evans RJ. Conserved cysteine residues in the extracellular loop of the human P2X(1) receptor form disulfide bonds and are involved in receptor trafficking to the cell surface. Mol Pharmacol. 2002;61:303–11. doi: 10.1124/mol.61.2.303. [DOI] [PubMed] [Google Scholar]
- 19.Clyne JD, Wang LF, Hume RI. Mutational analysis of the conserved cysteines of the rat P2X2 purinoceptor. J Neurosci. 2002;22:3873–80. doi: 10.1523/JNEUROSCI.22-10-03873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silberberg SD, Li M, Swartz KJ. Ivermectin Interaction with transmembrane helices reveals widespread rearrangements during opening of P2X receptor channels. Neuron. 2007;54:263–74. doi: 10.1016/j.neuron.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Stauffer DA, Karlin A. Electrostatic potential of the acetylcholine binding sites in the nicotinic receptor probed by reactions of binding-site cysteines with charged methanethiosulfonates. Biochemistry. 1994;33:6840–9. doi: 10.1021/bi00188a013. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong CM. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971;58:413–37. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong CM. Ionic pores, gates, and gating currents. Q Rev Biophys. 1974;7:179–210. doi: 10.1017/s0033583500001402. [DOI] [PubMed] [Google Scholar]
- 24.Choi KL, Mossman C, Aube J, Yellen G. The internal quaternary ammonium receptor site of Shaker potassium channels. Neuron. 1993;10:533–41. doi: 10.1016/0896-6273(93)90340-w. [DOI] [PubMed] [Google Scholar]
- 25.Cao L, Young MT, Broomhead HE, Fountain SJ, North RA. Thr339-to-serine substitution in rat P2X2 receptor second transmembrane domain causes constitutive opening and indicates a gating role for Lys308. J Neurosci. 2007;27:12916–23. doi: 10.1523/JNEUROSCI.4036-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flynn GE, Zagotta WN. Conformational changes in S6 coupled to the opening of cyclic nucleotide-gated channels. Neuron. 2001;30:689–98. doi: 10.1016/s0896-6273(01)00324-5. [DOI] [PubMed] [Google Scholar]
- 27.Contreras JE, Srikumar D, Holmgren M. Gating at the selectivity filter in cyclic nucleotide-gated channels. Proc Natl Acad Sci U S A. 2008;105:3310–4. doi: 10.1073/pnas.0709809105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Q, Miller C. Silver as a probe of pore-forming residues in a potassium channel. Science. 1995;268:304–7. doi: 10.1126/science.7716526. [DOI] [PubMed] [Google Scholar]
- 29.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–23. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 30.Doyle DA, et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 31.Migita K, Haines WR, Voigt MM, Egan TM. Polar residues of the second transmembrane domain influence cation permeability of the ATP-gated P2X(2) receptor. J Biol Chem. 2001;276:30934–41. doi: 10.1074/jbc.M103366200. [DOI] [PubMed] [Google Scholar]
- 32.Egan TM, Khakh BS. Contribution of calcium ions to P2X channel responses. J Neurosci. 2004;24:3413–20. doi: 10.1523/JNEUROSCI.5429-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yellen G. The voltage-gated potassium channels and their relatives. Nature. 2002;419:35–42. doi: 10.1038/nature00978. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel- Fab complex at 2.0 A resolution. Nature. 2001;414:43–8. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 35.Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–23. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- 36.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.